Abstract
Cyclic phosphatidic acid (CPA) is a naturally occurring analog of the growth factor-like phospholipid mediator, lysophosphatidic acid (LPA). The sn-2 hydroxy group of CPA forms a 5-membered ring with the sn-3 phosphate. CPA affects numerous cellular functions, including antimitogenic regulation of the cell cycle, induction of stress fiber formation, inhibition of tumor cell invasion and metastasis, and regulation of differentiation and survival of neuronal cells. Interestingly, many of these cellular responses caused by CPA oppose those of LPA despite the activation of apparently overlapping receptor populations. Since the early 1990s, studies on CPA actions gradually developed, and we are now beginning to understand the importance of this lipid. In this review, we focus on the current knowledge about CPA, including enzymatic formation of CPA, unique biological activities and biological targets of CPA, and we also explore metabolically stabilized CPA analogs.
Keywords: cyclic phosphatidic acid, transphosphatidylation, cancer cell invasion, tumor metastasis, ATX inhibitor, anti-cancer drugs
Introduction
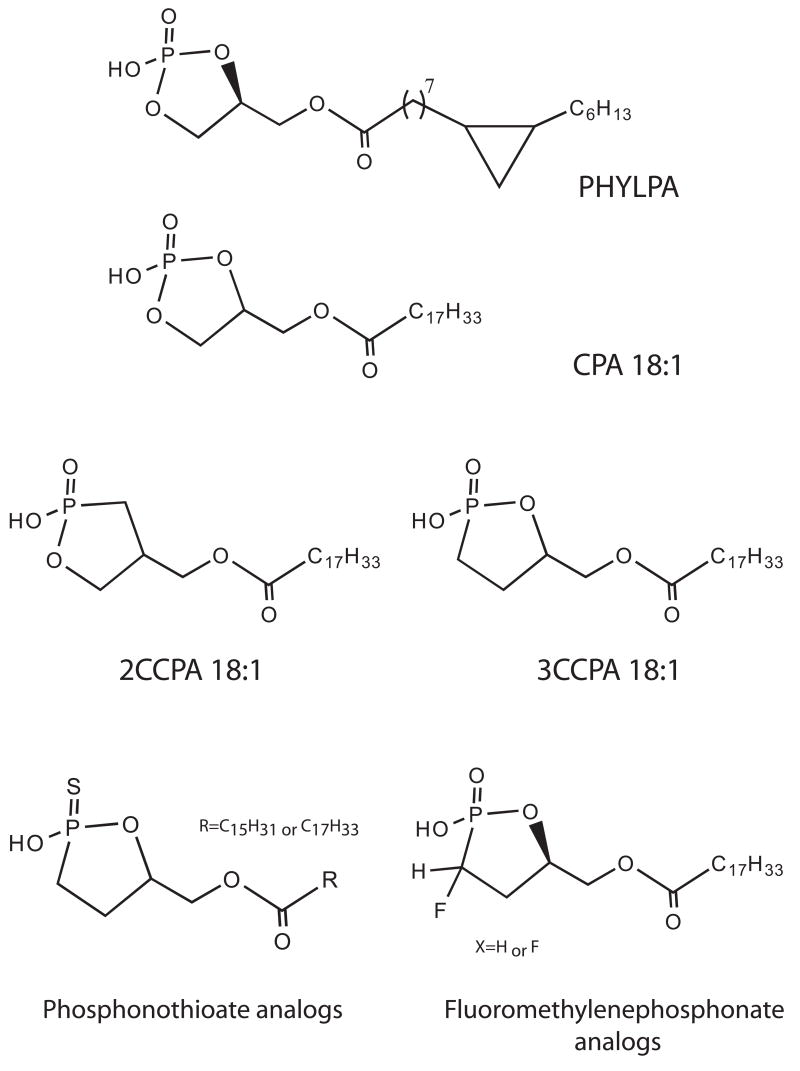
In 1992, the first cyclic phosphatidic acid (1-acyl-sn-glycerol-2,3-cyclic phosphate; CPA) species was isolated from the myxoamoebae of a true slime mold, Physarum polycephalum, and was named Physarum lysophosphatidic acid (PHYLPA) [1]. PHYLPA is structurally related to lysophosphatidic acid (LPA), chemically, the smallest and simplest glycerophospholipid. PHYLPA comprises cyclopropane-containing hexadecanoic acid at sn-1 position and a cyclic phosphate at sn-2 and sn-3 position of the glycerol carbons. A derivative of PHYLPA, having cyclopropane-containing octadecanoic acid, was also isolated from the same organism [2]. These substances inhibit eukaryotic DNA polymerase α and the proliferation of human fibroblasts cultured in a chemically defined serum free medium [3]. A variety of PHYLPA analogs with a cyclic phosphate and various fatty acyl chains were synthesized [4], and these compounds are now referred to as CPA. The biological activities of CPA and the structure-activity relationships indicated that the cyclic phosphate was necessary for the anti-proliferative activity [5]. Subsequent to the discovery of PHYLPA, CPA analogs with various fatty acyl chains were detected in human serum and some other organisms [6]. CPA activates LPA1–5 G-protein coupled receptors (GPCRs) [7–10]. However, the cellular effects of CPA can be remarkably different from those of LPA. CPA inhibits cell proliferation [3, 5, 11], induces actin stress fiber formation [11], promotes differentiation and survival of cultured embryonic hippocampal neurons [12], inhibits LPA-induced platelet aggregation [13], and inhibits cancer cell invasion and metastasis in vitro [14, 15] and in vivo [15, 16]. Recently, metabolically stabilized carba derivatives of CPA (CCPA) were described as a novel inhibitors of metastatic cancer [10, 15]. This finding provides the proof of concept for the anti-metastatic application of CCPA and led us to develop a second generation of CCPA analogs ([17], and see Prestwich et al. in this issue). CCPA and its analogs could be promising anti-cancer drug candidates but their anti-metastatic mechanism of action has not been fully elucidated. Further studies focusing on the molecular target(s) and the mechanism of inhibition of cancer cell invasion and metastasis by CPA will increase the feasibility of use of CPA derivatives in the treatment of cancer. This review summarizes current knowledge about CPA, including enzymatic formation of CPA, unique biological activities of CPA and also biological targets of CPA.
