Abstract
While many point-of-care (POC) diagnostic methods have been developed for blood-borne analytes, development of saliva-based POC diagnostics is in its infancy. We have developed a portable microfluidic device for detection of potential biomarkers of periodontal disease in saliva. The device performs rapid microfluidic chip-based immunoassays (<3–10 min) with low sample volume requirements (10 μL) and appreciable sensitivity (nM–pM). Our microfluidic method facilitates hands-free saliva analysis by integrating sample pretreatment (filtering, enrichment, mixing) with electrophoretic immunoassays to quickly measure analyte concentrations in minimally pretreated saliva samples. The microfluidic chip has been integrated with miniaturized electronics, optical elements, such as diode lasers, fluid-handling components, and data acquisition software to develop a portable, self-contained device. The device and methods are being tested by detecting potential biomarkers in saliva samples from patients diagnosed with periodontal disease. Our microchip-based analysis can readily be extended to detection of biomarkers of other diseases, both oral and systemic, in saliva and other oral fluids.
Keywords: microfluidics, periodontal disease, diagnostics, point-of-care, POC, immunoassay, lab-on-a-chip, saliva
Throughout the last decade, research studies using saliva as a diagnostic fluid have increased exponentially.1 The primary benefits of saliva-based tests, over more common blood tests, include easier, noninvasive saliva collection, and the lower costs associated with saliva testing.2–4 The increased interest in saliva diagnostics has also been spurred by rapidly accumulating evidence of correlation between saliva analyte levels and those in serum. Thus, saliva testing is not only a means to monitor oral health, but is now viewed as a potential window into the overall systemic health of an individual. Saliva diagnosis of autoimmune disorders (i.e., Sjögren’s syndrome), cardiovascular diseases, abnormal endocrine function, presence of infection (viral, bacterial), renal disease, cancer, and abuse of drugs are all areas that have benefited from using saliva as a diagnostic fluid.1 Saliva testing is also being explored for directing and monitoring treatment options. For example, drug doses can be monitored without inconvenient and costly visits to blood-drawing facilities.
Agencies such as the Office of the Surgeon General5 and the NIDCR6 have recognized the potential of saliva as a diagnostic fluid and have thus called for increased research and development of saliva-based testing. While the potential value of saliva as a diagnostic fluid has become more apparent, the adoption of saliva as a routine diagnostic fluid has met with three general types of barriers. As outlined by the 1999 NIDCR workshop for saliva diagnostics development, barriers to acceptance and use of saliva can be broadly characterized as follows: need for innovation in accurate analyte measurements in small volumes and development of standardized saliva collection methods; lack of investment in product development; and low technology adoption rates by clinicians and medical insurance companies.
Since 1999, substantial technological barriers to widespread use of saliva in diagnostics have begun to diminish. Recent development of techniques that combine the power of miniaturization with cutting-edge discoveries in fields once as distinct as biology and engineering is leading to rapid, high-throughput, automated, portable, low-cost, and efficient biochemical analyses with small sample volumes.
MICROFLUIDIC METHODS CONFER ADVANTAGES TO DIAGNOSTIC DESIGN AND USAGE PARADIGMS
With recent advances in proteomics and systems biology, it is evident that multi-parameter diagnostic approaches are needed for clinical use.7 Traditional single-marker approaches are based on the expectation that a change in the concentration of a single protein can unambiguously specify disease. In reality, diseases exhibit great heterogeneity between individuals; the same disease can be initiated by numerous factors and can cause a range of molecular changes. Thus, single-marker tests generally suffer from lack of sensitivity and specificity.8 Diagnostics developed for complex diseases require the analysis of multiple components to effectively (1) predict the onset of disease, (2) stratify disease (e.g., prostate cancer could be subcategorized as three or four distinct diseases), (3) indicate the progression of the disease, (4) direct treatment (identify resistance to drugs or potential adverse reactions, etc.), and (5) monitor treatment. For each type of disease, the informative set of markers will be different. To accomplish this goal, low-cost high-throughput methods that safely make use of small sample volumes are necessary.
Researchers have envisioned miniaturized diagnostic technologies that could accurately ascertain disease states using droplets (tens of microliters) of human body fluid. Recent reports describe lab-on-a-chip instruments that perform multiple operations in parallel in extralaboratory settings (i.e., field deployment, near-patient environments, and resource-poor settings).9–11 Current technologies not only provide paths toward such implementation, but are also presently being exploited and demonstrated. While recent reports show promise for microfluidic detection of analytes in various body fluids,11–14 few, if any, reports on detection of endogenous biomarkers in saliva using a semiportable instrument have been made. We believe that operating specifications for such instrumentation can be described by the following characteristics:
Saliva-based: Ready collection of samples by trained or untrained personnel.
