Abstract
Purpose
To determine whether the tubulin-binding drug noscapine could enhance radiosensitivity of GL261 glioma tumors by inhibiting tumor angiogenesis.
Methods and Materials
The human T98G and murine GL261 glioma cell lines treated with noscapine, radiation, or both were assayed for clonogenic survival. Mice with established GL261 hind limb tumors were treated with noscapine, radiation or both to evaluate the effect of noscapine on radioresponse. In a separate experiment with the same treatment groups, 7 days after radiation tumors were resected and immunostained to measure proliferation rate, apoptosis and angiogenic activity.
Results
Noscapine reduced clonogenic survival without enhancement of radiosensitivity in vitro. Noscapine combined with radiation significantly increased tumor growth delay: 5, 8, 13, 18 days for control, noscapine alone, radiation alone, and the combination treatment, respectively (p < 0.001). To assess the effect of the combination of noscapine plus radiation on the tumor vasculature, tubule formation by the murine endothelial 2H11 cells was tested. Noscapine with radiation significantly inhibited tubule formation compared with radiation alone. By immunohistochemistry, tumors treated with the combination of noscapine plus radiation showed a decrease in BrdU incorporation, an increase in apoptosis by TUNEL and a decrease in tumor vessel density compared with tumors treated with radiation alone.
Conclusion
Noscapine enhanced the sensitivity of GL261 glioma tumors to radiation resulting in a significant tumor growth delay. These findings are clinically relevant, particularly in view of the mild toxicity profile of this drug.
Keywords: Noscapine, glioma, GL261 mouse model, angiogenesis, radioresponse
Introduction
It is now well established that solid tumors need a functional vasculature in order to grow beyond a few millimeters in size (1). Tumors remain dormant in the absence of angiogenic stimulation, but once angiogenesis becomes initiated, tumors grow rapidly. Vascular endothelial growth factor (VEGF) is thought to be a critical angiogenic factor for endothelial cell proliferation and blood vessel formation. Treatment with bevacizumab, a monoclonal antibody against VEGF, has resulted in improved survival in colorectal cancer patients when combined with other chemotherapies and in glioblastoma patients when combined with camptothecin-11 (2, 3). As early as 1995, Teicher et al., showed that antiangiogenic agents combined with radiotherapy improved tumor oxygenation and increased treatment efficacy by killing both cancer and endothelial cells (4, 5).
Other antiangiogenic agents like angiostatin (6, 7), anti-VEGF antibody (8), receptor tyrosine kinase inhibitors (9-12), endostatin (13), and VEGF Trap (14) have been demonstrated to enhance radiotherapy's effects (reviewed 15, 16).
In addition to the established main mechanism of action of stabilizing microtubules, (17) taxanes also have an antiangiogenic effect. Taxanes have the ability to target endothelial cell proliferation, migration and differentiation into capillary-like tubes, all processes required for new blood vessel formation to supply a growing tumor (18-20). However, most tubulin binding agents tested to date in the clinic are associated with the risk of neurotoxicity, due to the high tubulin content of neuronal tissues. Other common side-effects include hypersensitivity reactions, cardiotoxicity, myelosuppression, alopecia and gastrointestinal toxicity.
Efforts to develop novel tubulin binding agents with improved toxicity profiles have resulted in a novel microtubule binding agent, noscapine, which has shown promise in this regard in both animal and human studies (21-23). Recently, we showed noscapine has antiangiogenic activity similar to taxanes (24). Noscapine downregulated hypoxia-mediated HIF-1α expression in human glioma cells, concomitantly with reduced secretion of the potent angiogenic cytokine, VEGF (24). In addition, noscapine inhibited tubule formation by human umbilical vein endothelial cells (HUVECs) in a dose-dependent manner. Based on these observations, and given its unique low toxicity profile, we hypothesized that noscapine might be a promising drug to combine with ionizing radiation. To test this hypothesis, we studied the effects of noscapine and radiation in vitro and in the GL261 glioma experimental tumor model.
Methods and Materials
Cell lines and reagents
The human glioma T98G, the murine glioma GL261 and the murine endothelial 2H11 cell lines were cultured in 5% CO2 and 95% air at 37°C in Dulbecco's Modified Eagles Medium (DMEM) (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA), 1% penicillin and streptomycin and 1% L-glutamine. Noscapine hydrochloride and Staurosporine (Sigma-Aldrich, Saint Louis, MO) were dissolved in DMSO and stock solutions (100 mM, 1 mM, respectively) were stored at -80°C.
