Abstract
Purpose
Transcriptional profiling demonstrated decreased expression of gastrokine 1 (GKN1) and the related trefoil factor interacting protein (TFIZ1/GKN2) in H. pylori infection. Decreased GKN1 and GKN2 mRNA expression has been reported in gastric adenocarcinoma. We have examined GKN1 and GKN2 protein expression in a large gastric cancer series, correlated expression with tumor subtype, and evaluated their utility as prognostic biomarkers.
Experimental Design
GKN1, GKN2 and the trefoil factors TFF1 and TFF3 were examined in tissue microarrays from 155 distal gastric adenocarcinomas. Immunohistochemical expression was correlated with clinical outcome. GKN1 and GKN2 expression was measured by real-time PCR and Western analysis in samples of gastric cancer and adjacent non-neoplastic mucosa.
Results
GKN1 was lost in 78% of diffuse and 42% of intestinal cancers (p<0.0001, diffuse versus intestinal). GKN2 expression was lost in 85% of diffuse and 54% of intestinal type cancers (p<0.002). GKN1 and GKN2 down-regulation were confirmed by western and real-time PCR analysis. Loss of either protein was associated with significantly worse outcome in intestinal-type tumors by univariate analysis; and GKN2 loss remained a predictor of poor outcome in multivariate analysis (p<0.033). TFF1 was lost in over 70%, and TFF3 was expressed in about 50% of gastric cancers.
Conclusions
Loss of GKN1 and GKN2 expression occurs frequently in gastric adenocarcinomas, especially in the diffuse subtype. GKN1 and GKN2 loss are associated with shorter overall survival in the intestinal subtype.
Keywords: biomarkers, gastric cancer, trefoil factor family, prognosis, Helicobacter pylori
INTRODUCTION
Gastric cancer is the second most common cause of cancer mortality worldwide 1 and is, except for cancers of the gastric cardia, attributable to chronic infection by the intragastric bacterium Helicobacter pylori in most cases 2. The more common “intestinal” subtype of gastric cancer 3 develops over decades through a preneoplastic sequence originating in chronic superficial gastritis (usually caused by H. pylori) that progresses through atrophic gastritis, intestinal metaplasia and dysplasia 4 5. The diffuse subtype 3 is also preceded by years of chronic H. pylori-associated gastritis, although the molecular pathways and histological changes involved in progression to cancer are less well characterized 5.
The molecular pathogenesis of gastric cancer is heterogeneous and few gastric cancer-specific markers have been discovered that have clinical diagnostic or prognostic potential. Among the best-characterized gastric tumor suppressor genes are p53 (mutated frequently in both diffuse and intestinal gastric cancer subtypes), RUNX3 (lost by gene deletion or promoter hypermethylation) 6, and E-cadherin (expression of which is lost commonly in diffuse gastric cancers 7.
The importance of H. pylori infection in promoting gastric cancer is recognized. Examining the expression of genes regulated specifically by H. pylori infection in vivo may help identify novel molecular pathways involved in gastric carcinogenesis and lead to the identification of novel gastric cancer-associated oncogenes and tumor suppressor genes. We recently determined the human gastric epithelial transcriptome of H. pylori infection using an Affymetrix expression microarry 8. Purified gastric epithelial cell populations free from contaminating stromal elements were isolated by laser capture microdissection of paired gastric endoscopic biopsies taken before and after H. pylori eradication. From this analysis we identified two related genes as being downregulated by H. pylori infection 8: GKN2 (also known as TFIZ19, GDDR 10 and blottin 11) and Gastrokine-1 (GKN1, also known as CA11, AMP-18, foveolin and TFIZ2 9, 12–14). These genes encode partially homologous proteins 9 whose expression has been reported to be decreased in small series of gastric cancers at the mRNA level for GKN2 10 and GKN1 13,14 and at the protein level for GKN1 only 13. Although the functions of GKN1 and GKN2 are not known, GKN2 is present as a heterodimer with the trefoil factor family (TFF) protein TFF1 in human gastric epithelium and in adherent gastric mucus, hence its earlier designation as trefoil interacting protein 1 9, 15. This suggests that GKN2 and possibly GKN1 may be involved in the intracellular or extracellular function of TFF1 9, 15. More recently an interaction between the murine orthologue of GKN2 and a fusion protein of murine TFF2 and alkaline phosphatase was demonstrated in vitro by ligand blot analysis 11. It is not known if GKN2 interacts with TFF2 in vivo. Gastrokine 1 was so named because it has been reported that it is induced by gastrin but there is no evidence as to whether or not TFIZ1/GKN2 may mediate gastrin action.
TFF proteins have important roles in gastric epithelial regeneration and cell turnover, and they have therefore been implicated in gastric carcinogenesis 16 17. TFF1 knockout mice develop gastric adenomas and carcinomas 18 and TFF1 is markedly down-regulated in human gastric cancer 19, 20 21 22, suggesting it is a tumor suppressor for human gastric cancer. Interestingly, the TFF2 knockout mouse does not develop gastric cancer 23. TFF2 down-regulation has been reported in some cases of human gastric adenocarcinoma 24. TFF3 expression is rare in non-neoplastic gastric mucosa but occurs in about 50% of gastric cancers. Interestingly overexpression of TFF3 has been associated with a worse prognosis in two separate series of gastric cancer cases. 25, 26 Furthermore, trefoil protein expression is altered during H. pylori infection 27, 28 and TFF1 binds to and may be a receptor for H. pylori 29. Taken together, these findings suggest that proteins that interact with TFF members may be important in the molecular pathogenesis of H. pylori-associated gastric carcinogenesis.