CPA in mammalian body fluids
To answer the question whether CPA is present in mammalian biological fluids, Kobayashi et al. extracted lipids from human serum albumin searching for CPA [6]. Preparative TLC and HPLC analysis revealed that CPA was extracted and purified from human serum albumin. Furthermore, ESI-MS/MS analysis determined that the most abundant molecular species was CPA 16:0, whereas, CPA 14:0 and CPA 18:0 were also present as minor species. These authors estimated the concentration of CPA 16:0 in human serum could be about 0.1 μM. This is less than one-tenth of the concentration of LPA, since the concentration of LPA bound to albumin in mammalian serum is 1–5 μM [18]. Nonetheless, the detection of CPA after the harsh conditions of albumin purification underlines the chemical stability of this lipid. Recently, Shan and colleagues developed an LC/ESI/MS/MS method and successfully quantified the level of two CPA species, CPA 16:0 and CPA 18:1 in human serum [19]. The average concentration of CPA 16:0 in the serum of pre-surgery ovarian cancer patients was significantly lower than that of either post-surgery ovarian cancer patients or normal subjects. These data suggest that CPA might exert some anti-cancer activity in human body and also that surgery can affect the serum CPA levels of ovarian cancer patients. Liliom et al. [20] also reported the evidence that CPA along with other growth factor-like glycerophosphate mediators are present in the aqueous humor and the lacrimal gland fluid of rabbit eyes. The amount of these mediators increases after injury.
Enzymatic formation of CPA
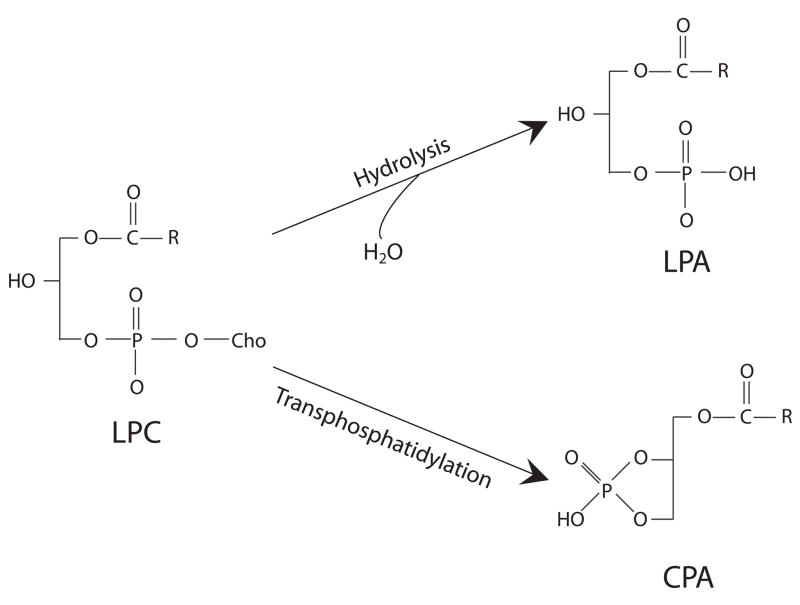
In 1996, Friedman et al. [21] demonstrated that CPA can be formed by intermolecular transphosphatidylation with the hydroxyl on sn-2 position of lysophosphatidylcholine (LPC) by the bacterial phospholipase D (PLD) from Streptomyces chromofuscus. However, the efficiency of transphospatidylation relative to hydrolysis differs among PLD enzymes and the assay conditions. Murakami-Murofushi et al. examined the formation of CPA from LPC by PLD derived from Actinomadurea sp. NO 362 and by PLD from Streptomyces chromofuscus, and found the Actinomadurea enzyme, under certain conditions, preferentially transphosphatidylates rather than hydrolyzes LPA and produces almost exclusively CPA [22]. These results raised the hypothesis that in blood a LPLD/PLD-like enzyme could be responsible for CPA production via transphosphatidylation of abundant LPC.
CPA-producing enzyme in blood
Based on the reaction by Actinomadurea PLD and the fact that LPC, a substrate of PLD, is the second most abundant phopholipid in serum [23], Tsuda et al. reported significant transphosphatidylation activity capable of CPA generation in fetal bovine serum [24]. Furthermore, they purified the enzyme and the peptide mass matching analysis revealed that the purified CPA biosynthetic enzyme shared several peptide fragments with autotaxin (ATX), a serum lysoPLD (LPLD) that also produces LPA [25, 26]. Immunoblot analysis confirmed that active fraction contains a 100-kDa protein that was immunoreactive with human ATX antibody. Moreover, recombinant ATX expressed in Sf9 cells also generated CPA in addition to LPA, and no significant CPA-producing activity was detected in ATX-depleted serum from bovine and human. However, the conditions used in this study (two-phase buffer) do not mimic the physiological milieu and for this reason, ATX may not be the only and most important source of CPA in blood.
