Abstract
Experiments were performed to determine the involvement of ATP-sensitive K+ channels (KATP channels) in the renal afferent arteriolar dilation that occurs during the hyperfiltration stage of insulin-dependent diabetes mellitus (IDDM). IDDM was induced in rats by streptozotocin (STZ) injection and adequate insulin was provided to maintain moderate hyperglycemia. Sham rats received vehicle treatments. Two weeks later, afferent arteriolar function was assessed using the in vitro blood-perfused juxtamedullary nephron technique. Baseline afferent arteriolar lumen diameter was greater in STZ rats (25.9±1.1 μm) than in Sham rats (20.8±1.0 μm). Glibenclamide (3–300 μM) had virtually no effect on afferent arterioles from Sham rats; however, this KATP antagonist caused concentration-dependent afferent arteriolar constriction in kidneys from STZ-treated rats, restoring lumen diameter to 20.6±1.7 μm (P>0.05 vs Sham baseline). In both groups of rats, pinacidil (a cyanoguanidine KATP agonist; 0.3–300 μM) evoked concentration-dependent afferent arteriolar dilation indicating the functional expression of KATP channels; however, lumen diameter was increased by 73% in STZ kidneys but only by 48% in Sham kidneys. The glibenclamide-sensitive afferent arteriolar dilator response to 1 μM PCO-400 (a benzopyran KATP agonist) was also accentuated in STZ kidneys. These observations suggest that increases in both the functional availability and basal activation of KATP channels promote afferent arteriolar vasodilation during the early stage of IDDM, changes that likely contribute to the etiology of diabetic hyperfiltration.
Keywords: glibenclamide, pinacidil, PCO-400, renal circulation, rat
Introduction
Extensive studies have explored the role of altered renal hemodynamics in the development of diabetic nephropathy. In both clinical and experimental settings, increased glomerular filtration rate and renal plasma flow are manifest during the early stages of IDDM. An elevated filtration fraction is usually apparent, and micropuncture studies have revealed reduced preglomerular vascular resistance with relatively normal postglomerular resistance (1). The pathophysiologic significance of hyperfiltration in early IDDM is underscored by evidence that ameliorating these early renal hemodynamic events, even in the absence of improved metabolic status, retards the progression of diabetic glomerulosclerosis and eventual development of renal failure (2,3).
Although numerous metabolic and hormonal abnormalities have been implicated in the pathogenesis of diabetic hyperfiltration, no single factor has been proven to be responsible for the early changes in renal vascular function. It seems unlikely that changes in any specific membrane receptor or vasoactive agonist would underlie an altered responsiveness to a broad spectrum of stimuli, the situation that appears evident in the early stage of IDDM (4,5). Bank and coworkers (6) have proposed that insulinopenia in IDDM might impair Ca2+ movement through renal vascular voltage-gated Ca2+ channels. Indeed, recent studies from our laboratory revealed that afferent arteriolar contractile responses to BAY K8644 (a dihydropyridine agonist) and K+-induced membrane depolarization are impaired during the hyperfiltration stage of IDDM, and that these changes are accompanied by suppressed afferent arteriolar [Ca2+] responses to membrane depolarization (7). Because voltage-gated Ca2+ channels are prominent in afferent arterioles, but functionally silent in efferent arterioles (8), altered expression or regulation of these channels would promote afferent arteriolar dilation and hyperfiltration in IDDM; however, dysregulation of membrane potential as a result of increased K+ conductance might also contribute to this phenomenon.
Among the K+ channels that have been identified in the cell membrane of arterial smooth muscle cells (9), K+ channels closed by intracellular ATP (KATP channels) are unique in that their activity may reflect the cellular metabolic state. KATP channels are composed of pore-forming (inwardly-rectifying K+ channel; KIR6.x) and regulatory (sulfonylurea receptor; SUR) subunits. Distinct combinations of KIR6.x and SUR subunits associate in a 1:1 stoichiometry to form tetrameric channels in specific cell types (10). The ATP-sensing mechanism and biophysical properties of KATP channels are intrinsic to the KIR6.x subunit, while the various SUR isoforms confer tissue-specific pharmacologic properties regarding interactions with sulfonylurea compounds and potassium channel openers (11). Previous studies have provided functional evidence that KATP channels are expressed the renal microcirculation (12–15), although the KIR6.x and SUR subunits comprising these channels have not been identified. Marcus and coworkers (16) have proposed that changes in KATP channel activity could engender glomerular hyperfiltration in IDDM. Although numerous pharmacologic studies have assessed KATP channel function in diverse vascular beds at various times during the progression of IDDM (17–21), the results have been inconsistent and the impact of KATP channels on renal afferent arteriolar function during the hyperfiltration stage of IDDM remains unexplored. Accordingly, the goal of the present study was to examine the hypothesis that the influence of KATP channels on afferent arteriolar tone is exaggerated during the early (hyperfiltration) stage of IDDM in the rat.
Materials and Methods
Animals
Male Sprague-Dawley rats were treated according to the NIH Guide for the Care and Use of Laboratory Animals. Rats were housed in pairs, provided ad libitum access to food and water, and maintained on a 12 h light/dark cycle.
Induction of Diabetes Mellitus
Rats were anesthetized with sodium methohexital (50 mg/kg, i.p.) to facilitate intravenous injection of streptozocin (STZ, Upjohn Co., Kalamazoo, MI, 65 mg/kg). Sham rats received vehicle. The rats were allowed to recover from anesthesia and housed overnight. The following morning, blood glucose levels were measured (Accu-Check III model 766, Boehringer Mannheim Co., Indianapolis, IN) and the rats were anesthetized again to allow either a) intraperitoneal implantation of an osmotic minipump (model 2002; Alzet, Palo Alto, CA) prepared for delivery of insulin (3.5 U·kg−1·day−1 Iletin II; Eli Lily, Indianapolis, IN), or b) subcutaneous insertion via a 16-G needle of a 2.3×2.0 mm sustained-release insulin implant (Linplant®; Linshin Canada, Scarborough, Ontario). In our hands, both modes of insulin delivery maintain moderate hyperglycemia during the ensuing 2-wk period. Sham rats received either diluent infusion via a minipump or a micro-recrystallized palmitic acid (vehicle) implant. All animals subjected to minipump implantation received penicillin G procaine (60,000 U i.m.) immediately after surgery. Thereafter, blood glucose concentration and body weight were measured twice weekly.
