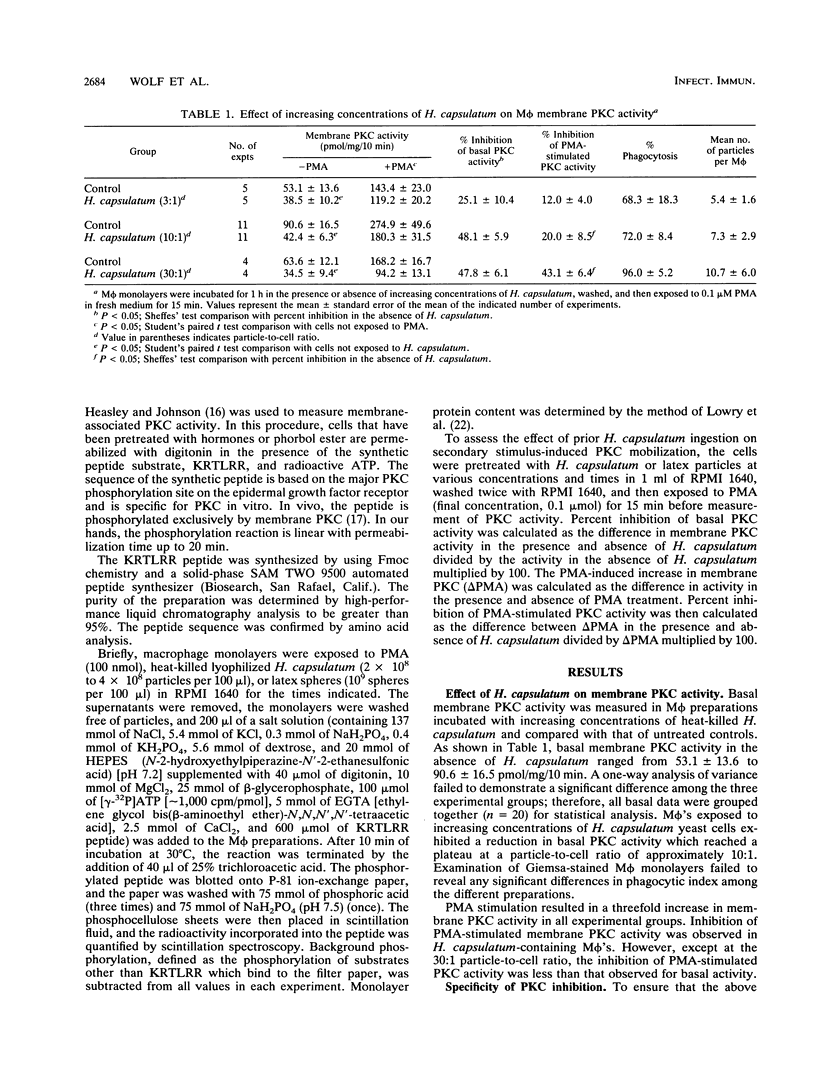
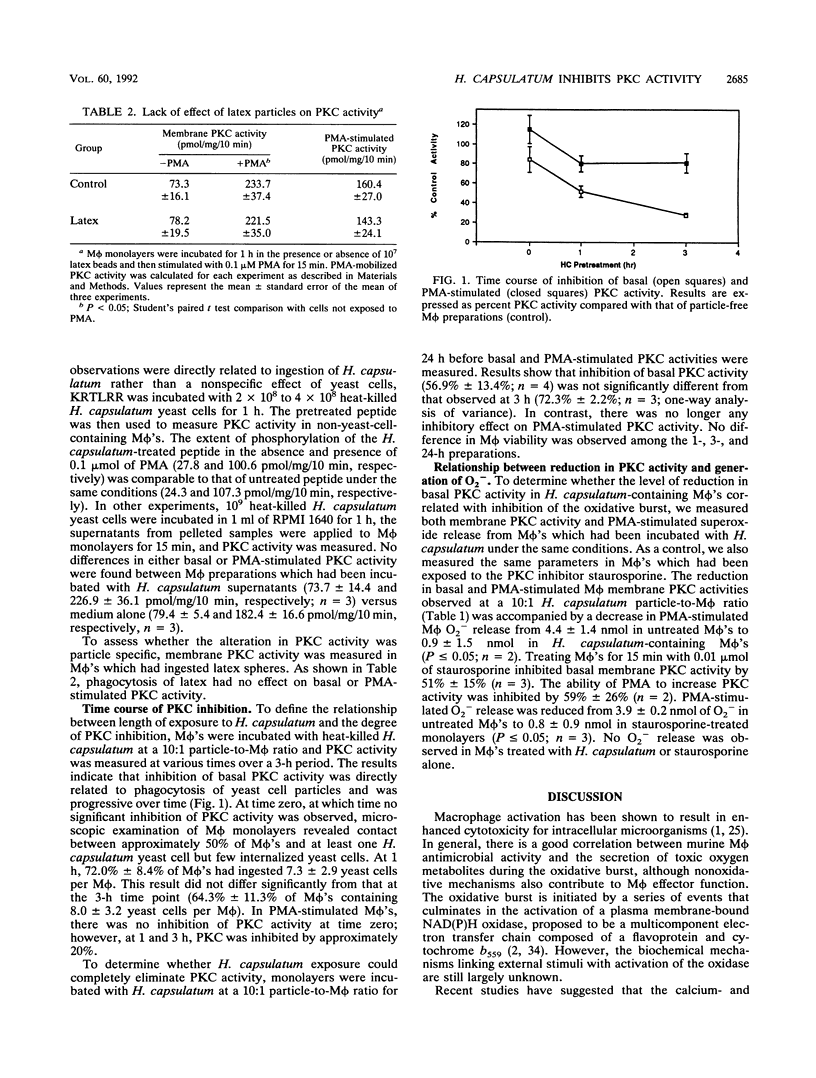
Abstract
Ingestion of Histoplasma capsulatum yeast cells inhibits the oxidative burst response of murine macrophages (M phi's). Since protein kinase C (PKC) is believed to be an integral part of the signal transduction pathway involved in the production of reactive oxygen intermediates, we investigated the relationship between PKC activity and oxidative burst inhibition in H. capsulatum-containing murine peritoneal M phi's. An inhibitory effect on both basal and phorbol myristate acetate-mobilized membrane PKC activities was observed in M phi's which had ingested H. capsulatum but not latex spheres. These results suggest that one way in which H. capsulatum may disrupt the oxidative burst is through a PCK-dependent mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr K., Laine R. A., Lester R. L. Carbohydrate structures of three novel phosphoinositol-containing sphingolipids from the yeast Histoplasma capsulatum. Biochemistry. 1984 Nov 6;23(23):5589–5596. doi: 10.1021/bi00318a032. [DOI] [PubMed] [Google Scholar]
- Barr K., Lester R. L. Occurrence of novel antigenic phosphoinositol-containing sphingolipids in the pathogenic yeast Histoplasma capsulatum. Biochemistry. 1984 Nov 6;23(23):5581–5588. doi: 10.1021/bi00318a031. [DOI] [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Mauël J. Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania spp. Infect Immun. 1987 Mar;55(3):587–593. doi: 10.1128/iai.55.3.587-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Wright S. D. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987 Jan 1;165(1):195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chan J., Fan X. D., Hunter S. W., Brennan P. J., Bloom B. R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991 May;59(5):1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Shuford W. W. Survival and growth of Yersinia pestis within macrophages and an effect of the loss of the 47-megadalton plasmid on growth in macrophages. Infect Immun. 1985 Jan;47(1):234–241. doi: 10.1128/iai.47.1.234-241.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. A., Jeng A. Y., Sharkey N. A., Blumberg P. M., Tauber A. I. Activation of the human neutrophil nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase by protein kinase C. J Clin Invest. 1985 Nov;76(5):1932–1938. doi: 10.1172/JCI112190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg L. G., Goldman W. E. Histoplasma capsulatum fails to trigger release of superoxide from macrophages. Infect Immun. 