Distinguishing features of LPA and CPA action
Although CPA is structurally close to LPA, CPA shows several distinct biological activities. The major physiological and pathophysiological effects of CPA are shown in Table 1.
Table 1.
Major biological effects of CPA
| Effects | References |
|---|---|
| Anti-mitogenic regulation of cell cycle | [3, 5, 20] |
| Actin polymerization and stress fiber formation | [20] |
| Inhibition of platelet aggregation | [13] |
| Activation of Cl− current | [59] |
| Enhancement of neurite outgrowth and survival | [12] |
| Mobilization of intracellular calcium | [3, 9, 10] |
| Inhibition of tumor cell invasion and metastasis | [10, 14–16, 22, 27, 60] |
i. Anti-mitogenic action
When CPA was added to cultures of human fibroblast cells, TIG-3 or TIG-7, in serum-free medium, proliferation was inhibited [3]. In both cell lines, LPA stimulates cell proliferation. In mouse fibroblast NIH3T3, pertussis toxin (PTX) pretreatment inhibited LPA-induced proliferation, whereas the anti-proliferative effect of CPA was unaffected. Thus, PTX-sensitive Gi protein is unlikely to be involved in the anti-proliferative effect induced by CPA.
ii. cAMP elevation
CPA elevates cyclic AMP (cAMP) level via a non-PTX-sensitive mechanism through the activation of a Ca2+-sensitive adenylyl cyclase [11, 22, 27]. In contrast, LPA possesses dual action in cAMP mobilization. LPA decreases cAMP level possibly through Gi-coupled LPA1–3 receptors [28, 29], whereas it increases cAMP level through LPA4 and LPA5 receptors [30, 31]. These results suggest that CPA could elevate cAMP through the activation of LPA4 and/or LPA5. cAMP has been recognized as a modulator of cell proliferation [32, 33], and the elevation of cAMP causes a marked growth-inhibiting effect [34, 35]. These observations led us to assume that cAMP elevation mediates the anti-proliferative action of CPA. However, another possibility for the cell cycle regulation by CPA has also been proposed [22]. CPA directly inhibits the cell cycle regulatory protein Cdc25, which in turn dephosphorylates the cyclin-dependent kinase 2 (Cdk2) resulting in the arrest of the cell cycle progression and the inhibition of cell proliferation. The contribution of these two mechanisms to the CPA-elicited anti-proliferative effect is not known at the time.
iii. Platelet inhibition
The structure-activity analysis of the effect of various LPA analogs on platelet aggregation has been reported [13]. Unlike LPA, CPA with an acyl chain inhibited LPA-induced platelet aggregation, whereas CPA with an alkyl chain was a weak agonist inducing only reversible platelet aggregation. Apparently, cyclization of the phosphate group greatly decreases the aggregating activity of CPA. However, since the alkyl analog of LPA has been found to act on platelets at around 20-fold lower concentrations [36, 37], the same difference was observed between the acyl and alkyl CPA analogs. Furthermore, acyl- and alkyl-CPA had only a marginal effect on platelet cAMP levels. This indicates that CPA does not inhibit platelet aggregation by stimulating adenylate cyclase.
iv. Neurotrophic effect
Since the brain tissue has been found to be the richest source of CPA (0.14 μmol per g wet weight of cerebrum) [12], and LPA has pronounced effect on neuronal cell morphology [38–40], the effects of CPA have been tested in primary neurons established from embryonic rat hippocampi [12]. Nanomolar concentrations of CPA promote neurite outgrowth and enhances the survival of the neuronal cells, suggesting that CPA mimics the effects of the neurotrophin NGF in cultured embryonic hippocampal neurons.
v. Inhibition of tumor cell invasion
Tumor cell invasion across the basement membrane is an important step in the cancer metastasis [41]. An in vitro culture system has been developed by Akedo and colleagues [42], in which the tumor cells are seeded on a mesothelial cell monolayer and a peritoneal dissemination is monitored by counting the number of invasion foci underneath the monolayer. This in vitro system is considered to be a good model for the first step of experimental cancerous peritonitis. Using this system, MM1 rat ascites hepatoma cells, OC-10 human lung cancer cells, PSN-1 human pancreatic adenocarcinoma cells and B16 mouse melanoma cells have been tested for the effect of CPA on transcellular migration [14]. Although LPA was a potent inducer of transmonolayer migration, CPA significantly inhibited transcellular migration. Among some naturally occurring CPA species, CPA 16:0 was the most potent inhibitor and 25 μM CPA 16:0 inhibited the LPA-induced invasion in all of these cell lines by 70–95% [14].
CPA is a stable LPA analog and it has been shown that in tissue culture medium over 75% remains intact up to 24 hrs after incubation [9]. Furthermore, the chemical breakdown of CPA did yield LPA [9]. Nonetheless, CPA could give rise to the formation of LPA by ring opening. To prevent the opening of the cyclic phosphate ring by phospholipases and phosphodiesterase, metabolically stabilized derivatives of CPA have been synthesized [15]. These derivatives are called carba-CPA (CCPA) because the phosphate oxygen is replaced with a methylene (CH2, carba) group at either the sn-2 or sn-3 position. 2CCPA 16:0 and 3CCPA 16:0 at 25 μM significantly inhibited serum- and LPA-induced transcellular migration of MM1 cells [15]. Furthermore, CCPA derivatives with unsaturated fatty acids, CCPA 18:1 or CCPA 16:1, inhibit tumor cell migration more strongly than CCPA 16:0. LPA elicits the transient activation of RhoA, which is an essential event in transcellular tumor cell migration [27]. CPA 16:0 and 2CCPA 16:1 inhibited LPA-induced RhoA activation, suggesting that the anti-metastatic effect of CCPA is achieved by the inhibition of RhoA activation.