The In Vitro Blood-Perfused Juxtamedullary Nephron Technique
Two weeks after induction of IDDM, acute experiments were performed using the in vitro blood-perfused juxtamedullary nephron technique (22). Most experiments utilized two animals, one as a blood donor and another as a kidney donor, although a single rat provided both tissues in some instances. After anesthetization with sodium pentobarbital (50 mg/kg, i.p.), a catheter was placed in the carotid artery. Arterial pressure was measured in some animals via this carotid cannula using a P23XL transducer (Gould Inc., Oxnard, CA) connected to a polygraph (Grass Instruments, Quincy, MA). Blood donors were bilaterally nephrectomized through flank incisions and their blood collected into a heparinized syringe. Kidney donors were laparotomized and the left kidney removed. A perfusion cannula introduced via the superior mesenteric artery into the right renal artery allowed renal perfusion with Tyrode's solution containing 52 g/l bovine serum albumin, a mixture of L-amino acids (23), and either 5 or 20 mM glucose (in Sham and STZ rats, respectively). The renal vein was incised to drain the perfusate and the animal was exsanguinated into a heparinized syringe; however, renal perfusion was maintained throughout the ensuing dissection procedure. The kidney was removed and cut longitudinally to expose the pelvic cavity, leaving the papilla intact within the perfused dorsal two-thirds of the organ. Small incisions were made in the lateral fornices, allowing the papilla to be reflected back and retained in that position by insect pins. The pelvic mucosa, adipose and connective tissues that normally cover the inside cortical surface were removed and the veins were cut open, thus exposing the tubules and microvasculature of juxtamedullary nephrons. The portions of the vasculature that give rise to arterioles associated with these juxtamedullary nephrons were isolated with tight ligatures.
Blood collected from the two rats was prepared for perfusion as described previously (7). The reconstituted blood was filtered through a 5-μm nylon mesh and its pH was measured (System 1302 pH/Blood Gas Analyzer, Instrumentation Laboratory, Lexington, MA) and adjusted to 7.40–7.44 by addition of NaHCO3, as necessary. Thereafter, the blood was stirred continuously in a closed reservoir that was maintained under pressure from a gas tank (95%O2:5%CO2). This arrangement provided both tissue oxygenation and the driving force for perfusion of the kidney. The Tyrode's perfusate was then replaced by the reconstituted blood perfusate. Kidneys from STZ rats were perfused with blood from STZ donors, while kidneys from Sham rats were perfused with blood from Sham donors. Perfusion pressure measured at the cannula tip in the renal artery was maintained at 110 mmHg throughout the experiment. The tissue was warmed to 37°C and its surface continuously bathed with Tyrode's solution containing 10 g/l bovine serum albumin, approximating the composition of renal interstitial fluid (24). Stock solutions containing vasoactive agents (100 mM glibenclamide in DMSO; 150 mM pinacidil in EtOH; 10 mM PCO-400 in DMSO) were stored at −20ºC until the day of the experiment, at which time there were diluted with Tyrode’s bath to the appropriate final concentrations.
Videometric techniques were utilized to measure arteriolar lumen diameter (7). The tissue was transilluminated on the fixed stage of a Nikon Optiphot microscope equipped with a water-immersion objective (×40, numerical aperture 0.55). Enhanced video images of the microvessels were displayed at a magnification of ×1,400 on a high resolution monitor (Conrac Display Systems, Covina, CA) and recorded simultaneously on videotape using a SuperVHS videocassette recorder (Panasonic, Secaucus, NJ). During the 15-min equilibration period, an afferent arteriole was selected for study based on visibility and acceptable blood flow. Only arterioles with rapid flow of erythrocytes were studied, and vessels were rejected on the basis of inadequate flow if the passage of single erythrocytes could be discerned. All experimental protocols were designed to assess arteriolar diameter at a single measurement site under several experimental conditions. When two vessels could be visualized within the same field of view, responses of both vessels were recorded simultaneously and analyzed separately during videotape playback. Afferent arteriolar lumen diameter was monitored at sites located <100 μm upstream from the glomerulus and >80 μm downstream from the interlobular artery.
Experimental Protocols
Afferent arteriolar diameter responses to glibenclamide
The effect of glibenclamide (Sigma Chemical, St. Louis, MO), a potent and selective KATP channel inhibitor (10), on afferent arteriolar tone was examined in kidneys from STZ and Sham rats. Afferent arteriolar diameter responses to this drug were assessed by sequential exposure of the tissue surface to the following solutions: 1) Tyrode’s bath alone, 10 min; 2) Tyrode’s solution containing 3, 10, 30, 100, and 300 μM glibenclamide for 5 min at each concentration; and 3) Tyrode’s solution alone, 15 min. Previous studies from our laboratory have documented the stability of perfused juxtamedullary afferent arteriolar diameter in normal rat kidney for at least 35 min (25,26). To exclude the possibility that renal arterioles from STZ rats are more susceptible to time-related degeneration than arterioles from normal kidneys, a time control study was performed on kidneys from 6 STZ rats. In this time control study, afferent arteriolar lumen diameter was monitored while the tissue surface was exposed to Tyrode’s solution alone for 50 min.
Impact of baseline diameter on afferent arteriolar responses to glibenclamide
Additional experiments were performed to determine if a non-specific effect of increased arteriolar baseline diameter per se could underlie the exaggerated response to glibenclamide observed in kidneys from STZ rats. Preliminary experiments revealed that treatment of kidneys from Sham rats with 5 μM sodium nitroprusside (SNP; Elkins-Sinn, Cherry Hill, NJ) evoked prompt and sustained afferent arteriolar dilation, achieving lumen diameters similar to those typically observed under baseline conditions in kidneys from STZ-treated rats. Accordingly, juxtamedullary afferent arterioles in kidneys from Sham rats were exposed sequentially to the following bathing solutions: 1) Tyrode’s solution alone, 10 min; 2) Tyrode’s solution containing 5 μM SNP, 5 min; 3) Tyrode’s solution containing both 5 μM SNP and increasing concentrations (3–300 μM) of glibenclamide, 5 min at each concentration; 4) Tyrode’s solution containing 5 μM SNP, 15 min; and 5) Tyrode’s solution alone.
Afferent arteriolar diameter responses to pinacidil
Afferent arteriolar responses to the cyanoguanidine KATP agonist, pinacidil (27,28), were assessed in kidneys from STZ and Sham rats. The protocol employed sequential exposure of the tissue surface to the following bathing solutions: 1) Tyrode’s solution alone, 10 min; 2) Tyrode’s solution containing 0.3, 1.0, 3.0, 10, 30, 100, and 300 μM pinacidil for 5 min at each concentration; and 3) Tyrode’s solution alone, 15 min. The duration of exposure to the drug was determined by preliminary experiments conducted using kidneys from normal rats (n=3), in which the dilator response of afferent arterioles to 300 μM pinacidil developed almost immediately, reached its maximum within 3 min, and was sustained for more than 40 min.