Based on our findings that GKN1 and GKN2 are two related gastric-specific proteins down-regulated by H. pylori infection 8 and previous reports of decreased expression of GKN1 and GKN2 in small series of gastric cancer cases 10, 13, 30, the aims of our study were to evaluate the down-regulation or loss of expression of GKN1 and GKN2 proteins and mRNA in a large collection of non-cardia gastric cancer cases from the United States. We investigated if the down-regulation of GKN1 and GKN2 occurred in both intestinal type and diffuse type cancers, and determined whether down-regulation or loss of expression of these proteins had any prognostic significance.
Because GKN2 interacts with TFF1 and because TFF3 has been reported to be a marker of poor prognosis in gastric cancer we also evaluated TFF1 and TFF3 expression in this same gastric cancer series. Our results demonstrate the loss of GKN1 and GKN2 expression in the majority of distal gastric cancer cases, and that loss of GKN1 or GKN2 in intestinal type gastric cancer is related to poor prognosis.
METHODS
Tissues
Gastric cancer tissue microarrays representing 155 distal (non-cardia) gastric cancers with their non-neoplastic resection margins and intestinal metaplasia were constructed from archival tissue blocks in the Department of Pathology, Rhode Island Hospital, as described previously 31. The cases were a consecutive series of patients with distal gastric cancer undergoing surgical resection in our hospital. Demographic details of the cases are described in the Table. Clinical and pathological data on these cases including information on staging, recurrence, treatment and survival were ascertained through the Rhode Island Tumor Registry, and by review of the Rhode Island Hospital medical records. Tumors were staged and graded according to the American Joint Committee on Cancer criteria 32. The study was approved by the Investigational Review Board of Rhode Island Hospital.
Immunohistochemistry
Immunostaining was performed on 5 μm paraffin-embedded tissue sections using antigen retrieval with citrate buffer and the Dako EnVision Plus horseradish peroxidase/diaminobenzene signal amplification detection system (catalog # K4007, Dako, Carpinteria, CA). The primary antisera were diluted 1:2500. These antibodies were raised in rabbits against the synthetic peptides LVKEKKLQGKGPGG and KYNPLESLIKDVDWF and were affinity purified on the basis of the immunoreactivity with the immunizing peptides. The specificity of the antibodies was confirmed by immunoneutralization with the immunizing peptides on gastric tissues, and by the absence of immunostaining of non-gastric tissues. Footnote 1 TFF1 expression was detected using monoclonal anti-pS2 antibody (catalog number 08-1162, Invitrogen, Carlsbad, CA) at a dilution of 1:25 and TFF3 expression was detected using a previously described monoclonal anti-TFF3 antibody raised against structurally-validated recombinant TFF3, at a dilution of 1:40. 33
Immunohistochemical staining was assessed semi-quantitatively by measuring both the intensity of the staining (0, 1, 2, or 3) and extent of staining (0, 0%; 1, 0–10%; 2, 10–50%; 3, 50–100%). The scores for the intensity and extent of staining were multiplied to give a weighted score for each case (maximum possible, 9). For the statistical analysis, the weighted scores were grouped in two categories where scores of 0–3 were considered negative and 4–9 positive.
Protein Extraction and Western Blotting
Snap frozen tissue samples were homogenized in RIPA buffer (Sigma-Aldrich, St. Louis, MO) with protease inhibitors (Complete Mini, EDTA free Protease Inhibitor Cocktail tablets, Roche). For Western blotting, 50 μg protein samples were run on 12% precast SDS-PAGE gels (Biorad Laboratories, CA) and transferred to Immobilon–P membranes (Millipore, Bedford, MA). The membrane was washed twice with TBS for 50 min, blocked with 5% fat free milk in TBS and then incubated with primary antibody (GKN1: rabbit affinity-purified polyclonal antibody at 1:20,000 dilution 9, TFIZ1/GKN2: rabbit unpurified polyclonal antibody at 1:20,000 dilution 9). For loading controls, blots were stripped and reprobed with mouse monoclonal antibody to GAPDH (Abcam, Cambridge, MA; 1:4,000 dilution). The secondary antibodies used were goat anti-rabbit IgG-HRP and goat anti-mouse IgG-HRP (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Immune detection was performed using enhanced chemiluminescence for detection of HRP (Pierce Biotechnology Inc., Rockford, IL) and quantified by densitometry using Kodak image software.