vi. Anti-metastatic action
A few recent studies have reported the anti-metastatic effect of CPA and CCPA [43] using the B16 melanoma model in C57BL/6 mice [14, 15] and the azoxymethane-induced intestinal tumor model in Wistar rats [16]. In the B16 mouse melanoma metastasis model, a single injection of CPA 16:0 concomitantly with the tumor cells into the tail vein of C57BL/6 mice suppressed pulmonary metastasis by approximately 50% at 0.2 mg/kg and 90% at 0.4 mg/kg three weeks after the injection [14]. A single injection of CCPA 16:1 and CCPA 18:1 also significantly suppressed pulmonary metastasis of B16 melanoma [15].
In another cancer metastasis animal model, the administration of the gastrointerstinal peptide bombesin significantly increased the metastasis to the peritoneum from intestinal adenocarcinomas induced by azoxymethane in rats [44]. When 3 or 6 mg/kg of CPA was delivered subcutaneously every other day for 30 weeks [16], CPA blocked bombesin-induced metastasis by up to 95% in that model. CPA, at either dose, significantly also decreased the incidence of lymphatic vessel invasion of adenocarcinomas. However, CPA had little or no effect on the body weight, location, histologic type, and depth of involvement or infiltrating growth pattern of the tumors. Although these milestone studies established the profound inhibitory effect of CCPA on metastasis, they fell short of identifying the molecular target(s) of CCPA. (see section ii. Lysophospholipase D, autotaxin)
vii. Respiration and cardiovascular functions
The effects of CPA and CCPA on cancer cell invasion and metastasis hint at the possible usage of these compounds for cancer treatment. For clinical use, it is important to identify the effects of systemically administered compound in vivo on autonomic functions. A study on the effect of CCPA on respiratory and cardiovascular functions in anesthetized rats has recently been reported [45]. Intravenous administration of 3CCPA 18:1 (0.13 or 0.39 mg/kg) increased in tidal volume and respiratory frequency, resulting in an increase in total ventilation. Heart rate was slightly decreased at the 0.39 mg/kg dose, while systemic blood pressure was not affected. Although LPA has a similar structure to CPA, LPA has a potent hypertensive action on systemic blood pressure [46, 47]. CCPA at these doses caused neither vasopressor nor tachycardiac effects, suggesting that systemic administration of CPA will not have major adverse effects.
Biological targets of CPA
i. Receptors
Seven LPA-specific cell surface receptors, LPA1–7, have been identified to date. The LPA1–3 receptors belong to the endothelial differentiation gene (EDG) family of G-protein-coupled receptors (GPCRs) and they are well-characterized. More details of the properties of LPA receptors and signaling can be found in previous reviews [29, 48] and also reviews in this issue. More recently LPA4–7 have been identified as LPA receptors [30, 49–51], which are more closely related to the purino-receptor cluster of GPCRs. Although, CPA is known to activate LPA1–5 receptors [7–10], CPA activates LPA1–4 receptors with a significantly higher EC50 concentration than LPA [10]; however, it has as high an efficacy as LPA for LPA5 receptor (Y Fujiwara, in preparation). Interestingly, LPA4 and LPA5 have been shown to elevate cAMP by LPA [30, 31]. These results lead us to hypothesize that an elevation of cAMP by CPA reported previously could be mediated by the activation of LPA4 or/and LPA5. However, the possibility of the existence of as-yet-unknown CPA selective receptor(s) cannot be ruled out. Identification and characterization of CPA receptors will be important to shed more light on the understanding of CPA actions and signaling.
ii. Lysophospholipase D, autotaxin
It has been more than two decades since LPLD activity that produced LPA from LPC was detected in rat plasma [23]. However, in contrast to the numerous studies on LPA signaling mediated by the LPA receptors, the enzymes regulating LPA production and degradation had not been characterized until recently. In 2002, plasma LPLD was purified and found to be identical to ATX [25, 47]. ATX was known to be a tumor motility-stimulating protein and ATX enhances the invasion and metastatic dissemination of tumors [52, 53]. Affymetrix gene chip assays of 12,000 genes revealed that ATX was one of the 40 most unregulated genes associated with highly metastatic cancers [54]. More interestingly, van Meeteren et al. recently showed that ATX is under feedback inhibition by its hydrolysis products LPA and sphingosine-1-phosphate [55]. Thus, LPA generated by ATX can inhibit its own production and this inhibition is overcome by the constitutive secretion of ATX in cancer cells. This finding gave us the incentive to screen various LPA analogs for the ability to inhibit ATX. Analysis of ATX activity by measuring ATX-mediated hydrolysis of fluorescent substrates revealed that 2- and 3-CCPA analogs strongly inhibited ATX activity and LPA production [10]. 2CCPA 16:1 was the most effective inhibitor with an IC50 of 140 nM, which is more than 100-fold lower than LPA in this assay system. LPA receptor activation studies showed that the 3CCPA analogs neither activate nor inhibit LPA1–4 receptors, and 2CCPA 16:1 and 2CCPA 18:1 were partial agonists of these receptors. The activation profiles of these analogs are important because LPA receptors have been reported to be associated with tumor cell invasion [56]. Cell invasion assay using matrigel-coated modified Boyden chamber showed that co-treatment of CCPA analogs with ATX and LPC resulted in a significant decrease of tumor cell invasion [10]. Furthermore, exogenously added LPA can overcome this inhibitory effect of CCPA on ATX-induced cell invasion, suggesting that CCPA elicits its inhibitory effect on cancer cell invasion by targeting ATX and decreasing the production of LPA without either activating or inhibiting LPA receptors.