Glibenclamide reversal of pinacidil-induced vasodilation
Additional experiments were performed to confirm that both pinacidil and glibenclamide influence afferent arteriolar function via actions on the same effector, presumably KATP channels. Afferent arterioles from normal rats (n=5) were exposed to the following bathing solutions: 1) Tyrode’s solution alone, 10 min; 2) Tyrode’s solution containing 30 μM pinacidil, 5 min; 3) Tyrode’s solution containing both 30 μM pinacidil and 100 μM glibenclamide, 15 min; and 4) Tyrode’s solution containing 30 μM pinacidil alone, 5 min.
Arteriolar responses to PCO-400 in the absence and presence of glibenclamide
A final set of experiments assessed the effect of IDDM on afferent arteriolar dilator responses to PCO-400 (BIOMOL, Plymouth Meeting, PA), a benzopyran KATP antagonist (29). Afferent arteriolar responses to 1 μM PCO-400 (a concentration selected based on preliminary experiments utilizing kidneys from normal rats) were monitored in kidneys from Sham and STZ rats before, during and after exposure to 100 μM glibenclamide.
Data Analysis
Microvessel lumen diameter was determined from videotaped images utilizing a digital image-shearing monitor (model 908, IPM, San Diego, CA). This device was calibrated using a stage micrometer (smallest division=2 μm) and yielded diameter measurements reproducible to within <1 μm. Lumen diameter was measured at 12-s intervals from a single site along the vessel length. The average diameter during the final 1-min of each treatment period was utilized for statistical analysis of responses. Data were analyzed by analysis of variance (ANOVA) for repeated measures or Friedman repeated measures ANOVA on ranks, as appropriate, followed by Newman-Keuls test. A P value <0.05 was considered statistically significant. All data are presented as the mean±SEM (n=number of arterioles, unless otherwise stated).
Results
Animal Characteristics
The STZ rats developed hyperglycemia within 24-hr and remained moderately hyperglycemic for the ensuing 2 wk period. Blood glucose concentration averaged 18.2±0.4 mM in 37 STZ rats and 4.7±0.1 mM in 21 Sham rats (P<0.01). At the time of the microvascular function studies, body weight averaged 303±4 g in STZ rats and 329±7 g in Sham rats (P<0.01). As reported previously (30), left kidney weight was significantly greater in STZ-treated rats (1.62±0.06 g wet wt.; 0.34±0.01 g dry wt.; n=22 rats) than in Sham rats (1.33±0.07 g wet wt.; 0.29±0.01 g dry wt.; n=15 rats), indicating renal hypertrophy at this stage of IDDM. Mean arterial pressure averaged 108±5 mmHg in Sham rats (n=8) and did not differ significantly in STZ rats (115±7 mmHg, n=6).
Afferent arteriolar function was assessed at sites 350±30 μm from the glomerulus in STZ-treated rats and 210±30 μm from the glomerulus in Sham rats (P<0.05). However, this difference loses its significance when corrected by total length of afferent arterioles, and measurement was conducted at 42±17% and 43±17% of the total length from the glomerulus in each group of rats. In accord with our previous observations (30,31), STZ-treated rats had significantly larger baseline afferent arteriolar diameter than Sham/Normal rats, with values averaging 25.6±0.9 (n=28) and 20.5±0.8 μm (n=26), respectively.
Afferent Arteriolar Diameter Responses to Glibenclamide
The basal impact of KATP channels on afferent arteriolar diameter in kidneys from Sham and STZ rats was evaluated based on responses to glibenclamide. Figure 1 depicts afferent arteriolar diameter responses to increasing concentrations of glibenclamide in both groups of rats. Although kidneys from Sham rats exhibited a modest but statistically significant decrease in afferent arteriolar diameter when exposed to the highest concentration of glibenclamide (300 μM; Δ=−1.7±0.9 μm; n=8; P<0.05), the drug had no effect on these vessels at lower concentrations. In contrast, glibenclamide evoked concentration-dependent reductions in afferent arteriolar lumen diameter in kidneys from STZ rats, with significant changes evident in response to 30 μM glibenclamide (Δ=−2.4 ±0.6 μm; n=7; P<0.05). During exposure of STZ kidneys to 300 μM glibenclamide, afferent diameter was 5.5±1.1 μm lower than the STZ baseline and similar to baseline values observed in Sham kidneys. Upon removal of glibenclamide from the bath, afferent arteriolar diameter was restored to 19.1±1.6 μm in Sham kidneys and 25.0±2.6 μm in STZ kidneys (P>0.05 vs baseline values). Time-control experiments revealed no significant change in afferent arteriolar diameter in kidneys from STZ rats within the time frame of these experiments (baseline diameter = 26.2±2.6 μm; diameter after 50 min exposure to Tyrode’s bathing solution = 27.0±2.8 μm; n=7). Thus, while glibenclamide had little impact on afferent arterioles in kidneys from Sham rats, the observation of significant afferent constrictor responses to glibenclamide in kidneys from STZ rats suggests that open KATP channels exert a basal dilator influence on the afferent arteriole in IDDM.
Figure 1.
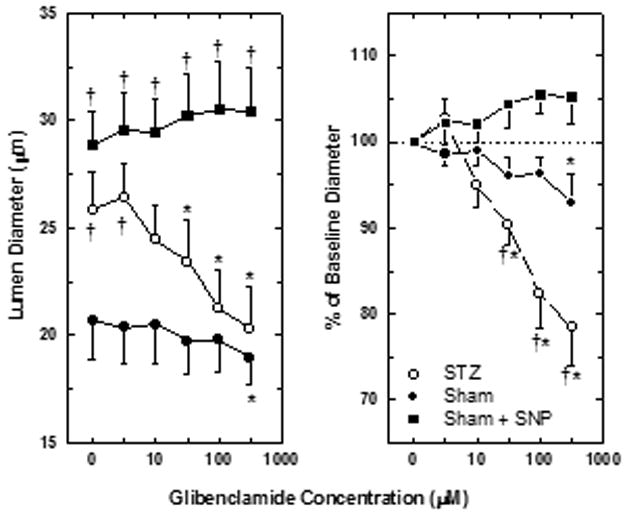
Juxtamedullary afferent arteriolar responses to glibenclamide in kidneys from Sham rats (closed symbols) and STZ-treated rats (open symbols). In some kidneys from Sham rats, arterioles were pre-dilated by exposure to 5 μM sodium nitroprusside (SNP). Left panel depicts lumen diameters (μm). Right panel expresses the same data as percent of baseline diameter. Glibenclamide concentrations were not corrected for binding to bovine serum albumin in the bathing solution. Values are means±SEM. *P<0.05 vs baseline (no glibenclamide); †P<0.05 vs arterioles from Sham rats.