Microfluidic: Requires small volumes of saliva.
Multiplexed detection: Simultaneous analysis of multiple analytes for accurate assessment of complex diseases.
Portable and easy to use: Point-of-care (POC) testing with simple user interface.
Rapid: Simultaneous protein measurements within a routine clinical visit.
Low cost: Feasible widespread screening, diagnosis, and monitoring.
Key advances in bioanalytical instrument design, both at Sandia and elsewhere, have relied upon microfluidic technologies to enable sensitive and fast analyses, especially for manipulation of sub-microliter fluid volumes. Such significant technological advances in miniaturization (at the micro- and nanoscales) have led to the advent of valuable new diagnostic formats, known as “lab-on-a-chip” devices.
Realizing the potential miniaturization and automation made possible through the lab-on-a-chip paradigm requires integration of various functionalities. Incorporation of unit functionalities (fluid containment and fluid handling in channels similar in dimensions to a human hair; hands-free operation of sample preparation steps; automated bioanalytical assays; integrated optical systems for fluorescence-based detection; and subsequent reporting of assay results) is essential to conduct sophisticated analyses. Sandia has recently reported one of the first lab-on-a-chip systems to demonstrate full integration and hand-portable operation.9 Innovation regarding portable, rapid, specific bioanalytical methods and associated hardware has been undertaken at Sandia for applications ranging from biotoxin detection9,15,16 to proteomics17–19 to cell sorting20,21 and clinical diagnostics.22
Building on technologies introduced above, our group is actively developing an integrated microfluidic platform for oral diagnostics (IMPOD). An image of an early hardware version of the portable diagnostic is based on that developed at Sandia previously9 and is shown in Figure 1. A descriptive summary of the technology is given in Table 1. IMPOD, as well as quantitative, portable instrumentation like it, has the potential to be translated to clinical settings for use in rapid, near-patient analysis of human saliva and oral fluids. The methods and technologies presented here also have applicability to nonoral diagnostic fluids, as well as other local and systemic diseases.
FIGURE 1.
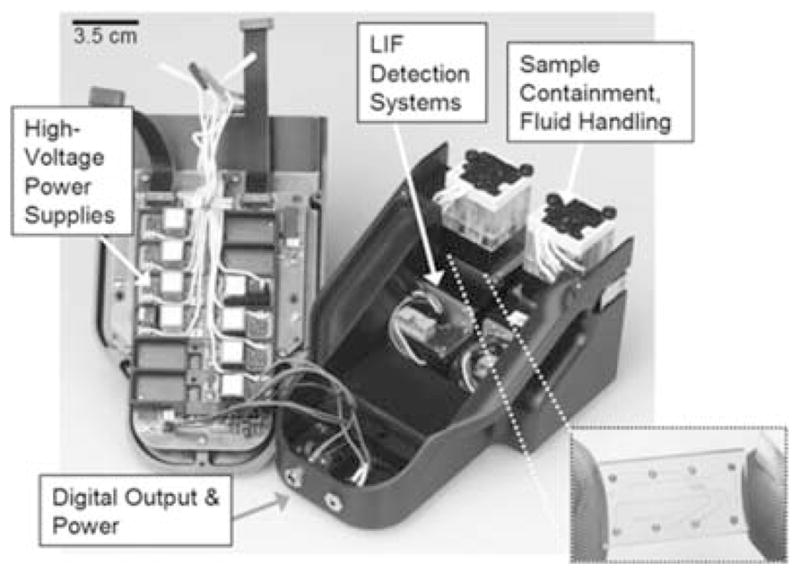
Image of early generation IMPOD with hardware components labeled. Inset shows glass microdevice used in performing immunoassays.
TABLE 1.
Summary of IMPOD characteristics
| Automated operation |
|---|
| Manual volume-independent sample introduction via syringe or pipette (no volume dependence, if > 50 μL) |
| Automated sample preparation and associated timing |
| Integrated high-voltage power supply (drives mixing, preconcentration, and electrophoresis) |
| Automated assay and replicate runs (timing) |
| Electronic data collection via software interface |
| Sample introduction and pre-processing |
| Off-chip: centrifugation and dilution |
| On-chip: debris removal (filtering); sample preconcentration; rapid mixing |
| Microfluidic immunoassay specificity and selectivity |
| Multiplexing: serial format demonstrated; parallel format in testing stage |
| “Capture chemistry”: high-affinity, fluorescent receptors provide specificity |
| Tunable sieving matrix provides assay selectivity and resolution |
| Analysis of saliva from subjects in both healthy |
| and periodontal-diseased categories |
| Gold-standard results comparison via validation assays (ELISA, protein microarray) |
| Miniaturized detection system |
| Fluorescent receptor molecules provide specific detection signal |
| Diode laser induced fluorescence excitation |
| Photomultiplier tube (PMT) fluorescence detection |
| Data analysis |
| Software-based data analysis (research stage) |
MICROFLUIDIC ELECTROPHORETIC IMMUNOASSAYS AS CORE ANALYTICAL METHOD
Use of microfluidic technologies naturally confers assay speed, low-reagent volume consumption, and streamlined sample preparation and assay integration to the IMPOD diagnostic platform. By incorporating photopolymerized cross-linked polyacrylamide gels within a microfluidic device and using immunoassays, we have further conferred high-resolution sieving-based performance and fine specificity to the microanalytical platform.