It is important to point out that addition of LPC 18:1, the substrate of ATX, does not induce transcellular migration in MM1 cells [43], suggesting that the LPLD activity of ATX generated by these cells does not appear to produce sufficient LPA 18:1 to elicit cell migration. Therefore, the mechanism of inhibition described in this cell line does not solely depend on ATX inhibition and point out a multiplicity of targets.
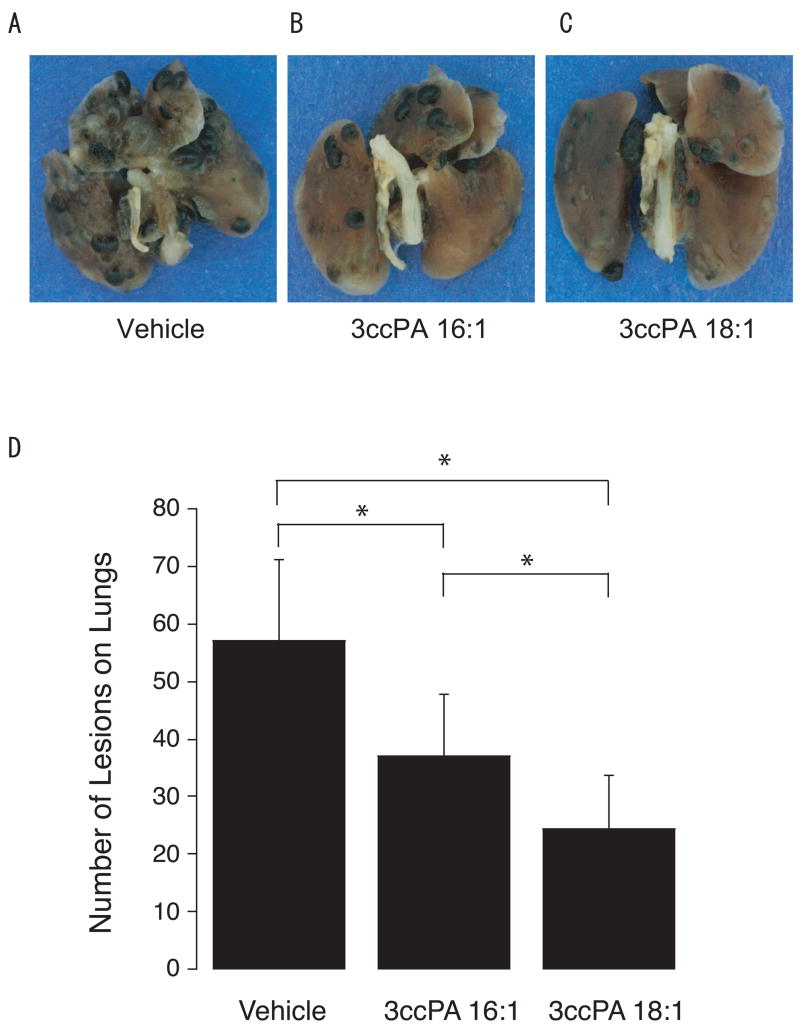
B16F10 mouse melanoma cells are widely used as a model to assess the therapeutic effect of anti-metastatic compounds in vivo. In this model, intraperitoneal injections 3CCPA 16:1 and 3CCPA 18:1 blocked B16F10 tumor cell-derived lung metastasis by 35 and 57%, respectively, with only two 0.25 mg/kg doses administrated at 15 min and 48 hrs after inoculation. These data suggest that CCPA inhibited the formation of metastatic foci. Taken together these results validate ATX as a target of anti-metastatic treatment, and provide a proof of concept for the anti-metastatic effect of the CCPA analogs.
Summary and Future Direction
Over the last 15 years, our understanding of CPA actions has progressed gradually. CPA has emerged as a potential anti-metastatic drug candidate but the mechanisms responsible for this effect remain unresolved. Clinically, the promising drug candidates would be the compounds that inhibit both ATX and LPA receptor subtypes that promote invasion of cancer cells. Molecular pharmacological studies are underway by several groups, aiming to identify novel non-lipid ligand scaffolds using in silico screening and to synthesize antagonists that selectively block lysophospholipid targets ([17, 57] and see Prestwich et al. in this issue). Further studies on molecular target(s) as well as the mechanism of these compounds will increase the feasibility of the compounds in the treatment of cancer metastasis.
Figure 1. Structures of PHYLPA, CPA, CCPA and CCPA analogs.
For details of phosphonothioate and fluoromethylene phosphonate analogs, see [58] and Prestwich et al. in this issue
Figure 2.
Enzymatic synthesis of CPA from LPC by PLD
Figure 3.
Inhibition of metastasis in vivo: B16F10 melanoma cells were injected in the tail vein of C57BL/6 mice, followed by i.p. injections of vehicle (PBS) (A), 3CCPA 16:1 (B) or 3CCPA 18:1 (C). Animals were sacrificed 3 weeks later and lung nodules were counted (D). (* p < 0.01 ANOVA). For details see [10].
Acknowledgments
This work was supported by American Heart Association Postdoctoral Fellowship 0625325B (to Yuko Fujiwara), National Institutes of Health grant R01 HL 061469 and CA 092160 (to Gabor Tigyi).