Impact of Baseline Diameter on Responses to Glibenclamide
Figure 1 also shows the effect of glibenclamide on pre-dilated afferent arterioles in kidneys from Sham rats. In these experiments, exposure to 5 μM SNP increased arteriolar diameter from 21.3±2.6 to 28.9±1.5 μm (n=4), a value that does not differ significantly from baseline afferent diameter in kidneys from STZ rats. During continued exposure to SNP, increasing concentrations of glibenclamide had no effect on these pre-dilated vessels (Δ=1.6±0.9 μm during treatment with 300 μM glibenclamide; NS vs SNP alone). Removal of glibenclamide from the SNP-containing bath did not alter afferent diameter (31.2±1.9 μm), while subsequent removal SNP from the bath restored diameter to a value similar to baseline (22.3,±2.4 μm). Thus, the afferent arteriolar constrictor response to glibenclamide in kidneys from STZ rats cannot be attributed to a nonspecific effect of reduced basal arteriolar tone per se.
Afferent Arteriolar Diameter Responses to Pinacidil
Additional experiments were performed to determine if the potentiated afferent arteriolar response to glibenclamide in STZ kidneys reflects opening of recruitable KATP channels that are normally present but quiescent under basal conditions. This issue was evaluated on the basis of afferent arteriolar diameter responses to increasing concentrations of pinacidil (Figure 2). In kidneys from STZ-treated rats, 1 μM pinacidil evoked a significant increase in afferent arteriolar diameter (Δ=4.1±1.2 μm; n=8), while this concentration of pinacidil had no effect on afferent diameter in kidneys from Sham rats (Δ=0.0±0.4 μm; n=9). Moreover, responses to 300 μM pinacidil were significantly greater in STZ rats (Δ=18.1±2.1 μm) than in Sham rats (Δ=10.1±1.2 μm). In a subset of 4 kidneys from Sham rats, subsequent exposure to 1 mM pinacidil did not evoke any further increase in arteriolar diameter, indicating that the maximum response to this agonist had been achieved. Thus, while pinacidil evoked a concentration-dependent increase in afferent arteriolar lumen diameter in kidneys from both groups of rats, vessels from STZ-treated rats were more sensitive to the drug and the magnitude of the response was augmented substantially compared to vessels from Sham rats. Removal of pinacidil from the superfusate bath restored afferent arteriolar diameter to 22.3±1.3 μm in Sham kidneys and 25.1±2.4 μm in STZ kidneys (both P>0.05 vs baseline), indicating that the observed responses did not reflect a time-related loss of arteriolar tone. The observation of exaggerated dilator responses to pinacidil in afferent arterioles from STZ kidneys suggests that the functional availability of KATP channels may be increased in IDDM.
Figure 2.
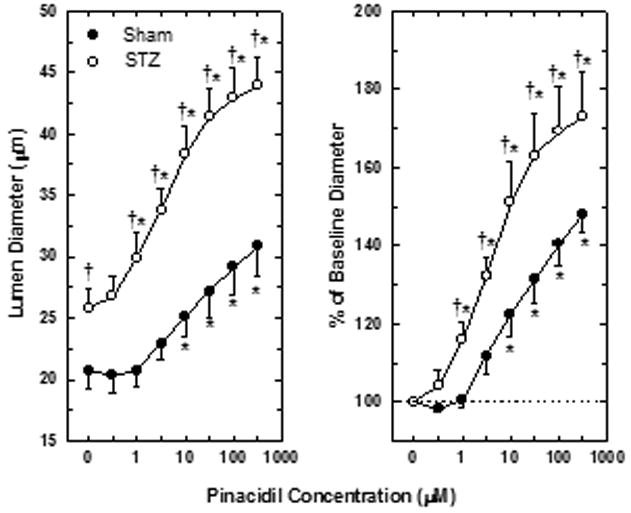
Juxtamedullary afferent arteriolar responses to pinacidil in kidneys from Sham rats (closed symbols) and STZ-treated rats (open symbols). Left panel depicts lumen diameter in μm. Right panel expresses same data as percent of baseline diameter. Pinacidil concentrations were not corrected for binding to bovine serum albumin in the bathing solution. Values are means±SEM. *P<0.05 vs baseline (no pinacidil); †P<0.05 vs arterioles from Sham rats.
Glibenclamide Reversal of Pinacidil-Induced Vasodilation
Figure 3 documents the ability of glibenclamide to reverse the afferent arteriolar dilator response to pinacidil in kidneys from normal rats. Exposure to 30 μM pinacidil increased afferent arteriolar lumen diameter from 17.5±0.9 to 24.5±1.6 μm (P<0.05; n=5). Subsequent addition of 100 μM glibenclamide to the pinacidil-containing bath significantly reduced arteriolar diameter to 21.6±1.8 μm within 5 min. After 15 min of simultaneous exposure to both drugs, arteriolar diameter averaged 18.2±0.8 μm (NS vs initial baseline). When glibenclamide was removed from the pinacidil-containing bath, the vessels dilated again to 23.6±1.6 μm (P<0.05 vs baseline value; NS vs initial response to pinacidil alone).
Figure 3.
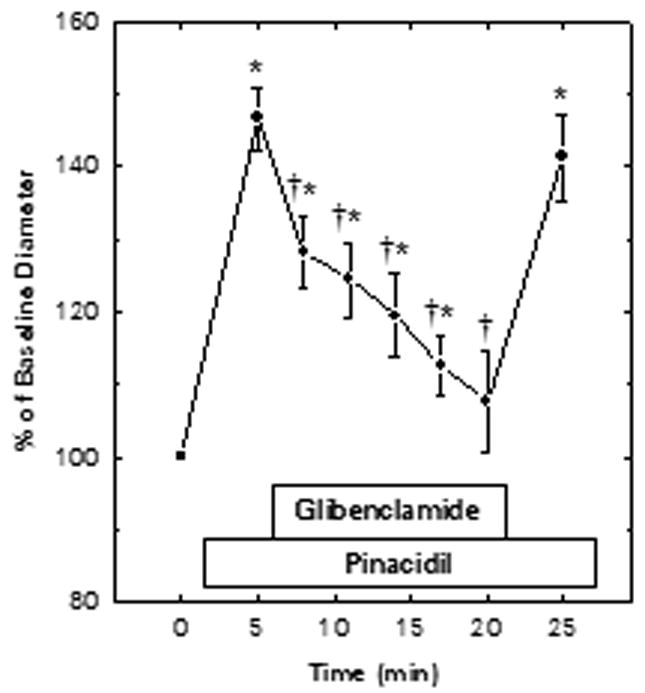
Glibenclamide reversal of pinacidil-induced dilation of juxtamedullary afferent arterioles in kidneys from normal rats. Pinacidil (30 μM) and glibenclamide (100 μM) were added to the bath at the times indicated. Concentrations were not corrected for binding to bovine serum albumin in the bathing solution. Baseline diameter averaged 17.5±0.9 μm. Values are means±SEM. *P<0.05 vs baseline (prior to exposure to pinacidil or glibenclamide); †P<0.05 vs pinacidil alone (5 min time point).