RNA extraction and quantification by real-time PCR
In a randomly selected subset of 30 cases (11 diffuse gastric cancer cases and 19 intestinal gastric cancer cases) RNA was extracted from representative 10 micron thick sections of formalin-fixed paraffin-embedded samples using methods similar to those described previously 8 except that there was no laser capture microdissection step and the Paradise Whole Transcript Reagent system from Molecular Devices, Sunnyvale, CA was used. Transcript levels were evaluated by real-time PCR and the data normalized for beta-actin expression as described previously 8.
Statistical Analysis
Statistical analysis was performed using the SPSS 10 program for Windows. The correlation between the immunohistochemical expression scores and patient survival after surgery was estimated using the Kaplan-Meier method followed by a univariate comparison between the groups using the log-rank test. In order to adjust for potential confounding variables and to single out independent predictors of survival, a multivariate analysis of survival was performed using the Cox’s proportional hazard model using a forward stepwise mode. Associations between GKN1, GKN2, TFF1 and TFF3 expression and the tumor histological type was tested using the Chi square or the Fisher’s exact test.
Quantitative PCR results were expressed as a ratio of the transcript of interest to beta-actin in the tumor tissue divided by a similar ratio in the non-neoplastic tissue of the same patient. These values (tumor/“normal”) were then normalized to the group average for each histological tumor subtype.
Comparisons between the results for intestinal versus diffuse cancer subtype were assessed by the Mann-Whitney U test. Two-tailed p values of 0.05 or less were considered statistically significant.
RESULTS
Expression of GKN1 and GKN2 in normal, and loss in neoplastic gastric tissues
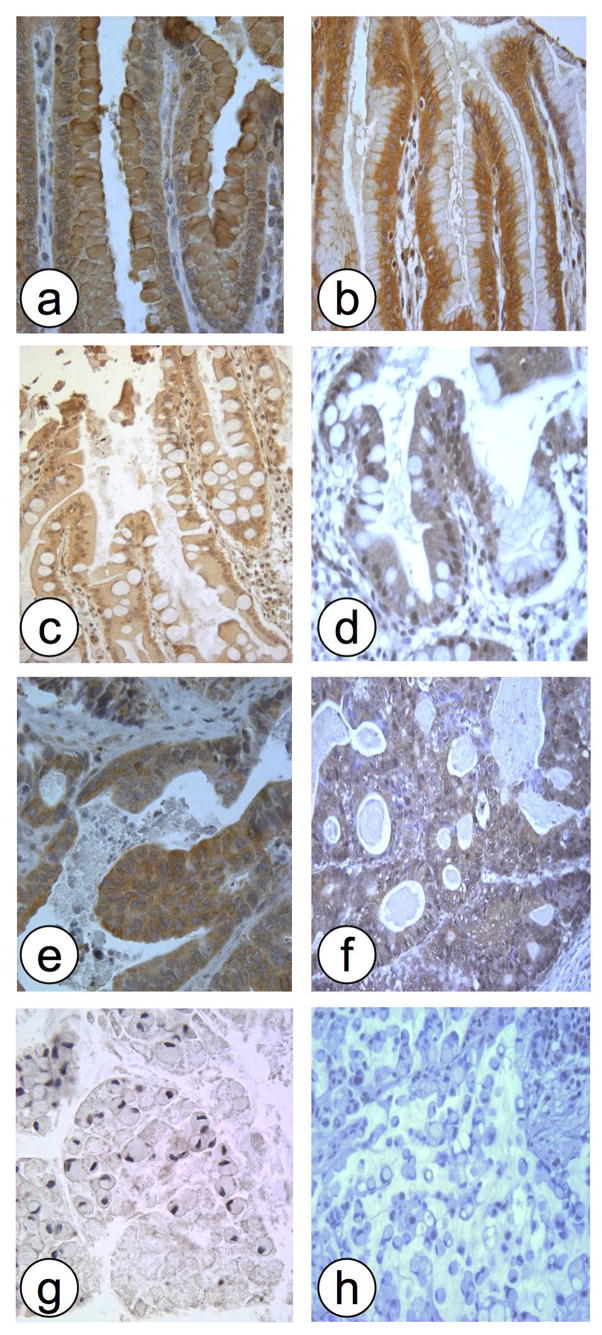
GKN1 and GKN2 were expressed at high levels in non-neoplastic gastric epithelial cells but not in adjacent stromal or inflammatory cells. The immunoreaction was strongest in the superficial gastric foveolar cells, with weaker staining of the epithelium in the neck region and deeper glands. GKN1 and GKN2 staining was diffuse throughout the cytoplasm, sparing the nucleus. Occasional membranous accentuation of GKN1 was seen. GKN1 was expressed within mucous vacuoles (fig 1). Areas of intestinal metaplasia also expressed cytoplasmic GKN1 and GKN2, whereas expression of both proteins was reduced or absent in gastric cancers.
Fig 1.
Representative photomicrographs of immunohistochemical staining of gastric tissue, demonstrating expression of GKN1 (a,c,e,g) and GKN2 (b,d,f,h) in gastric epithelial cells. a,b: normal mucosa; c,d: intestinal metaplasia; e,f: gastric cancer (intestinal type); g,h: gastric cancer (diffuse type). All original magnifications × 200.