I would like to thank my supervisor during my graduate course, Professor Kimiko Murofushi (Ochanomizu University, Tokyo, Japan), who introduced me to the many interesting projects that CPA offers. I would also like to express my gratitude to Professor Gabor Tigyi (University of Tennessee), who provided me with extensive and supportive training during my Ph.D. studies and continuing research.
Abbreviation used
- CPA
cyclic phosphatidic acid
- GPCR
G-protein coupled receptors
- LPA
lysophosphatidic acid
- CCPA
carba-CPA
- PLD
phospholipase D
- LPC
lysophosphatidyl choline
- ATX
autotaxin
- PTX
pertussis toxin
- cAMP
cyclic AMP
- EDG
endothelial differentiation gene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murakami-Murofushi K, Shioda M, Kaji K, Yoshida S, Murofushi H. Inhibition of eukaryotic DNA polymerase alpha with a novel lysophosphatidic acid (PHYLPA) isolated from myxoamoebae of Physarum polycephalum. J Biol Chem. 1992;267:21512–7. [PubMed] [Google Scholar]
- 2.Takahashi Y, Shimada Y, Shioda M, Yoshida S, Murofushi H, Murakami-Murofushi K. Isolation of a new species of Physarum lysophosphatidic acid, PHYLPA, and its effect on DNA polymerase activity. Cell Struct Funct. 1993;18:135–8. doi: 10.1247/csf.18.135. [DOI] [PubMed] [Google Scholar]
- 3.Murakami-Murofushi K, Kaji K, Kano K, Fukuda M, Shioda M, Murofushi H. Inhibition of cell proliferation by a unique lysophosphatidic acid, PHYLPA, isolated from Physarum polycephalum: signaling events of antiproliferative action by PHYLPA. Cell Struct Funct. 1993;18:363–70. doi: 10.1247/csf.18.363. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi S, Tokunoha Ryosuke, Shibasaki Masakatsu, Shinagawa Rumi, Murakami-Murofushi Kimiko. Synthesis of 1-O-acylglycerol 2, 3-cyclic phosphate: Determination of the absolute structure of PHYLPA, a specific inhibitor of DNA polymerase α. Tetrahedron Letters. 1993;34:4047–4050. [Google Scholar]
- 5.Murakami-Murofushi K, Kobayashi S, Onimura K, Matsumoto M, Shioda M, Yoshida S, Shoji M, Murofushi H. Selective inhibition of DNA polymerase-alpha family with chemically synthesized derivatives of PHYLPA, a unique Physarum lysophosphatidic acid. Biochim Biophys Acta. 1995;1258:57–60. doi: 10.1016/0005-2760(95)00097-v. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, Tanaka-Ishii R, Taguchi R, Ikezawa H, Murakami-Murofushi K. Existence of a bioactive lipid, cyclic phosphatidic acid, bound to human serum albumin. Life Sci. 1999;65:2185–91. doi: 10.1016/s0024-3205(99)00483-x. [DOI] [PubMed] [Google Scholar]
- 7.Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett. 2000;478:159–65. doi: 10.1016/s0014-5793(00)01827-5. [DOI] [PubMed] [Google Scholar]
- 8.Fischer DJ, Murakami-Murofushi K, Tigyi G. Characterization of endogenous and heterologously expressed LPA receptor subtypes. Ann N Y Acad Sci. 2000;905:287–9. doi: 10.1111/j.1749-6632.2000.tb06562.x. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara Y, Sardar V, Tokumura A, Baker D, Murakami-Murofushi K, Parrill A, Tigyi G. Identification of residues responsible for ligand recognition and regioisomeric selectivity of lysophosphatidic acid receptors expressed in mammalian cells. J Biol Chem. 2005;280:35038–50. doi: 10.1074/jbc.M504351200. [DOI] [PubMed] [Google Scholar]
- 10.Baker DL, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, Murofushi H, Uchiyama A, Murakami-Murofushi K, Koh E, Bandle RW, Byun HS, Bittman R, Fan D, Murph M, Mills GB, Tigyi G. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J Biol Chem. 2006;281:22786–93. doi: 10.1074/jbc.M512486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer DJ, Liliom K, Guo Z, Nusser N, Virag T, Murakami-Murofushi K, Kobayashi S, Erickson JR, Sun G, Miller DD, Tigyi G. Naturally occurring analogs of lysophosphatidic acid elicit different cellular responses through selective activation of multiple receptor subtypes. Mol Pharmacol. 1998;54:979–88. doi: 10.1124/mol.54.6.979. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara Y, Sebok A, Meakin S, Kobayashi T, Murakami-Murofushi K, Tigyi G. Cyclic phosphatidic acid elicits neurotrophin-like actions in embryonic hippocampal neurons. J Neurochem. 2003;87:1272–83. doi: 10.1046/j.1471-4159.2003.02106.x. [DOI] [PubMed] [Google Scholar]
- 13.Gueguen G, Gaige B, Grevy JM, Rogalle P, Bellan J, Wilson M, Klaebe A, Pont F, Simon MF, Chap H. Structure-activity analysis of the effects of lysophosphatidic acid on platelet aggregation. Biochemistry. 1999;38:8440–50. doi: 10.1021/bi9816756. [DOI] [PubMed] [Google Scholar]