Effect of IDDM on Responses to PCO-400
The ability of KATP agonists to elicit exaggerated, sulfonylurea-sensitive afferent arteriolar vasodilation in IDDM was confirmed in studies utilizing PCO-400, a KATP opener that is structurally distinct from pinacidil (28). In these experiments, baseline afferent arteriolar diameter averaged 19.3±0.5 μm (n=5) in kidneys from Sham rats and 24.6±1.9 μm in STZ kidneys (n=6; P<0.05 vs Sham). In each arteriole studied, diameter responses to PCO-400 were monitored before, during and after glibenclamide treatment. PCO-400 responses obtained after removal from glibenclamide from the bath did not differ significantly from those evident prior to glibenclamide treatment. Therefore, the average response for each vessel was employed for data analysis and preparation of Figure 4, which summarizes responses to PCO-400 and the glibenclamide-sensitivity of these responses in Sham and STZ kidneys. In kidneys from Sham rats, 1 μM PCO-400 significantly increased afferent arteriolar diameter by 2.6±0.7 μm. Although 100 μM glibenclamide failed to alter baseline diameter in Sham kidneys, it abolished the dilator response to PCO-400 (Δ=0.4±0.6 μm). In kidneys from STZ rats, 1 μM PCO-400 increased afferent arteriolar diameter by 6.2±1.3 μm (P<0.05 vs Sham kidneys). During exposure of STZ kidneys to 100 μM glibenclamide, afferent diameter was 3.0±0.8 μm lower than the STZ baseline (P<0.05) and did not differ from baseline values observed in Sham kidneys. Glibenclamide exposure also abolished the vasodilator response to PCO-400 in STZ kidneys (Δ=−0.2±0.3 μm).
Figure 4.
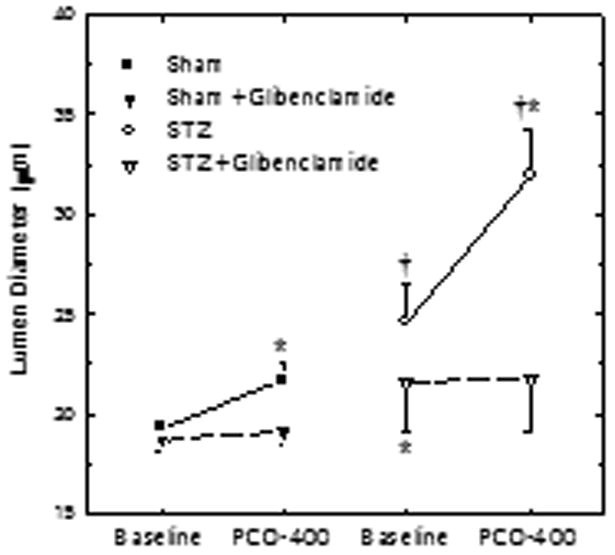
Effect of IDDM on glibenclamide-sensitive afferent arteriolar diameter responses to PCO-400 in kidneys from Sham (closed symbols) and STZ rats (open symbols). Responses to 1 μM PCO-400 were monitored in each vessel both in the absence (circles) and presence (inverted triangles) of 100 μM glibenclamide. Values are means±SEM. *P<0.05 vs baseline in the absence of glibenclamide; †P<0.05 vs Sham.
Discussion
Renal afferent arteriolar tone is critically dependent upon smooth muscle membrane potential, which regulates Ca2+ influx through voltage-gated channels. Ca2+ influx through this pathway is curtailed during the hyperfiltration stage of IDDM, suggesting a defect in the expression or regulation of the L-type Ca2+ channel (7). The open probability of the L-type Ca2+ channel is determined chiefly by membrane potential, a primary determinant of which is membrane K+ conductance. An increase in K+ conductance should hyperpolarize the cell membrane, diminish the open probability of voltage-gated Ca2+ channels and promote vasodilation. The present studies examined the possibility that afferent arteriolar dilation in IDDM might reflect an augmented impact of KATP channels on basal tone. The strategy sought to detect differential responses to pharmacological KATP channel manipulation in kidneys from Sham- and STZ-treated rats. The moderately hyperglycemic model of IDDM employed in this study exhibits glomerular hyperfiltration at both the whole kidney level in vivo and the single juxtamedullary nephron level in vitro (30), and typically displays polydipsia and polyuria (32). However, mean arterial pressure is not significantly altered in these animals.
Because of the heteromultimeric nature of KATP channels, the standard sulfonylurea blockers and the structurally heterogeneous array of potassium channel openers display a wide spectrum of affinities toward KATP channels expressed in varied tissues. Nevertheless, these well-characterized pharmacologic tools have yielded substantial insight into the functional role of KATP channels in a variety of physiologic and pathophysiologic settings. The native SUR2B/KIR6.2 channel expressed in most vascular smooth muscle is inhibited by 20 μM glibenclamide (the most potent sulfonylurea, a derivative of which was exploited in the initial purification of the SUR subunit) and activated by low micromolar concentrations of pinacidil (10,33). In the present study, significant effects of glibenclamide on afferent arteriolar diameter were observed in kidneys from STZ rats only at bath concentrations of 30–300 μM. Glibenclamide binds with high affinity to bovine serum albumin (present in our superfusate bath at a concentration of 10 g/l), such that only 1–10% of total drug is available in free form in media similar to that employed in the present study (34,35). Accordingly, the maximum free glibenclamide concentration achieved in our experiments was in the range of 3–30 μM, which should block vascular smooth muscle KATP channels while exerting little effect on other classes of ion channel. Available data indicates that 50–90% of pinacidil is available in free form in albumin-containing solutions (36). Our studies reveal that the effect of pinacidil on juxtamedullary afferent arteriolar diameter is rapid in onset and fully reversed by glibenclamide within 15 min. A similar time course for achieving maximal inhibition of the pinacidil response has been reported to occur in rabbit superior mesenteric artery (37) and was suggested to indicate a requirement for glibenclamide to enter the lipid bilayer and move laterally within the membrane to access its site of action (38). Glibenclamide and pinacidil bind to the SUR subunit of the channel at separate cytoplasmic sites, and the prolonged time course for glibenclamide reversal of pinacidil-induced dilation may reflect the fact that cyanoguanidine binding inhibits sulfonylurea binding in a negative allosteric manner (17). Overall, the pharmacologic properties of glibenclamide and pinacidil at free concentrations achieved in the present study are consistent with the well-established and specific actions of these agents on vascular KATP channels. The ability of glibenclamide to restore afferent arteriolar diameter after dilation by pinacidil, and to abrogate dilator responses to PCO-400 in both Sham and STZ rats, lends further credence to the contention that these agents act on a common target in our experimental setting — presumably KATP channels in afferent arteriolar smooth muscle cells.