1987 Jan;55(1):29–34. doi: 10.1128/iai.55.1.29-34.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer B., Störkel S., Loos M. Contributions of C1q, bacterial lipopolysaccharide, and porins during attachment and ingestion phases of phagocytosis by murine macrophages. Infect Immun. 1986 Mar;51(3):807–815. doi: 10.1128/iai.51.3.807-815.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez A. M., Rhodes J. C., Deepe G. S., Jr Antigenicity and immunogenicity of an extract from the cell wall and cell membrane of Histoplasma capsulatum yeast cells. Infect Immun. 1991 Jan;59(1):330–336. doi: 10.1128/iai.59.1.330-336.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Detection of nerve growth factor and epidermal growth factor-regulated protein kinases in PC12 cells with synthetic peptide substrates. Mol Pharmacol. 1989 Mar;35(3):331–338. [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Regulation of protein kinase C by nerve growth factor, epidermal growth factor, and phorbol esters in PC12 pheochromocytoma cells. J Biol Chem. 1989 May 25;264(15):8646–8652. [PubMed] [Google Scholar]
- Imamichi T., Koyama J. Two distinct types of Fc receptor for IgG on guinea pig macrophages activate the NADPH oxidase through different signal-transduction pathways. Biochem Biophys Res Commun. 1990 Oct 15;172(1):223–228. doi: 10.1016/s0006-291x(05)80197-4. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Adams D. O., Hamilton T. A. Regulation of respiratory burst in murine peritoneal macrophages: differential sensitivity to phorbol diesters by macrophages in different states of functional activation. Cell Immunol. 1986 Jul;100(2):400–410. doi: 10.1016/0008-8749(86)90039-0. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Johnston R. B., Jr Deactivation of the respiratory burst in activated macrophages: evidence for alteration of signal transduction. J Immunol. 1986 Apr 1;136(7):2605–2612. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McNeely T. B., Turco S. J. Inhibition of protein kinase C activity by the Leishmania donovani lipophosphoglycan. Biochem Biophys Res Commun. 1987 Oct 29;148(2):653–657. doi: 10.1016/0006-291x(87)90926-0. [DOI] [PubMed] [Google Scholar]
- Myers M. A., McPhail L. C., Snyderman R. Redistribution of protein kinase C activity in human monocytes: correlation with activation of the respiratory burst. J Immunol. 1985 Nov;135(5):3411–3416. [PubMed] [Google Scholar]