Combining the specificity and selectivity typical of conventional immunoassays with high-efficiency separations achieved by microfluidic methods, our demonstrated technology efficiently separates receptor-bound and unbound species, resulting in quantitation of only those analytes of interest. A schematic description of the assay protocol is in Figure 2. Such an approach avoids problems associated with open-channel protein electrophoresis. Polyacrylamide gel electrophoresis (PAGE) has been used for decades for protein analysis and does not exhibit nonspecific adsorption of proteins. Channel surfaces are coated with linear polyacrylamide23,24 using photoinitiated polymerization. Cross-linked polyacrylamide gels are made in situ by photopolymerization using methods reported by our group.25,26
FIGURE 2.
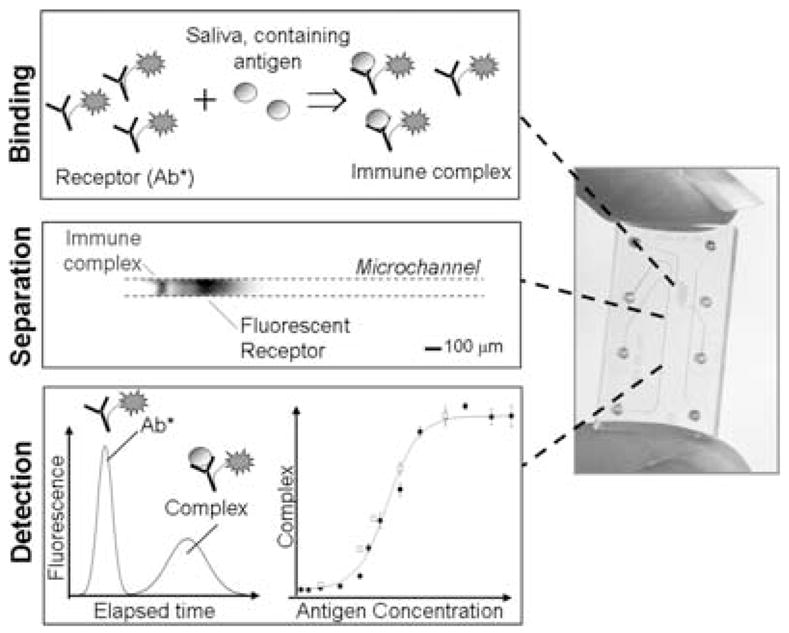
Microchip-based electrophoretic immunoassays. (left) Protocol schematic: Binding/Preconcentration: The sample is mixed with a known amount of fluorescently labeled antibody. The mixing is done off-chip or on-chip. During initial methods development, we carry out the mixing outside the chip (requires ~15 min). Upon optimization of immunoassay conditions, the sample and the labeled antibody are added to different reservoirs in the sample manifold and are mixed (in <3 min) electrokinetically on-chip. Separation: Mixed sample is electrophoretically injected into a separation channel. The antibody-bound antigen and unbound antibody are separated based on size and charge by native polyacrylamide gel electrophoresis. Detection & Analysis: A laser-induced fluorescence detector detects the unbound antibody and bound antibody (i.e., immune complex) peaks. Migration times are recorded and peak areas are calculated. The immunoassay is repeated with samples containing known amounts of analyte to generate a multipoint calibration curve. The calibration curve is used to estimate the concentration of analyte in the unknown sample. (right) Image of glass microdevice.
Electrophoretic immunoassays offer a number of advantages over conventional radioimmunoassays or enzyme-linked immunosorbent assays (ELISA).27 Capillary electrophoresis is a highly effective separation method allowing separation of bound from unbound species in a single step, thus eliminating the multiple wash steps required in conventional immunoassays. Immobilization of reporter molecules (antibodies) on sensor surfaces is unnecessary. Electrophoretic immunoassays are typically performed in an open or surface-modified microfluidic channel.28–31 In contrast to conventional capillary zone electrophoresis approaches, we have relied upon sieving–gelbased electrophoretic immunoassays, as open channel (or free solution) immunoassays suffer from a number of disadvantages:
Difficulty in attaining adequate species discrimination with electrophoresis-based immunoassays on account of the small differences in charge-to-mass characteristics between large antibodies and immune complexes.