In normal rats, systemic infusion of KATP blockers has been reported to increase (39) or not alter renal vascular resistance and glomerular filtration rate (40–42). Reductions in medullary blood flow have also been reported (43,44). The effects of these agents on renal hemodynamics in vivo can be complicated by alterations in arterial pressure or reflex-mediated responses that allow maintained arterial pressure. Overall, however, the literature indicates that KATP blockers exert little direct impact on renal vascular resistance under basal conditions. The ineffectiveness of glibenclamide as an afferent arteriolar constrictor in kidneys from Sham rats in the present study, during perfusion in vitro at a constant pressure, provides further indirect evidence that the open probability of KATP channels in afferent arteriolar smooth muscle is low under normal physiological conditions. Less than 2% of the KATP channels in rabbit mesenteric arterial smooth muscle cells are estimated to be open in the absence of activators and in the presence of physiological intracellular ATP (9), a situation that may also transpire in the renal afferent arteriole. However, our observation of afferent arteriolar dilation in response to structurally-distinct KATP agonists, pinacidil and PCO-400, in kidneys from Sham rats confirms previous reports that this vascular segment is responsive to potassium channel openers (14,15) and, thus, endowed with a recruitable pool of KATP channels.
In contrast to afferent arterioles from Sham rats, vessels from STZ-treated rats exhibited significant constriction in response to glibenclamide. This response could not be attributed to an acute degenerative change of diabetic vessel function, because arteriolar diameter was stable in time control experiments. It also seemed possible that the larger basal afferent arteriolar diameter in juxtamedullary nephrons of STZ-treated rats, characteristic of the hyperfiltration stage of IDDM (30), could have enhanced responsiveness to glibenclamide indirectly through stretch-induced changes in membrane potential and/or tangential wall stress. Because neither SNP nor nitric oxide has been reported to interfere with the glibenclamide binding to (or effects on) the KATP channel, the failure of glibenclamide to elicit constriction of SNP-dilated arterioles from Sham rats makes it unlikely that the constrictor response to this agent observed in STZ rats is nonspecifically related to reduced arteriolar tone. The inability of glibenclamide to constrict SNP-dilated arterioles from Sham rats is also consistent with the contention that the renal vasodilator response to nitric oxide does not involve opening of KATP channels (45). Overall, the responses to glibenclamide observed in the present study suggest that KATP channels are quiescent in normal rats but exert a vasodilator influence on afferent arteriolar tone during the hyperfiltration stage of diabetes.
We reasoned that a diabetes-induced opening of a significant component of the normal pool of afferent arteriolar KATP channels might fully explain the response to glibenclamide in STZ rats, thus reducing the population of closed KATP channels capable of responding to potassium channel openers. Deducing that this phenomenon should be indicated by a diminished vasodilator response to potassium channel openers, we were surprised to find that afferent arterioles from STZ rats exhibited exaggerated responses to these compounds. In spite of the larger baseline diameter in afferent arterioles from STZ rats, the dilator responses to both pinacidil and PCO-400 exceeded those of Sham rats in terms of both % change and absolute change in diameter. These observations suggest that the functional availability (expression or density) of afferent arteriolar KATP channels may be increased during the early stage of IDDM, or that the impact of these channels on membrane potential is exaggerated. The validity of these intriguing scenarios cannot be discerned from the results of the present study, requiring exploration at the electrophysiological and molecular levels.
In addition to reversing renal vascular responses to potassium channel openers (15,39,46–48), glibenclamide inhibits afferent arteriolar dilator responses to glycolytic inhibition (15) and hypoxia (12). These observations indicate the potential for coupling afferent arteriolar smooth muscle metabolism to microvascular function via changes KATP channel activity, as has been suggested to occur in a variety of vascular beds. The possible relevance of this putative metabolic coupling to regulation of afferent arteriolar tone in IDDM was first suggested by Marcus and coworkers (16), who documented diminished glucose uptake (associated with decreased expression of insulin-responsive GLUT4 transporter mRNA and polypeptide) in afferent arterioles from diabetic rats. Glucose transport is rate-limiting for glycolysis in vascular smooth muscle (49) and glycolytic inhibition evokes afferent arteriolar dilation through a glibenclamide-sensitive process (15). Accordingly, Marcus and coworkers (16) postulated that the effect of IDDM to decrease glucose uptake into afferent arteriolar smooth muscle might decrease cytosolic ATP levels, allowing opening of resident KATP channels, membrane hyperpolarization and reduced membrane excitability, closing of voltage-gated calcium channels and vasodilation. It is also possible that KATP channel expression is promoted by the state of oxidative stress that is evident in the renal cortex even at this early stage of IDDM (50). Although the precise underlying mechanism remains to be established, our observation that glibenclamide restored afferent arteriolar diameter in STZ rats to a value not different from that observed in Sham rats supports the contention that opening of KATP channels may be a primary factor contributing to the afferent arteriolar dilation that underlies diabetic hyperfiltration. However, we note that a recent study failed to discern any influence of the putative KATP blocker U37883A on renal hemodynamics and glomerular filtration rate in anesthetized rats with STZ-induced IDDM (41). Because of the potential indirect (systemic) influences on renal function in clearance studies performed in anesthetized rats, the problems inherent with in vitro studies of arteriolar function, and uncertainties about the efficacy and specificity of the pharmacological agents employed in these studies, further investigation is required to more fully evaluate the role of afferent arteriolar KATP channels in engendering diabetic hyperfiltration.
One final observation deserves comment, although it is somewhat tangential to the original aims of the study. The enormous dilation evoked by pinacidil in afferent arterioles from STZ rats refutes our previous suggestion (offered on the basis of diminished responses to supramaximal concentrations of SNP) that afferent arterioles are nearly maximally dilated during diabetic hyperfiltration (30). The magnitude of the dilator response to pinacidil observed in STZ kidneys is substantially greater than afferent arteriolar responses to any dilator stimulus that we have imposed on in vitro blood-perfused juxtamedullary nephrons. We cannot yet distinguish between the possible involvement of specific pathophysiological alterations in electromechanical coupling events or the role of arteriolar hypertrophy in this phenomenon, although we note that renal hypertrophy is already evident at this stage in our IDDM model.
In summary, experiments were performed to test the postulate that the impact of KATP channels on renal afferent arteriolar tone is exaggerated during the hyperfiltration stage of IDDM. Glibenclamide did not alter afferent arteriolar diameter in normal (Sham) rats, although potassium channel openers (pinacidil and PCO-400) evoked afferent arteriolar dilation, supporting the contention that these vessels are endowed with KATP channels that are normally quiescent. In contrast, 2-wk after induction of IDDM, glibenclamide reduced afferent arteriolar diameter (to values similar to those observed in normal rats), suggesting that open KATP channels exert an arteriolar dilator influence under these conditions. Moreover, the dilator responses to pinacidil and PCO-400 were exaggerated in kidneys from diabetic rats, consistent with an increased functional expression of KATP channels and/or an enhanced impact of these channels on membrane potential and, hence, arteriolar tone. We conclude that the early stage of IDDM in the rat is associated with the apparent emergence of a KATP-dependent vasodilator influence on renal afferent arteriolar tone, the consequences of which can be expected to contribute to diabetic hyperfiltration.