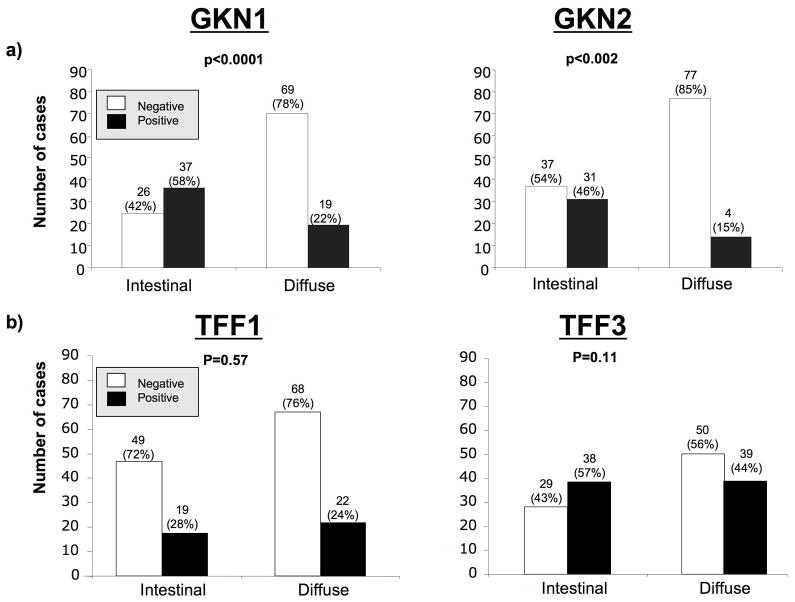
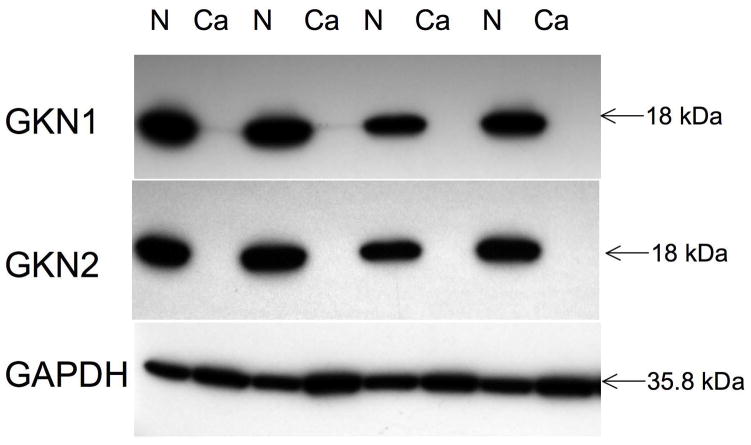
Overall GKN1 expression was negative in 78% of diffuse type cancers and in 42% of intestinal type cancers (p<0.0001, comparing diffuse with intestinal cancer expression, Fisher’s exact test), and GKN2 was lost in 85% of diffuse type cancers and 54% of intestinal type cancers (p<0.002) (fig 2A). Overall, loss of GKN1 was positively correlated with loss of GKN2 (r =0.24, p=0.009). A marked reduction or total loss of GKN1 and GKN2 expression was confirmed by immunoblot in 4 cases of diffuse type gastric cancer, comparing the tumor tissue with the non-neoplastic resection margins of the same cases (fig 3).
Fig 2.
Summary of immunostaining of gastric cancer cases, grouped into (a) those cancers staining negative (score 0–3) or positive (score 4–9) for GKN1 or GKN2, and (b) those staining negative (score 0–3) or positive (score 4–9) for TFF1 or TFF3. P values refer to difference between proportions of the staining expression of these markers in the intestinal and diffuse cancer types.
Fig 3.
Western blot of tissue lysates from 4 cases of diffuse type gastric cancer (Ca) with adjacent non-neoplastic mucosa (N) showing marked loss/absence of GKN1 and GKN2 in the cancers. GAPDH expression (bottom panel) serves as a control, demonstrating equal protein loading across all lanes.