Vulnerability to analyte dispersion arising from difficult-to-control hydrodynamic flow.
Difficulty associated with integration of open channel components with complex chip-based systems (e.g., sample preprocessing functionalities).
The microfluidic components that form the core of IMPOD integrate multiple functions (e.g., sample injection, mixing and incubation with antibodies, sample enrichment, and separation of bound and unbound immune complexes). The immunoassays themselves take minutes to complete (compared to hours for conventional quantitative ELISA) and are capable of analyzing sample volumes of less than 10 μL. Integration of parallel analyses using multichannel chips and spectral multiplexing for simultaneous multianalyte analysis is currently under way. Integrated sample enrichment improves the detection limit to the low picomolar range.32
PROTEIN BIOMARKERS FOR CLINICAL DIAGNOSIS OF PERIODONTAL DISEASE
Saliva is a fluid that can be easily collected, contains locally derived and systemically derived biomarkers of periodontal disease, and hence may offer the basis for a patient-specific biomarker assessment for periodontitis and other systemic diseases. Periodontitis is a group of inflammatory diseases affecting the connective tissue attachment and supporting bone around the teeth. Once initiated, the disease progresses with the loss of tooth-supporting collagen fibers and attachment to the cemental surface, apical migration of the junctional epithelium, formation of deepened periodontal pockets, and resorption of alveolar bone. If left untreated, the disease continues with progressive bone destruction, leading to tooth mobility and subsequent tooth loss. Periodontal disease afflicts over 50% of the adult population in the United States, with approximately 10% displaying severe disease concomitant with early tooth loss. Recent evidence suggests a strong association among periodontal disease, cardiovascular disease, and pulmonary and other serious systemic diseases.
Because of the noninvasive and undemanding nature of saliva collection, analysis of saliva may be especially beneficial in the determination of current periodontal status and a means of monitoring the response to treatment. Studies report that the determination of inflammatory mediator levels in biological fluids is a good indicator of inflammatory activity.33 Accordingly, studies related to the pathogenesis of periodontal disease examine the link between biochemical and immunological markers in saliva and the extent of periodontal destruction—with potential for predicting future disease progression. Oral fluid biomarkers studied for periodontal diagnosis include proteins of host origin (i.e., enzymes and immunoglobulins), phenotypic markers, host cells, hormones, bacteria and bacterial products, ions and volatile compounds. There are a variety of other biomarkers of skeletal homeostasis. These biomarkers may have significant potential in other bone metabolic diseases, such as osteoporosis, rheumatoid arthritis, and osteolytic bone metastases.
Risk factors are considered to be modifiers of the nature of disease. Associated with host susceptibility and a variety of local and systemic conditions, risk factors influence the initiation and progression of periodontitis and alter biomarker levels. Longitudinal studies of biomarkers play an important role in life sciences and have begun to assume a greater role in diagnosis, monitoring of therapy outcomes, and drug discovery. The challenge for biomarkers is to allow earlier detection of the disease evolution and more robust assessment of therapeutic efficacy. For biomarkers to assume their rightful role, a greater understanding of the mechanism of disease progression and therapeutic intervention is essential. Consequently, there is a need for the development of new diagnostic tests that can detect the presence of active disease, forecast future disease progression, and evaluate the response to periodontal therapy, thereby improving the clinical management of periodontal patients. The diagnosis of active phases of periodontal disease and the identification of patients at risk for active disease represent challenges for both clinical investigators and practitioners.
ONGOING CLINICAL MEASUREMENTS USE IMPOD
Our group has recently demonstrated the ability to measure putative protein biomarkers in whole saliva, gingival crevicular fluid (GCF), and other oral fluids. We have completed a pilot analysis in a recent cross-sectional investigation of periodontal disease and health, as well as recently published evaluation of protein biomarkers in oral fluids during periodontal reconstructive therapy using local34,35 or systemic drug delivery.36 Our studies employing the IMPOD diagnostic have shown the reproducible assessment of putative periodontal disease biomarkers.37 Figure 3 shows IMPOD analysis of saliva for two cytokines, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). While these results demonstrate IMPOD-based measurement of spiked cytokines in whole saliva, the data show how rapid the analyses are (completed in less than 250 sec) and that the assays are quantitative—allowing generation of calibration curves for measurement of endogenous saliva in pilot samples.
FIGURE 3.