- 14.Mukai M, Imamura F, Ayaki M, Shinkai K, Iwasaki T, Murakami-Murofushi K, Murofushi H, Kobayashi S, Yamamoto T, Nakamura H, Akedo H. Inhibition of tumor invasion and metastasis by a novel lysophosphatidic acid (cyclic LPA) Int J Cancer. 1999;81:918–22. doi: 10.1002/(sici)1097-0215(19990611)81:6<918::aid-ijc13>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama A, Mukai M, Fujiwara Y, Kobayashi S, Kawai N, Murofushi H, Inoue M, Enoki S, Tanaka Y, Niki T, Kobayashi T, Tigyi G, Murakami-Murofushi K. Inhibition of transcellular tumor cell migration and metastasis by novel carba-derivatives of cyclic phosphatidic acid. Biochim Biophys Acta. 2007;1771:103–12. doi: 10.1016/j.bbalip.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishihara R, Tatsuta M, Iishi H, Baba M, Uedo N, Higashino K, Mukai M, Ishiguro S, Kobayashi S, Murakami-Murofushi K. Attenuation by cyclic phosphatidic acid of peritoneal metastasis of azoxymethane-induced intestinal cancers in Wistar rats. Int J Cancer. 2004;110:188–93. doi: 10.1002/ijc.20069. [DOI] [PubMed] [Google Scholar]
- 17.Durgam GG, Tsukahara R, Makarova N, Walker MD, Fujiwara Y, Pigg KR, Baker DL, Sardar VM, Parrill AL, Tigyi G, Miller DD. Synthesis and pharmacological evaluation of second-generation phosphatidic acid derivatives as lysophosphatidic acid receptor ligands. Bioorg Med Chem Lett. 2006;16:633–40. doi: 10.1016/j.bmcl.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291(Pt 3):677–80. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan L, Li S, Jaffe K, Davis L. Quantitative determination of cyclic phosphatidic acid in human serum by LC/ESI/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;862:161–7. doi: 10.1016/j.jchromb.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Liliom K, Guan Z, Tseng JL, Desiderio DM, Tigyi G, Watsky MA. Growth factor-like phospholipids generated after corneal injury. Am J Physiol. 1998;274:C1065–74. doi: 10.1152/ajpcell.1998.274.4.C1065. [DOI] [PubMed] [Google Scholar]
- 21.Friedman P, Haimovitz R, Markman O, Roberts MF, Shinitzky M. Conversion of lysophospholipids to cyclic lysophosphatidic acid by phospholipase D. J Biol Chem. 1996;271:953–7. doi: 10.1074/jbc.271.2.953. [DOI] [PubMed] [Google Scholar]
- 22.Murakami-Murofushi K, Uchiyama A, Fujiwara Y, Kobayashi T, Kobayashi S, Mukai M, Murofushi H, Tigyi G. Biological functions of a novel lipid mediator, cyclic phosphatidic acid. Biochim Biophys Acta. 2002;1582:1–7. doi: 10.1016/s1388-1981(02)00131-2. [DOI] [PubMed] [Google Scholar]
- 23.Tokumura A, Harada K, Fukuzawa K, Tsukatani H. Involvement of lysophospholipase D in the production of lysophosphatidic acid in rat plasma. Biochim Biophys Acta. 1986;875:31–8. [PubMed] [Google Scholar]
- 24.Tsuda S, Okudaira S, Moriya-Ito K, Shimamoto C, Tanaka M, Aoki J, Arai H, Murakami-Murofushi K, Kobayashi T. Cyclic phosphatidic acid is produced by autotaxin in blood. J Biol Chem. 2006;281:26081–8. doi: 10.1074/jbc.M602925200. [DOI] [PubMed] [Google Scholar]
- 25.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–33. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–42. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 27.Mukai M, Nakamura H, Tatsuta M, Iwasaki T, Togawa A, Imamura F, Akedo H. Hepatoma cell migration through a mesothelial cell monolayer is inhibited by cyclic AMP-elevating agents via a Rho-dependent pathway. FEBS Lett. 2000;484:69–73. doi: 10.1016/s0014-5793(00)02129-3. [DOI] [PubMed] [Google Scholar]
- 28.van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- 29.Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem. 2004;279:20555–8. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 30.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–97. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 31.Yanagida K, Ishii S, Hamano F, Noguchi K, Shimizu T. LPA4/p2y9/GPR23 mediates rho-dependent morphological changes in a rat neuronal cell line. J Biol Chem. 2007;282:5814–24. doi: 10.1074/jbc.M610767200. [DOI] [PubMed] [Google Scholar]
- 32.Pastan IH, Johnson GS, Anderson WB. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Graves M, Lawrence John C. Insulin, growth factors, and cAMP Antagonism in the signal transduction pathways. Trends in Endocrinology and Metabolism. 1996;7:43–50. doi: 10.1016/1043-2760(95)00204-9. [DOI] [PubMed] [Google Scholar]
- 34.Sevetson BR, Kong X, Lawrence JC., Jr Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 1993;90:10305–9. doi: 10.1073/pnas.90.21.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw TJ, Keszthelyi EJ, Tonary AM, Cada M, Vanderhyden BC. Cyclic AMP in ovarian cancer cells both inhibits proliferation and increases c-KIT expression. Exp Cell Res. 2002;273:95–106. doi: 10.1006/excr.2001.5426. [DOI] [PubMed] [Google Scholar]
- 36.Tokumura A, Sinomiya J, Kishimoto S, Tanaka T, Kogure K, Sugiura T, Satouchi K, Waku K, Fukuzawa K. Human platelets respond differentially to lysophosphatidic acids having a highly unsaturated fatty acyl group and alkyl ether-linked lysophosphatidic acids. Biochem J. 2002;365:617–28. doi: 10.1042/BJ20020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G, Siess W. Subtype-selective antagonists of lysophosphatidic Acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108:741–7. doi: 10.1161/01.CIR.0000083715.37658.C4. [DOI] [PubMed] [Google Scholar]