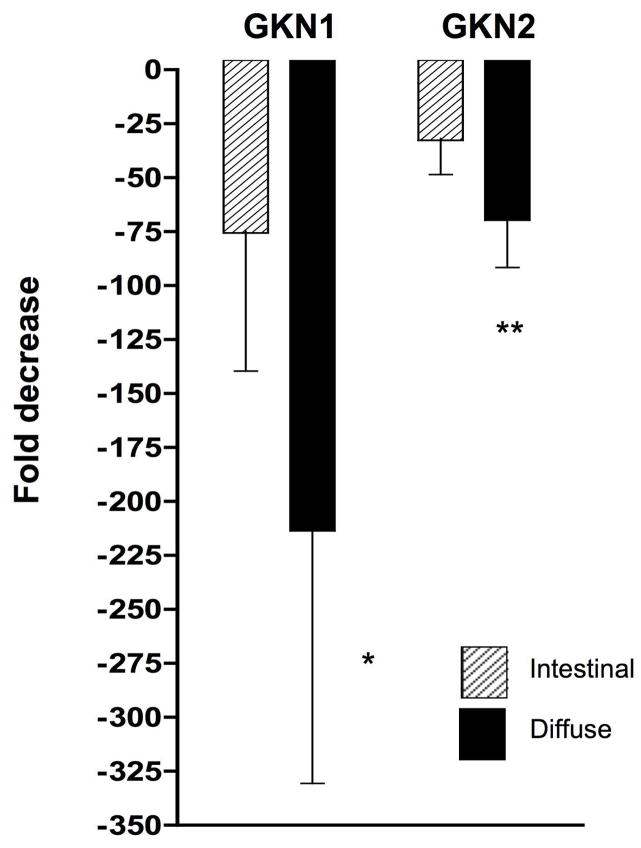
The loss of GKN1 and GKN2 expression was verified at the mRNA level by real time PCR analysis on 30 representative gastric cancer cases. GKN1 mRNA was decreased 80 ± 64 fold (mean ± SEM) in 19 intestinal type tumors (p = 0.54, paired Wilcoxon test) and 218 ± 117 fold (p = 0.007) in 11 cases of diffuse type tumors (fig 4). GKN2 mRNA was decreased 37 ± 16 fold (mean ± SEM) in 19 intestinal type tumors (p = 0.14, paired Wilcoxon test) and 74 ± 22 fold (p = 0.004) in 11 cases of diffuse type tumors (fig 4).
Fig 4.
Expression of GKN1 and GKN2 mRNA in tumors compared with non-neoplastic resection margins. Data expressed as mean fold decrease in the tumor epithelium relative to non-neoplastic gastric tissue. * p< 0.004, ** p<0.007.
Association between loss of GKN1 and TFIZ1/GKN2 and prognosis
To determine the clinical significance of loss of GKN1 and GKN2 expression on prognosis after surgery in gastric cancer, clinical outcome data on the cases in the gastric tissue microarray were collected and correlated with patient demographic data, stage, grade and type of tumor and treatment using Kaplan-Meier analysis. For both intestinal-type and diffuse-type tumors, tumor stage at diagnosis was highly significantly correlated with survival in Cox’s multivariate analysis (p<0.001 for intestinal, p<0.008 for diffuse).
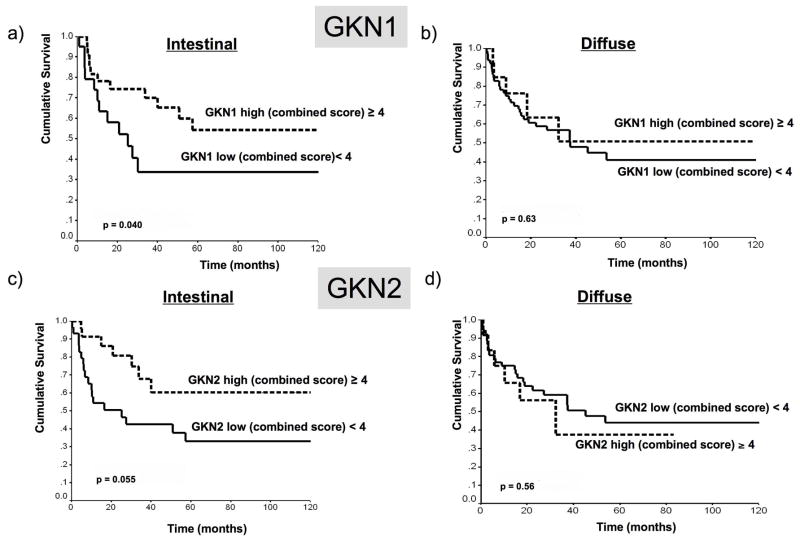
Loss of GKN1 and of GKN2 were each associated with worse outcome in intestinal tumor subtypes in univariate analysis (fig 5). After adjustment for other possible confounding variables, loss of GKN2 remained a statistically significant independent predictor of survival in intestinal type cancers by multivariate analysis (p<0.033).
Fig 5.
Kaplan-Meier plots of immunohistochemical scores of GKN1 (a, b) and GKN2 (c,d) expression in intestinal-type (a,c) and diffuse type (b,d) gastric cancers, demonstrating that intestinal tumors with low GKN1 and GKN2 expression were associated with significantly worse prognosis. Univariate analyses.