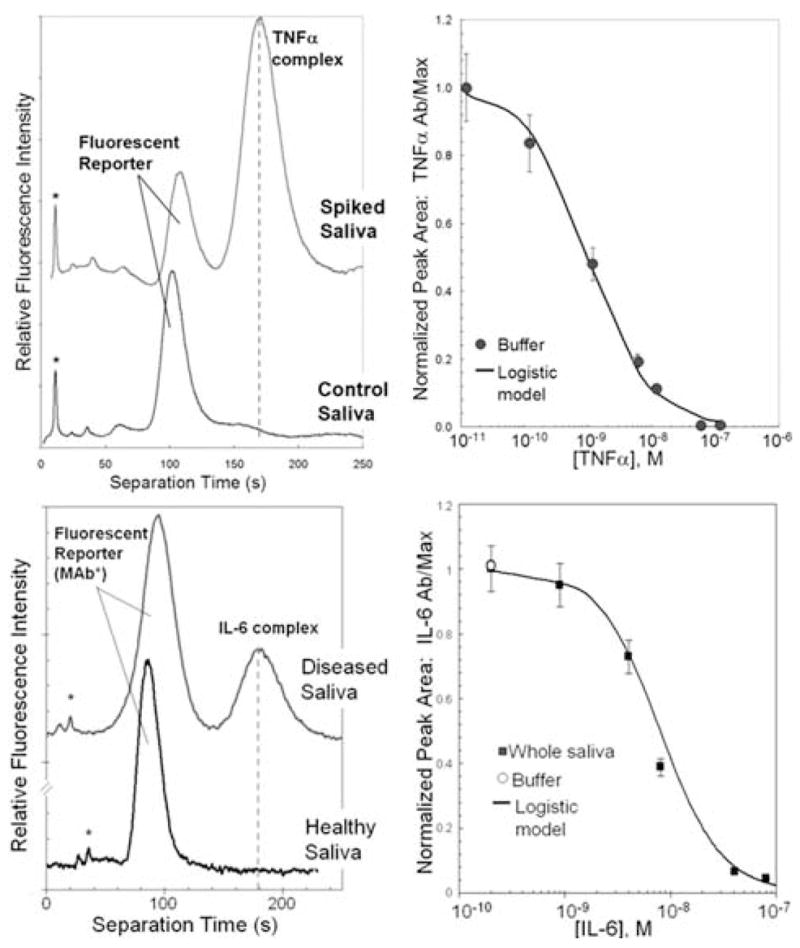
IMPOD analysis is quantitative and completes in less than four minutes. (Top) Electropherograms for exogenous TNF-α analysis in whole saliva. Companion dose-response curve acquired in model buffer system. (Bottom) IL-6 measurements from IMPOD analysis of spiked whole saliva. Companion dose-response curve from spiked saliva.
Low nanomolar detection limits are not always adequate for screening of low-abundance disease markers.38 Our group has demonstrated experience with development of on-chip functional components (e.g., dialysis membranes,39 buffer exchange membranes,40 and pressure-actuated valving18,41) using photolithographically controlled polymerization. We employ these fabrication methods to create size-exclusion membranes within microfluidic channels for sample concentration or enrichment. The pore size of the miniaturized size-exclusion membrane is controlled via the formulation of the precursor solution (monomer and cross-linker).
Our group has shown that the incorporation of photolithographically fabricated size-exclusion membranes has extended the sensitivity of protein-sizing assays to the femtomolar level.32 When implemented with our electrokinetic immunoassays, online sample enrichment allows rapid identification (<10 min) of protein markers in human saliva at clinically relevant concentrations (10−12 M) without the need for additional off-chip sample preparation. Trapping of analytes in the volume near the membrane obviates the need for off-chip sample incubation and reporter-binding steps. Figure 4 shows results from the high-sensitivity IMPOD immunoassay technique, compared to immunoassays not incorporating sample enrichment, for analysis of C-reactive protein (CRP). An appreciable increase in assay sensitivity is observed when IMPOD operates using sample enrichment. Further, the duration of sample (as well as antibody) enrichment can be used to “tune” the assay sensitivity and dynamic range in real time, thus making a single assay applicable to measurement of both high-and low-abundance protein species in a given diagnostic sample.
FIGURE 4.