- Nathan C. F. Secretion of oxygen intermediates: role in effector functions of activated macrophages. Fed Proc. 1982 Apr;41(6):2206–2211. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W. A., Fujiki T., Rossi M. W., Korchak H. M., Johnston R. B., Jr Influence of calcium on the subcellular distribution of protein kinase C in human neutrophils. Extraction conditions determine partitioning of histone-phosphorylating activity and immunoreactivity between cytosol and particulate fractions. J Biol Chem. 1989 May 15;264(14):8361–8365. [PubMed] [Google Scholar]
- Reca M. E. Reduction of a tetrazolium salt in determining growth activity of yeast-phase Histoplasma capsulatum. Appl Microbiol. 1968 Feb;16(2):236–238. doi: 10.1128/am.16.2.236-238.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur R. A., Newman S. L. The respiratory burst response to Histoplasma capsulatum by human neutrophils. Evidence for intracellular trapping of superoxide anion. J Immunol. 1990 Jun 15;144(12):4765–4772. [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Role of phagocytosis in the activation of macrophages. J Exp Med. 1978 Dec 1;148(6):1449–1457. doi: 10.1084/jem.148.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Hunter S. W., Brennan P. J., Krahenbuhl J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988 May;56(5):1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Mycobacterium leprae-burdened macrophages are refractory to activation by gamma interferon. Infect Immun. 1987 Feb;55(2):446–450. doi: 10.1128/iai.55.2.446-450.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tamoto K., Hazeki K., Nochi H., Mori Y., Koyama J. Phosphorylation of NADPH-cytochrome c reductase in guinea pig peritoneal macrophages stimulated with phorbol myristate acetate. FEBS Lett. 1989 Feb 13;244(1):159–162. doi: 10.1016/0014-5793(89)81183-4. [DOI] [PubMed] [Google Scholar]
- Wilson E., Olcott M. C., Bell R. M., Merrill A. H., Jr, Lambeth J. D. Inhibition of the oxidative burst in human neutrophils by sphingoid long-chain bases. Role of protein kinase C in activation of the burst. J Biol Chem. 1986 Sep 25;261(27):12616–12623. [PubMed] [Google Scholar]
- Wolf J. E., Abegg A. L., Travis S. J., Kobayashi G. S., Little J. R. Effects of Histoplasma capsulatum on murine macrophage functions: inhibition of macrophage priming, oxidative burst, and antifungal activities. Infect Immun. 1989 Feb;57(2):513–519. doi: 10.1128/iai.57.2.513-519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. E., Kerchberger V., Kobayashi G. S., Little J. R. Modulation of the macrophage oxidative burst by Histoplasma capsulatum. J Immunol. 1987 Jan 15;138(2):582–586. [PubMed] [Google Scholar]