Expression of TFF1 and TFF3 in gastric cancer and correlation with prognosis
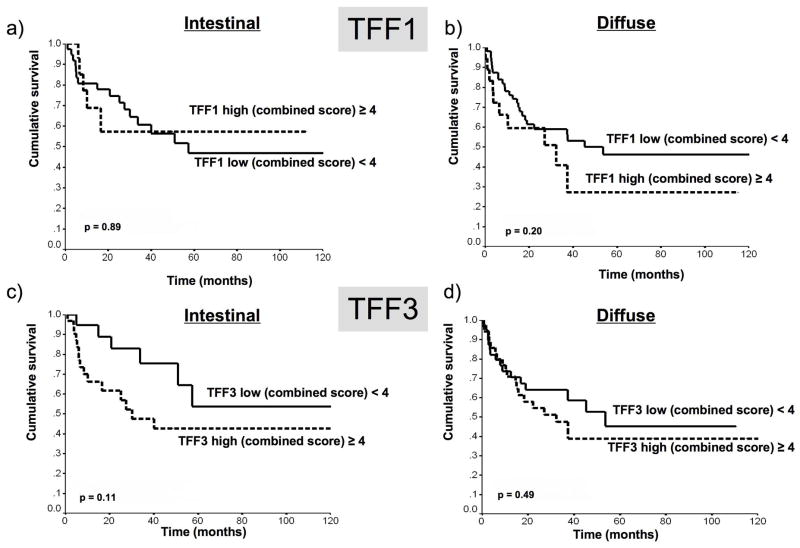
TFF1 expression was lost in the majority of gastric cancer cases and TFF3 was aberrantly expressed in about half of the gastric cancers, approximately equally in both intestinal and diffuse histological subtypes (fig 2B). No significant difference was seen in the proportion of patients with TFF1 loss or TFF3 gain between the two histological gastric cancer subtypes. There was no correlation between TFF1 loss and prognosis in univariate analysis, but there was a trend for high TFF3 expression to be associated with worse survival in univariate analysis in gastric cancers of the intestinal type but not in diffuse type tumors (p=0.11, figure 6.)
Fig 6.
Kaplan-Meier plots of immunohistochemical scores of TFF1 (a, b) and TFF3 (c,d) expression in intestinal type (a,c) and diffuse type (b,d) gastric cancers, demonstrating that intestinal tumors with high TFF3 expression were associated with significantly worse prognosis. Univariate analyses.
DISCUSSION
Our results demonstrate that loss of GKN1 and GKN2 expression is common in gastric cancer, particularly of the diffuse type. Loss of GKN1 and GKN2 was found at both the mRNA and protein levels, and GKN1 and GKN2 loss was associated with a particularly poor prognosis following surgery for intestinal type gastric cancer. In addition, our findings demonstrate that high TFF3 expression may also be a marker of poor survival in intestinal-type gastric cancer, which is consistent with two previous publications that did not stratify for tumor type 25, 26. In contrast, TFF1 loss did not confer any particular prognostic significance.
Down-regulation of GKN2 in gastric cancer was originally described in the Chinese literature by Du et al, 10 who cloned the gene from a subtraction library designed to screen down-regulated genes in gastric cancer. Sequence analysis indicates that GKN2 encodes a putative 184 amino acid protein with a BRICHOS domain of about 100 amino acids that has been found in a variety of functionally unrelated proteins implicated in dementia, in pulmonary surfactants and also in GKN1 34. The function of the BRICHOS domain is unknown but it may be involved in targeting proteins to the secretory pathway, in acting as an intracellular chaperone, and in regulating apoptotic pathways 15. The gene encoding GKN2 was also identified independently by two groups searching for novel proteins that interact with TFF members. Westley and colleagues 9 immunopurified GKN2 using a TFF1 specific antibody from human gastric mucosal cells. They showed that GKN2 is present in a heterodimer with TFF1 that is stabilized through an intermolecular disulfide bond between cysteine residues in the carboxy-terminus of TFF1 and in the BRICHOS domain of GKN2, hence the earlier designation of this protein as trefoil interacting protein 1. Recently in an unrelated proteomics approach Otto et al. 11, using ligand blot analysis with a fusion protein of murine TFF2 and alkaline phosphatase, identified the mouse orthologue of GKN2 which they called blottin. It remains to be determined if GKN2 interacts with TFF2 in vivo.
As discussed above, the only human protein with significant homology to GKN2 is GKN1 (24% identical, and 56% similar at the protein level) 9. GKN1 and GKN2 genes have the same intron and exon structures, and are located in close proximity on the same chromosomes in the genomes of both mice and humans. The frequent loss of expression in distal gastric cancer identified in the present study suggested that this gene locus might show loss of heterozygosity in gastric cancer, however no deletions or loss of heterozygosity at this locus on chromosome 2p14 have been identified in gastric cancer 35,36. GKN1 (also termed CA11, AMP-18, foveolin and TFIZ2 9, 12–14) was also first identified as being a potential gastric tumor suppressor through differential display techniques, comparing gastric cancer with normal gastric mucosa 14. Subsequently, GKN1 was found to be decreased or absent in 41 of 50 gastric cancer cases with no relationship to subtype, grade or stage 30. Independently, using a similar approach, Oien et al 13 noted GKN1 mRNA expression to be markedly decreased or absent in 8 of 8 gastric cancer cases examined, of both intestinal and diffuse histological subtypes.
In the present study, we examined a large number of cases of both histological subtypes of gastric cancer by immunohistochemistry and verified the findings by RT-PCR and Western transfer analysis. Importantly, in the present study, changes in expression were correlated with overall survival. The prognostic importance of GKN1 and GKN2 down-regulation was based upon the review of the medical records of patients who underwent a variety of different treatments, outside the context of a clinical trial. While the multivariate analysis accounted for treatment variations to some degree, it will be important to evaluate prospectively the prognostic significance of GKN1 and GKN2 in the context of a clearly defined clinical trial.