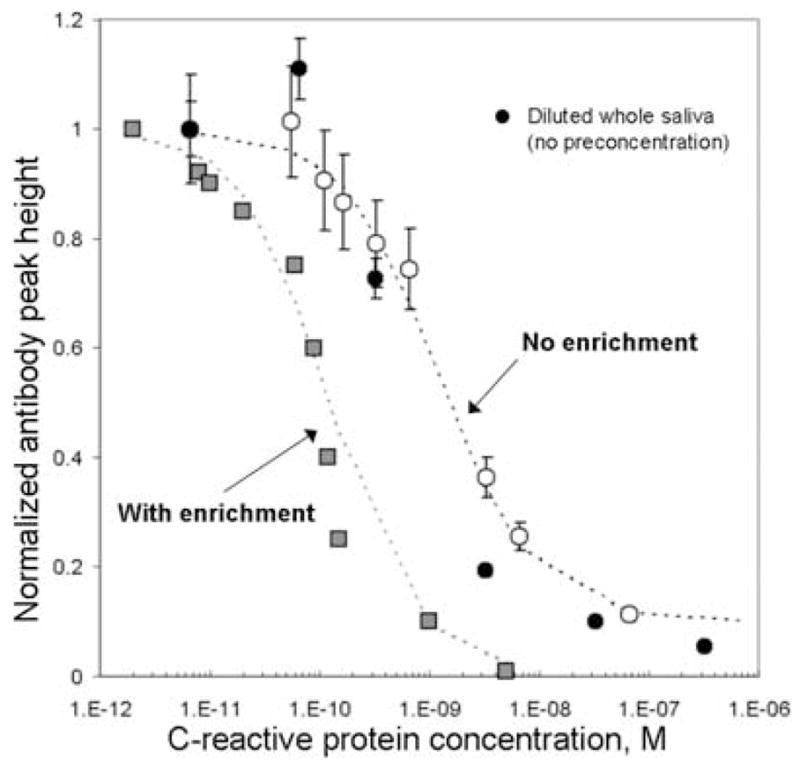
On-chip sample enrichment extends the C-reactive protein dynamic range and increases the sensitivity of IMPOD. Buffer samples spiked with known concentrations of C-RP were analyzed with (solid squares) and without (hollow circles) on-chip sample enrichment. Measurement of C-RP in spiked whole saliva, without enrichment, is shown for comparison (solid circles).
A key, defining feature of our microfluidic approach is development of an instrument that readily measures multiple analytes simultaneously. Such an approach is essential for early and accurate diagnosis of a chronic inflammatory disease, such as periodontitis, where measuring overall “composite profile” or “signature” of a set of biomarkers may have significantly higher diagnostic value than measurement of individual analytes. In addition to measuring endogenous protein content in saliva using the IMPOD approach, our group is assessing a large panel of putative biomarkers for relevance to periodontal disease. Data currently being generated from these proteomic measurements allow us to gauge the predictive ability of putative disease biomarkers (cytokines, enzymes, and bone collagen fragments). Currently, the use of digital subtraction radiography and longitudinal clinical parameter assessment is being compared to oral fluid samples (saliva, GCF) to develop the appropriate metrics in evaluating the interrelationship of disease biomarkers to various fluid components.
Analyte classes, shown here in order of progression of periodontal disease, are currently being evaluated for their predictive value in diagnosis of periodontal disease by our group and others: (1) inflammatory mediators and host-response modifiers (Th1/Th2 cytokines and CRP), (2) host-derived enzymes (collagenases, gelatinases), (3) connective and bone breakdown proteins (osteocalcin, osteonectin, laminin, C-telopeptide pyridinoline cross-links [ICTP]). Our preliminary proteomic studies suggest that at least three analytes (IL-1B, MMP-8, and ICTP) have significant correlation with the progression of periodontal disease. Each of these three analytes is implicated in a different stage of periodontal disease progression. Further work regarding identification and validation of these protein biomarkers is currently under way by our group.
CONCLUSIONS
Over the past 3 years, our collaborative project has focused on the development of a novel diagnostic strategy for the evaluation of human periodontal disease. A goal of periodontal diagnostic procedures is to provide the clinician with useful information regarding the present periodontal disease type, location, and severity. These findings serve as a basis upon which treatment planning is formulated and provide useful information during periodontal maintenance and disease monitoring phases of treatment. Traditional periodontal diagnostic parameters used by clinicians include several semisubjective assessment parameters, many of which develop during advanced disease. Advances in oral and diagnostic research are moving toward methods by which periodontal risk can be identified and quantified by objective measures, such as biomarkers that can be determined in a rapid fashion, such as is the case with our IMPOD instrument.
Ongoing work centers on identification and validation of panels of protein biomarkers, with particular emphasis on identifying groups of proteins that have relevance to all stages of periodontal disease progression—both as a means to enable early diagnosis of periodontal disease and as a means to assess the activity of disease in a particular patient or site. Our group is working to incorporate the capability for multiplex analyses (i.e., assaying a single sample for multiple biomarkers in parallel). Lastly, the research reported here lays the groundwork for extension of these methods to other diagnostic fluids and illnesses. Clinical and engineering approaches, such as those described in this work, present compelling advantages for furthering personalized medicine in the 21st century.