- 38.Jalink K, Eichholtz T, Postma FR, van Corven EJ, Moolenaar WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 1993;4:247–55. [PubMed] [Google Scholar]
- 39.Tigyi G, Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992;267:21360–7. [PubMed] [Google Scholar]
- 40.Fukushima N, Weiner JA, Chun J. Lysophosphatidic acid (LPA) is a novel extracellular regulator of cortical neuroblast morphology. Dev Biol. 2000;228:6–18. doi: 10.1006/dbio.2000.9930. [DOI] [PubMed] [Google Scholar]
- 41.Liotta LA. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 42.Akedo H, Shinkai K, Mukai M, Mori Y, Tateishi R, Tanaka K, Yamamoto R, Morishita T. Interaction of rat ascites hepatoma cells with cultured mesothelial cell layers: a model for tumor invasion. Cancer Res. 1986;46:2416–22. [PubMed] [Google Scholar]
- 43.Imamura F, Horai T, Mukai M, Shinkai K, Sawada M, Akedo H. Induction of in vitro tumor cell invasion of cellular monolayers by lysophosphatidic acid or phospholipase D. Biochem Biophys Res Commun. 1993;193:497–503. doi: 10.1006/bbrc.1993.1651. [DOI] [PubMed] [Google Scholar]
- 44.Iishi H, Tatsuta M, Baba M, Yamamoto R, Taniguchi H. Enhancement by bombesin of colon carcinogenesis and metastasis induced by azoxymethane in Wistar rats. Int J Cancer. 1992;50:834–9. doi: 10.1002/ijc.2910500529. [DOI] [PubMed] [Google Scholar]
- 45.Hotta H, Kagitani F, Murakami-Murofushi K. Cyclic phosphatidic acid stimulates respiration without producing vasopressor or tachycardiac effects in rats. Eur J Pharmacol. 2006;543:27–31. doi: 10.1016/j.ejphar.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 46.Tokumura A, Yotsumoto T, Masuda Y, Tanaka S. Vasopressor effect of lysophosphatidic acid on spontaneously hypertensive rats and Wistar Kyoto rats. Res Commun Mol Pathol Pharmacol. 1995;90:96–102. [PubMed] [Google Scholar]
- 47.Tokumura A. Physiological and pathophysiological roles of lysophosphatidic acids produced by secretory lysophospholipase D in body fluids. Biochim Biophys Acta. 2002;1582:18–25. doi: 10.1016/s1388-1981(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 48.Fukushima N, Chun J. The LPA receptors. Prostaglandins Other Lipid Mediat. 2001;64:21–32. doi: 10.1016/s0090-6980(01)00105-8. [DOI] [PubMed] [Google Scholar]
- 49.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–28. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- 50.Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363:861–6. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 51.Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40:329–34. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- 52.Nam SW, Clair T, Campo CK, Lee HY, Liotta LA, Stracke ML. Autotaxin (ATX), a potent tumor motogen, augments invasive and metastatic potential of ras-transformed cells. Oncogene. 2000;19:241–7. doi: 10.1038/sj.onc.1203263. [DOI] [PubMed] [Google Scholar]
- 53.Nam SW, Clair T, Kim YS, McMarlin A, Schiffmann E, Liotta LA, Stracke ML. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res. 2001;61:6938–44. [PubMed] [Google Scholar]
- 54.Euer N, Schwirzke M, Evtimova V, Burtscher H, Jarsch M, Tarin D, Weidle UH. Identification of genes associated with metastasis of mammary carcinoma in metastatic versus non-metastatic cell lines. Anticancer Res. 2002;22:733–40. [PubMed] [Google Scholar]
- 55.van Meeteren LA, Ruurs P, Christodoulou E, Goding JW, Takakusa H, Kikuchi K, Perrakis A, Nagano T, Moolenaar WH. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem. 2005;280:21155–61. doi: 10.1074/jbc.M413183200. [DOI] [PubMed] [Google Scholar]
- 56.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–91. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 57.Parrill AL, Echols U, Nguyen T, Pham TC, Hoeglund A, Baker DL. Virtual screening approaches for the identification of non-lipid autotaxin inhibitors. Bioorg Med Chem. 2007 doi: 10.1016/j.bmc.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 58.Xu Y, Jiang G, Tsukahara R, Fujiwara Y, Tigyi G, Prestwich GD. Phosphonothioate and fluoromethylene phosphonate analogues of cyclic phosphatidic acid: Novel antagonists of lysophosphatidic acid receptors. J Med Chem. 2006;49:5309–15. doi: 10.1021/jm060351+. [DOI] [PubMed] [Google Scholar]
- 59.Liliom K, Murakami-Murofushi K, Kobayashi S, Murofushi H, Tigyi G. Xenopus oocytes express multiple receptors for LPA-like lipid mediators. Am J Physiol. 1996;270:C772–7. doi: 10.1152/ajpcell.1996.270.3.C772. [DOI] [PubMed] [Google Scholar]
- 60.Mukai M, Iwasaki T, Tatsuta M, Togawa A, Nakamura H, Murakami-Murofushi K, Kobayashi S, Imamura F, Inoue M. Cyclic phosphatidic acid inhibits RhoA-mediated autophosphorylation of FAK at Tyr-397 and subsequent tumor-cell invasion. Int J Oncol. 2003;22:1247–56. [PubMed] [Google Scholar]