Previous studies have demonstrated decreased expression of GKN1 8,37 and TFIZ1/GKN2 8 in H. pylori-associated gastritis, the lesion that usually precedes the development of non-cardia gastric cancer 2. It is likely that H. pylori is directly responsible, because gastric inflammation due to injury by non-steroidal anti-inflammatory drugs is not accompanied by reduced expression of GKN1 or GKN2. Footnote 2 It is of interest to determine whether the changes in expression of GKN1 and GKN2 in gastric cancer cases are related to prior H. pylori infection. Establishing evidence of H. pylori infection in patients with gastric cancer is dependent on the measurement of serum antibodies to H. pylori because the bacteria are not normally present in the stomach at diagnosis. This is because the gastric environment becomes increasingly hostile to H. pylori survival during multi-stage gastric carcinogenesis 38. Since serum is not available from the patients in our study, direct correlation between GKN1 and GKN2 expression in gastric cancer and prior H. pylori infection will require a prospective study.
In summary, our results extend and confirm the studies of down-regulation of GKN1 and GKN2 mRNA in distal gastric cancer. Expression of the related gastric epithelial proteins GKN1 and GKN2 was reduced in the majority of cases of gastric cancer, especially in the diffuse subtype. Loss of expression of these proteins is associated with a worse prognosis in intestinal-type gastric cancers. It is particularly noteworthy that GKN2 is an independent marker of prognosis. Further studies to demonstrate the mechanisms responsible and the clinical biomarker potential of GKN1 and GKN2 would be of value.
Table 1.
Clinicopathological Characteristics of 155 Gastric Carcinoma Patients
| Variable | (n) |
|---|---|
| Age at Surgery (yr) | |
| Mean | 72 |
| Range | 31–96 |
| Gender | |
| Male | 81 |
| Female | 74 |
| Tumor Type | |
| Intestinal | 61 |
| Diffuse* | 94 |
| Tumor stage | |
| I | 37 |
| II | 44 |
| III | 34 |
| IV | 40 |
| Tumor differentiation | |
| Well | 3 |
| Moderate | 41 |
| Poor | 101 |
| Not defined | 7 |
| Vital Statistics | |
| Alive | 56 |
| Dead, gastric cancer | 70 |
| Dead, unrelated | 8 |
| Information unavailable | 21 |
Acknowledgments
Financial support: NIH grants R01CA111533 and P20RR17695 to SFM, P20RR17695 to MBR.
Footnotes
May FEB, Griffin SM and Westley BR, manuscript in preparation.
Gao J, Moss SF & Resnick MB – unpublished data.
References
- 1.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 (Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 3.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal type carcinoma: an attempt at a histoclinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 4.Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 5.Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003;3:592–600. doi: 10.1038/nrc1141. [DOI] [PubMed] [Google Scholar]
- 6.Li QL, Ito K, Sakakura C, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–24. doi: 10.1016/s0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 7.Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ. 2004:327–49. [PubMed] [Google Scholar]
- 8.Resnick MB, Sabo E, Meitner PA, et al. Global analysis of the human gastric epithelial transcriptome altered by Helicobacter pylori eradication in vivo. Gut. 2006;55:1717–24. doi: 10.1136/gut.2006.095646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westley BR, Griffin SM, May FEB. Interaction between TFF1, a gastric tumor suppressor trefoil protein, and TFIZ1, a brichos domain-containing protein with homology to SP-C. Biochemistry. 2005;44:7967–75. doi: 10.1021/bi047287n. [DOI] [PubMed] [Google Scholar]
- 10.Du JJ, Dou KF, Peng SY, et al. Down-regulated full-length novel gene GDDR and its effect on gastric cancer. Zhonghua Yi Xue Za Zhi. 2003;83:1166–8. [PubMed] [Google Scholar]
- 11.Otto WR, Patel K, McKinnell I, et al. Identification of blottin: a novel gastric trefoil factor family-2 binding protein. Proteomics. 2006;6:4235–45. doi: 10.1002/pmic.200500911. [DOI] [PubMed] [Google Scholar]
- 12.Martin TE, Powell CT, Wang Z, et al. A novel mitogenic protein that is highly expressed in cells of the gastric antrum mucosa. Am J Physiol Gastrointest Liver Physiol. 2003;285:G332–43. doi: 10.1152/ajpgi.00453.2002. [DOI] [PubMed] [Google Scholar]