Acknowledgments
The authors thank V. VanderNoot, R. Renzi and J. Stamps at Sandia National Laboratories. The authors also thank M. McReynolds, K. Ghandi, and D. Degrasse at Caliper Life Sciences. At the University of Michigan, the authors gratefully acknowledge J. Kinney, C. Ramseier, L. Rayburn, and J. Sugai.
This work was supported by the U.S. National Institute of Dental and Craniofacial Research (Grant NIDCR U01-DE014961). Sandia is a multiprogram laboratory operated by Sandia Corp., a Lockheed Martin Co., for the United States Department of Energy under Contract DE-AC04-94AL85000.
References
- 1.Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002;8:69–76. doi: 10.1034/j.1601-0825.2002.1o834.x. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson DB. Current diagnostic uses of saliva. J Dent Res. 1987;66:420–424. doi: 10.1177/00220345870660020601. [DOI] [PubMed] [Google Scholar]
- 3.Mandel ID. The diagnostic uses of saliva. J Oral Path. 1990;19:119–125. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 4.Malamud D. Saliva as a diagnostic fluid. Br Med J. 1992;305:207–208. doi: 10.1136/bmj.305.6847.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HHS. Oral health in America. A report of the Surgeon General. U.S. Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; Rockville, MD: 2000. [Google Scholar]
- 6.NIDCR. Workshop on Development of New Technologies for Saliva and Other Oral Fluid-Based Diagnostics. Airlie House Conference Center; Virginia. September 12–14, 1999.1999. [Google Scholar]
- 7.Weston AD, Hood L. Systems biology, proteomics, and the future of health care: toward predictive, preventative, and personalized medicine. J Proteome Res. 2004;3:179. doi: 10.1021/pr0499693. [DOI] [PubMed] [Google Scholar]
- 8.Anderson NL, Anderson NG. The human plasma proteome—History, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 9.Renzi RF, et al. Hand-held microanalytical instrument for chip-based electrophoretic separations of proteins. Anal Chem. 2005;77:435–441. doi: 10.1021/ac049214f. [DOI] [PubMed] [Google Scholar]
- 10.Sia SK, et al. An integrated approach to a portable and low-cost immunoas-say for resource-poor settings. Angewandte Chemie Int Ed. 2004;43:498. doi: 10.1002/anie.200353016. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan V, V, Pamula K, Fair RB. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip. 2004;4:310. doi: 10.1039/b403341h. [DOI] [PubMed] [Google Scholar]
- 12.Hatch A, et al. A rapid diffusion immunoassay in a T-sensor. Nat Biotechnol. 2001;19:461–465. doi: 10.1038/88135. [DOI] [PubMed] [Google Scholar]
- 13.Yang CY, et al. Detection of picomolar levels of interleukin-8 in human saliva by SPR. Lab Chip. 2005;5:1017. doi: 10.1039/b504737d. [DOI] [PubMed] [Google Scholar]
- 14.Christodoulides N, et al. Application of microchip assay system for the measurement of C-reactive protein in human saliva. Lab Chip. 2005;5:261–269. doi: 10.1039/b414194f. [DOI] [PubMed] [Google Scholar]
- 15.Bailey CG, et al. Chip-based, multiple-channel, total-analysis system. Abstr Papers Am Chem Soc. 2000;219:U85. [Google Scholar]
- 16.Fruetel JA, et al. Microchip separations of protein biotoxins using an integrated hand-held device. Electrophoresis. 2005;26:1144. doi: 10.1002/elps.200406194. [DOI] [PubMed] [Google Scholar]
- 17.Herr AE, Singh AK. Photopolymerized cross-linked polyacrylamide gels for on-chip protein sizing. Anal Chem. 2004;76:4727–4733. doi: 10.1021/ac049686u. [DOI] [PubMed] [Google Scholar]
- 18.Reichmuth DS, Shepodd TJ, Kirby BJ. Microchip HPLC of peptides and proteins. Anal Chem. 2005;77:2997. doi: 10.1021/ac048358r. [DOI] [PubMed] [Google Scholar]
- 19.Throckmorton DJ, Shepodd TJ, Singh AK. Electrochromatography in microchips: reversed-phase separation of peptides and amino acids using photopatterned rigid polymer monoliths. Anal Chem. 2002;74:784–789. doi: 10.1021/ac011077o. [DOI] [PubMed] [Google Scholar]
- 20.Cummings EB, Singh AK. Dielectrophoresis in microchips containing arrays of insulating posts: theoretical and experimental results. Anal Chem. 2003;75:4724. doi: 10.1021/ac0340612. [DOI] [PubMed] [Google Scholar]