Acknowledgments
We thank Dr. Steven C. Sansom for helpful suggestions concerning the manuscript. This work was supported by grants from the Juvenile Diabetes Foundation International (194113) and the National Institutes of Health (DK39202). The Nebraska Affiliate of the American Heart Association provided fellowship support for H. Ikenaga (post-doctoral) and J. P. Bast (pre-doctoral).
References
- 1.Hostetter TH, Troy JL, Brenner BM. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 1981;19:410–415. doi: 10.1038/ki.1981.33. [DOI] [PubMed] [Google Scholar]
- 2.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986;77:1925–1930. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 4.Anderson S, Brenner BM. Pathogenesis of diabetic glomerulopathy: Hemodynamic considerations. Diabetes/Metab Rev. 1988;4:163–177. doi: 10.1002/dmr.5610040206. [DOI] [PubMed] [Google Scholar]
- 5.Bank N. Mechanisms of diabetic hyperfiltration. Kidney Int. 1991;40:792–807. doi: 10.1038/ki.1991.277. [DOI] [PubMed] [Google Scholar]
- 6.Bank N, Lahorra MA, Aynedjian HS. Acute effect of calcium and insulin on hyperfiltration of early diabetes. Am J Physiol. 1987;252:E13–E20. doi: 10.1152/ajpendo.1987.252.1.E13. [DOI] [PubMed] [Google Scholar]
- 7.Carmines PK, Ohishi K, Ikenaga H. Functional impairment of renal afferent arteriolar voltage-gated calcium channels in rats with diabetes mellitus. J Clin Invest. 1996;98:2564–2571. doi: 10.1172/JCI119075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casellas D, Carmines PK. Control of the renal microvasculature: cellular and integrative perspectives. Current Opin Nephrol Hyperten. 1996;5:57–63. doi: 10.1097/00041552-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 10.Babenko AP, Aguilar-Bryan L, Bryan J. A view of SUR/KIR6X, KATP channels. Ann Rev Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- 11.Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes. 1996;45:1439–1445. doi: 10.2337/diab.45.10.1439. [DOI] [PubMed] [Google Scholar]
- 12.Loutzenhiser RD, Parker MJ. Hypoxia inhibits myogenic reactivity of renal afferent arterioles by activating ATP-sensitive K+ channels. Circ Res. 1994;74:861–869. doi: 10.1161/01.res.74.5.861. [DOI] [PubMed] [Google Scholar]
- 13.Reslerova M, Loutzenhiser R. Renal microvascular actions of calcitonin gene-related peptide. Am J Physiol Renal Physiol. 1998;274:F1078–F1085. doi: 10.1152/ajprenal.1998.274.6.F1078. [DOI] [PubMed] [Google Scholar]
- 14.Reslerova M, Loutzenhiser R. Divergent mechanisms of ATP-sensitive K+ channel-induced vasodilation in renal afferent and efferent arterioles: Evidence of L-type Ca2+ channel-dependent and -independent actions of pinacidil. Circ Res. 1995;77:1114–1120. doi: 10.1161/01.res.77.6.1114. [DOI] [PubMed] [Google Scholar]
- 15.Lorenz JN, Schnermann J, Brosius FC, Briggs JP, Furspan PB. Intracellular ATP can regulate afferent arteriolar tone via ATP-sensitive K+ channels in the rabbit. J Clin Invest. 1992;90:733–740. doi: 10.1172/JCI115945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus RG, England R, Nguyen K, Charron MJ, Briggs JP, Brosius FC., III Altered renal expression of the insulin-responsive glucose transporter GLUT4 in experimental diabetes mellitus. Am J Physiol. 1994;267:F816–F824. doi: 10.1152/ajprenal.1994.267.5.F816. [DOI] [PubMed] [Google Scholar]
- 17.Glocker S, Quast U. Binding and effects of P1075, an opener of ATP-sensitive K+ channels, in the aorta from streptozotocin-treated diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:210–215. doi: 10.1007/pl00005043. [DOI] [PubMed] [Google Scholar]
- 18.Kersten JR, Brooks LA, Dellsperger KC. Impaired microvascular response to graded coronary occlusion in diabetic and hyperglycemic dogs. Am J Physiol. 1995;268:H1667–H1674. doi: 10.1152/ajpheart.1995.268.4.H1667. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]
- 20.Cameron NE, Cotter MA. Impaired contraction and relaxation in aorta from streptozotocin-diabetic rats: role of polyol pathway. Diabetologia. 1992;35:1011–1019. doi: 10.1007/BF02221675. [DOI] [PubMed] [Google Scholar]
- 21.Mayhan WG, Faraci FM. Responses of cerebral arterioles in diabetic rats to activation of ATP-sensitive potassium channels. Am J Physiol. 1993;265:H152–H157. doi: 10.1152/ajpheart.1993.265.1.H152. [DOI] [PubMed] [Google Scholar]
- 22.Casellas D, Navar LG. In vitro perfusion of juxtamedullary nephrons in rats. Am J Physiol. 1984;246:F349–F358. doi: 10.1152/ajprenal.1984.246.3.F349. [DOI] [PubMed] [Google Scholar]
- 23.Carmines PK, Navar LG. Disparate effects of Ca channel blockade on afferent and efferent arteriolar responses to ANG II. Am J Physiol. 1989;256:F1015–F1020. doi: 10.1152/ajprenal.1989.256.6.F1015. [DOI] [PubMed] [Google Scholar]
- 24.Selen G, Persson AEG. Hydrostatic and oncotic pressures in the interstitium of dehydrated and volume expanded rats. Acta Physiol Scand. 1983;117:75–81. doi: 10.1111/j.1748-1716.1983.tb07180.x. [DOI] [PubMed] [Google Scholar]
- 25.Ohishi K, Carmines PK, Inscho EW, Navar LG. EDRF-angiotensin II interactions in rat juxtamedullary afferent and efferent arterioles. Am J Physiol. 1992;263:F900–F906. doi: 10.1152/ajprenal.1992.263.5.F900. [DOI] [PubMed] [Google Scholar]
- 26.Harrison-Bernard LM, Carmines PK. Juxtamedullary microvascular responses to arginine vasopressin in rat kidney. Am J Physiol. 1994;267:F249–F256. doi: 10.1152/ajprenal.1994.267.2.F249. [DOI] [PubMed] [Google Scholar]
- 27.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 28.Soria B. Potential role of the pharmacology of K+ channels in therapeutics. In: Soria B, Ceña V, editors. Ion Channel Pharmacology. New York; Oxford Univ. Press: 1998. pp. 229–245. [Google Scholar]