- 13.Oien KA, McGregor F, Butler S, et al. Gastrokine 1 is abundantly and specifically expressed in superficial gastric epithelium, down-regulated in gastric carcinoma, and shows high evolutionary conservation. J Pathol. 2004;203:789–97. doi: 10.1002/path.1583. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa Y, Mukai H, Hino F, Asada K, Kato I. Isolation of two novel genes, down-regulated in gastric cancer. Jpn J Cancer Res. 2000;91:459–63. doi: 10.1111/j.1349-7006.2000.tb00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton JL, Allen A, Westley BR, May FEB. The human trefoil peptide, TFF1, is present in different molecular forms that are intimately associated with mucus in normal stomach. Gut. 2000;46:312–20. doi: 10.1136/gut.46.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–32. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 17.Regalo G, Wright NA, Machado JC. Trefoil factors: from ulceration to neoplasia. Cell Mol Life Sci. 2005;62:2910–5. doi: 10.1007/s00018-005-5478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefebvre O, Chenard MP, Masson R, et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274:259–62. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- 19.Henry JA, Bennett MK, Piggott NH, Levett DL, May FEB, Westley BR. Expression of the pNR-2/pS2 protein in diverse human epithelial tumours. Br J Cancer. 1991;64:677–82. doi: 10.1038/bjc.1991.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller W, Borchard F. pS2 protein in gastric carcinoma and normal gastric mucosa: association with clincopathological parameters and patient survival. J Pathol. 1993;171:263–9. doi: 10.1002/path.1711710406. [DOI] [PubMed] [Google Scholar]
- 21.Park WS, Oh RR, Park JY, et al. Somatic mutations of the trefoil factor family 1 gene in gastric cancer. Gastroenterology. 2000;119:691–8. doi: 10.1053/gast.2000.16483. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto J, Yasui W, Tahara H, et al. DNA hypermethylation at the pS2 promoter region is associated with early stage of stomach carcinogenesis. Cancer Lett. 2000;149:125–34. doi: 10.1016/s0304-3835(99)00349-3. [DOI] [PubMed] [Google Scholar]
- 23.Farrell JJ, Taupin D, Koh TJ, et al. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109:193–204. doi: 10.1172/JCI12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katoh M. Trefoil factors and human gastric cancer (review) Int J Mol Med. 2003;12:3–9. [PubMed] [Google Scholar]
- 25.Yamachika T, Werther JL, Bodian C, et al. Intestinal trefoil factor: a marker of poor prognosis in gastric carcinoma. Clin Cancer Res. 2002;8:1092–9. [PubMed] [Google Scholar]
- 26.Dhar DK, Wang TC, Tabara H, et al. Expression of trefoil factor family members correlates with patient prognosis and neoangiogenesis. Clin Cancer Res. 2005;11:6472–8. doi: 10.1158/1078-0432.CCR-05-0671. [DOI] [PubMed] [Google Scholar]
- 27.Van De Bovenkamp JH, Korteland-Van Male AM, Buller HA, Einerhand AW, Dekker J. Infection with Helicobacter pylori affects all major secretory cell populations in the human antrum. Dig Dis Sci. 2005;50:1078–86. doi: 10.1007/s10620-005-2708-4. [DOI] [PubMed] [Google Scholar]
- 28.Xia HH, Yang Y, Lam SK, et al. Aberrant epithelial expression of trefoil family factor 2 and mucin 6 in Helicobacter pylori infected gastric antrum, incisura, and body and its association with antralisation. J Clin Pathol. 2004;57:861–6. doi: 10.1136/jcp.2003.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clyne M, Dillon P, Daly S, et al. Helicobacter pylori interacts with the human single-domain trefoil protein TFF1. Proc Natl Acad Sci U S A. 2004;101:7409–14. doi: 10.1073/pnas.0308489101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiozaki K, Nakamori S, Tsujie M, et al. Human stomach-specific gene, CA11, is down-regulated in gastric cancer. Int J Oncol. 2001;19:701–7. doi: 10.3892/ijo.19.4.701. [DOI] [PubMed] [Google Scholar]
- 31.Resnick MB, Gavilanez M, Newton E, et al. Claudin expression in gastric adenocarcinomas: a tissue microarray study with prognostic correlation. Hum Pathol. 2005;36:886–92. doi: 10.1016/j.humpath.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 32.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6. Springer; 2002. pp. 99–106. [Google Scholar]
- 33.Lafontaine PO, Arnal M, Buron N, et al. Trefoil factor family mRNA and protein expression in pterygium. Int J Oncol. 2005;27:997–1003. [PubMed] [Google Scholar]
- 34.Sanchez-Pulido L, Devos D, Valencia A. BRICHOS: a conserved domain in proteins associated with dementia, respiratory distress and cancer. Trends Biochem Sci. 2002;27:329–32. doi: 10.1016/s0968-0004(02)02134-5. [DOI] [PubMed] [Google Scholar]
- 35.Ottini L, Falchetti M, Lupi R, Rizzolo P, Agnese V, Colucci G, Bazan V, Russo A. Patterns of genomic instability in gastric cancer: clinical implications and perspectives. Ann Oncol. 2006;17 (Suppl 7):97–102. doi: 10.1093/annonc/mdl960. [DOI] [PubMed] [Google Scholar]
- 36.Chetty R, Naidoo R, Tarin M, Sitti C. Chromosome 2p, 3p, 5q and 18q status in sporadic gastric cancer. Pathology. 2002;34:275–81. doi: 10.1080/00313020220131354. [DOI] [PubMed] [Google Scholar]
- 37.Nardone G, Rippa E, Martin G, et al. Gastrokine 1 expression in patients with and withoutHelicobacter pylori infection. Dig Liver Dis. 2007;39:122–9. doi: 10.1016/j.dld.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Karnes WE, Jr, Samloff IM, Siurala M, et al. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology. 1991;101:167–74. doi: 10.1016/0016-5085(91)90474-y. [DOI] [PubMed] [Google Scholar]