- 21.Barrett LM, et al. Dielectrophoretic manipulation of particles and cells using insulating ridges in faceted prism microchannels. Anal Chem. 2005;77:6798–6804. doi: 10.1021/ac0507791. [DOI] [PubMed] [Google Scholar]
- 22.Herr AE, Davenport AA, Singh AK. Microchip immunoassays using photodefined polyacrylamide for rapid native gel electrophoresis of immune complexes. Anal Chem. 2005;77:585–590. doi: 10.1021/ac0489768. [DOI] [PubMed] [Google Scholar]
- 23.Hjerten S, et al. Reversed-phase chromatography of proteins and peptides on compressed continuous beds. Chromatographia. 1993;37:287. [Google Scholar]
- 24.Hjerten S, Zhu MD. Adaptation of the equipment for high-performance electrophoresis to isoelectric-focusing. J Chromatogr. 1985;346:265–270. [Google Scholar]
- 25.Herr AE, Singh AK. Photopolymerized cross-linked polyacrylamide gels for on-chip protein sizing. Anal Chem. 2004;76:4727–4733. doi: 10.1021/ac049686u. [DOI] [PubMed] [Google Scholar]
- 26.Han J, Singh AK. Rapid protein separations in ultra-short microchannels: microchip sodium dodecyl sulfate-polyacrylamide gel electrophoresis and isoelectric focusing. J Chromatogr A. 2004;1049:205. [PubMed] [Google Scholar]
- 27.Schmalzing D, Nashabeh W. Capillary electrophoresis based immunoassays: a critical review. Electrophoresis. 1997:2184–2193. doi: 10.1002/elps.1150181209. [DOI] [PubMed] [Google Scholar]
- 28.Shimura K, Karger BL. Affinity probe capillary electrophoresis: analysis of recombinant human growth-hormone with a fluorescent-labeled antibody fragment. Anal Chem. 1994;66:9–15. doi: 10.1021/ac00073a004. [DOI] [PubMed] [Google Scholar]
- 29.Schultz NM, Kennedy RT. Rapid immunoassays using capillary electrophoresis with fluorescence detection. Anal Chem. 1993;1:3161–3165. [Google Scholar]
- 30.Cheng SB, et al. Development of a multichannel microfluidic analysis system employing affinity capillary electrophoresis for immunoassay. Anal Chem. 2001;73:1472–1479. doi: 10.1021/ac0007938. [DOI] [PubMed] [Google Scholar]
- 31.Wang YC, et al. Enhancement of the sensitivity of a capillary electrophoresis immunoassay for estradiol with laser-induced fluorescence based on a fluorescein-labeled secondary antibody. Anal Chem. 2001;15:5616–5619. doi: 10.1021/ac010537a. [DOI] [PubMed] [Google Scholar]
- 32.Hatch AV, et al. Integrated preconcentration-sizing of proteins in mi-crochips using photopatterned polyacrylamide gels. Anal Chem. 2006;78:4976–4984. doi: 10.1021/ac0600454. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis—A review. J Clin Periodontol. 2000;27:453–465. doi: 10.1034/j.1600-051x.2000.027007453.x. [DOI] [PubMed] [Google Scholar]
- 34.Cooke JA, et al. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng. 2006;12:1441–1450. doi: 10.1089/ten.2006.12.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarment DP, et al. Effects of rhPDGF-BB on bone marrow turnover during periodontal repair. J Clin Periodontol. 2006;33:135–140. doi: 10.1111/j.1600-051X.2005.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gapski R, et al. Effect of systemic matrix metalloproteinase inhibition on periodontal wound repair: a proof of concept trial. J Periodontol. 2004;75:441–452. doi: 10.1902/jop.2004.75.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herr AE, et al. Microfluidic immunoassays as rapid saliva-based clinical diagnostics. 2007 doi: 10.1073/pnas.0607254104. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozmeric N. Advances in periodontal disease markers. Clin Chim Acta. 2004;343:1–16. doi: 10.1016/j.cccn.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 39.Song S, et al. Microchip dialysis of proteins using in situ photopatterned nanoporous polymer membranes. Anal Chem. 2004;76:2367–2373. doi: 10.1021/ac035290r. [DOI] [PubMed] [Google Scholar]
- 40.Song S, Singh AK, Kirby BJ. Electrophoretic concentration of proteins at laser-patterned nanoporous membranes in microchips. Anal Chem. 2004;76:4589. doi: 10.1021/ac0497151. [DOI] [PubMed] [Google Scholar]
- 41.Kirby BJ, et al. Microfluidic routing of aqueous and organic flows at high pressures: fabrication and characterization of integrated polymer microvalve elements. Lab Chip. 2005;5:184. doi: 10.1039/b413199a. [DOI] [PubMed] [Google Scholar]