- 29.Small RC, Berry JL, Foster RW, Blarer S, Quast U. Analysis of the relaxant action of SDZ PCO 400 in airway smooth muscle from the ox and guinea-pig. Eur J Pharmacol. 1992;219:81–88. doi: 10.1016/0014-2999(92)90583-p. [DOI] [PubMed] [Google Scholar]
- 30.Ohishi K, Okwueze MI, Vari RC, Carmines PK. Juxtamedullary microvascular dysfunction during the hyperfiltration stage of diabetes mellitus. Am J Physiol. 1994;267:F99–F105. doi: 10.1152/ajprenal.1994.267.1.F99. [DOI] [PubMed] [Google Scholar]
- 31.Ohishi K, Carmines PK. Superoxide dismutase restores the influence of nitric oxide on renal arterioles in diabetes mellitus. J Am Soc Nephrol. 1995;5:1559–1566. doi: 10.1681/ASN.V581559. [DOI] [PubMed] [Google Scholar]
- 32.Schoonmaker GC, Fallet RW, Carmines PK. Superoxide anion curbs nitric oxide modulation of afferent arteriolar ANG II responsiveness in diabetes mellitus. Am J Physiol. 2000;278:F###–F###. doi: 10.1152/ajprenal.2000.278.2.F302. In Press. [DOI] [PubMed] [Google Scholar]
- 33.Dunne MJ. Properties of the ATP-regulated K+ channel in pancreatic b-cells. In: Soria B, Ceña V, editors. Ion Channel Pharmacology. New York; Oxford Univ. Press: 1998. pp. 208–228. [Google Scholar]
- 34.Panten U, Burgfeld J, Goerke F, Rennicke M, Schwanstecher M, Wallasch A, Zuenkler BJ, Lenzen S. Control of insulin secretion by sulfonylureas, meglitinide and diazoxide in relation to their binding to sulfonylurea receptor in pancreatic islets. Biochem Pharmacol. 1989;38:1217–1229. doi: 10.1016/0006-2952(89)90327-4. [DOI] [PubMed] [Google Scholar]
- 35.Pearson JG. Pharmacokinetics of glyburide. Am J Med. 1985;79:67–71. doi: 10.1016/s0002-9343(85)80010-3. [DOI] [PubMed] [Google Scholar]
- 36.Eilertsen E, Hart JW, Magnussen MP, Sorensen H, Arrigoni-Martelli E. Pharmacokinetics and distribution of the new antihypertensive agent pinacidil in rat, dog and man. Xenobiotica. 1982;12:177–185. doi: 10.3109/00498258209046792. [DOI] [PubMed] [Google Scholar]
- 37.Meisheri KD, Khan SA, Martin JL. Vascular pharmacology of ATP-sensitive K+ channels: interactions between glyburide and K+ channel openers. J Vasc Res. 1993;30:2–12. doi: 10.1159/000158969. [DOI] [PubMed] [Google Scholar]
- 38.Ashcroft FM. Adenosine-triphosphate-sensitive potassium channels. Annu Rev Neuroscience. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- 39.Gardiner SM, Kemp PA, March JE, Fallgren B, Bennett T. Effects of glibenclamide on regional haemodynamic actions of alpha-trinositol and its influence on responses to vasodilators in conscious rats. Br J Pharmacol. 1996;117:507–515. doi: 10.1111/j.1476-5381.1996.tb15219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mimuro T, Kawata T, Onuki T, Hashimoto S, Tsuchiya K, Nihei H, Koike T. The attenuated effect of ATP-sensitive K+ channel opener pinacidil on renal haemodynamics in spontaneously hypertensive rats. European J Pharmacol. 1998;358:153–160. doi: 10.1016/s0014-2999(98)00573-1. [DOI] [PubMed] [Google Scholar]
- 41.Vallon V, Albinus M, Blach D. Effect of KATP channel blocker U37883A on renal function in experimental diabetes mellitus in rats. J Pharmacol Exp Ther. 1998;286:1215–1221. [PubMed] [Google Scholar]
- 42.Bailey MA, Walter SJ. Renal effects of glibenclamide: a micropuncture study. J Pharmacol Exp Ther. 1998;285:464–467. [PubMed] [Google Scholar]
- 43.Sadowski J, Kompanowska-Jezierska E, Dobrowolski L, Walkowska A, Badzynska B. Simultaneous recording of tissue content and blood flow in rat renal medulla: evidence on interdependence. Am J Physiol. 1997;273:F658–F662. doi: 10.1152/ajprenal.1997.273.4.F658. [DOI] [PubMed] [Google Scholar]
- 44.Parekh N, Zou AP. Role of prostaglandins in renal medullary circulation: Response to different vasoconstrictors. Am J Physiol. 1996;271:F653–F658. doi: 10.1152/ajprenal.1996.271.3.F653. [DOI] [PubMed] [Google Scholar]
- 45.Mieyal P, Fulton D, McGiff JC, Quilley J. NO-independent vasodilation to acetylcholine in the rat isolated kidney utilizes a charybdotoxin-sensitive, intermediate-conductance Ca++-activated K+ channel. J Pharmacol Exp Ther. 1998;285:659–664. [PubMed] [Google Scholar]
- 46.Ludens JH, Clark MA, Smith MP, Humphrey SJ. Renal and vascular effects of chemically distinct ATP-sensitive K+ channel blockers in rats. J Cardiovasc Pharmacol. 1995;25:404–409. doi: 10.1097/00005344-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Iwamoto T, Nishimura N, Moriya T, Sukamoto T. Differential vasorelaxant effects of K+-channel openers and Ca2+-channel blockers on canine isolated arteries. J Pharm Pharmacol. 1993;45:292–297. doi: 10.1111/j.2042-7158.1993.tb05555.x. [DOI] [PubMed] [Google Scholar]
- 48.Kawata T, Mimuro T, Onuki T, Tsuchiya K, Nihei H, Koike T. The KATP channel opener nicorandil: Effect on renal hemodynamics in spontaneously hypertensive and Wistar Kyoto rats. Kidney Int. 1998;54:S231–S233. doi: 10.1046/j.1523-1755.1998.06758.x. [DOI] [PubMed] [Google Scholar]
- 49.Lynch RM, Paul RJ. Glucose uptake in porcine carotid artery: relation to alterations in active Na+-K+ transport. Am J Physiol. 1984;247:C433–C440. doi: 10.1152/ajpcell.1984.247.5.C433. [DOI] [PubMed] [Google Scholar]
- 50.Carmines PK, Pollock JS, Ishii N, Patel KP, Lane PH, Bian K, Murad F. Tyrosine nitration accompanies increased nitric oxide and superoxide production in renal cortex during the hyperfiltration stage of diabetes mellitus. [Abstract] J Am Soc Nephrol. 1999;10:393A. [Google Scholar]