Clonogenic and apoptosis assays
Glioma cells (5 × 105) were plated one day prior to the addition of different concentrations of noscapine (0-75 μM). After 24 h of drug exposure, untreated and drug-treated cultures were irradiated with a 60Co source at different single doses (0, 2.5, 5.0 and 7.5 Gy), at room temperature with drug present in the media, harvested and plated in duplicate in 6-well plates at cell concentrations estimated to yield 20-100 colonies/well. The 2H11 endothelial cells (10 × 105) were plated one day prior to the addition of different concentrations of noscapine (0-150 μM) for 6 h, harvested and were plated in duplicate in 6-well plates or stained with Annexin V to determine numbers of apoptotic cells. In some experiments 2H11 cells were irradiated with a single dose of radiation (3 Gy) at room temperature with drug (100 μM) present in the medium. After 11 days, colonies were fixed in 4% paraformaldehyde, stained with 0.5% crystal violet and colonies counted. Clonogenic survival curves were generated by combining data from at least two independent experiments and fitting the average survival levels by least-squares regression using the linear-quadratic model (25).
To monitor apoptosis, the Annexin V apoptosis detection kit (BD Biosciences, San Jose, CA) was used following the manufacturer's recommendations. Flow cytometry was performed on a Becton Dickinson FACScan (San Jose, CA).
In vitro tubule formation assay
Matrigel (150 μl) (BD Biosciences) was added to the wells of a 48-well plate and solidified at 37°C for 30 min. The murine 2H11 cells (2 × 104) were added in 200 μl of medium and incubated for 6 h with or without different doses of noscapine (0-150 μM). In other experiments the 2H11 endothelial cells were untreated, treated with a single dose of radiation (3 Gy), a single dose of noscapine (100 μM) or the combination. After 6 h following treatment, 2H11 cells were seeded on Matrigel-coated wells in the absence of noscapine for an additional 6 h to allow tubule formation. The extent of tubule formation was quantified as described (24). The assay was run in duplicates and repeated two times with similar results. Data are expressed as the number of branched points per well under each culture condition (mean ± SD).
In vivo studies
Female 6-8-week-old C57BL/6 mice were obtained from Taconic (Germantown, NY) and maintained in facilities approved by the American Association for Accreditation of Laboratory Animal Care, under an approved protocol by the Institutional Animal Care and Use Committee.
Mice were injected subcutaneously in the hind limb with 2.0 × 106 GL261 cells. When tumors reached 7-8 mm in diameter, mice were randomly assigned to groups (N=5) to receive sham treatment, noscapine (150 mg/kg by gavage once daily), a single fraction of radiation (25 Gy), or the combination. Radiation was delivered to mice anesthetized and positioned on a dedicated plexiglass tray by a 60Co source (Theratron 780-C, AECL Medical, Canada) with a dose rate of 81.15 cGy/min. Tumors were treated with a 5 × 5-cm field at a source-skin distance (SSD) of 80 cm with remainder of the body shielded with a lead block. Noscapine treatment was initiated 3 days prior to radiation to allow for an effective plasma concentration before the delivery of radiation (26). Tumor growth was measured twice weekly using vernier calipers, and tumor volume was calculated using the formula (length × width2)/2. Mice were sacrificed when tumors reached 2000 mm3. The experiment was repeated twice, with similar results. In a third experiment, mice from each treatment group were sacrificed at day 7 after radiation (3-4 mice per group). Tumors were embedded in OCT (Miles Laboratories, Pittsburgh, PA) and kept at -80°C until use.
Immunohistochemistry
Frozen tumors were sectioned (8 μm), mounted onto slides and kept at -20°C until use. To detect BrdU positive tumor cells, 2 h prior to sacrifice the mice were injected intraperitoneally with 0.01 ml/gm BrdU (Zymed, San Francisco, CA). Sections were fixed with cold acetone for 10 min at -20°C, air dried for 15 min, hydrated for 10 min in PBS and permeabilized in 1% Triton-X-100 for 8 min at room temperature. DNA was denatured with 2N HCl at 37°C for 30 min. Slides were washed in 0.1 M Tris-HCl (pH 7.5) for 5 min, blocked in blocking buffer (5% normal goat serum, 1% BSA, 0.1% Triton-X-100, 0.05% Tween-20 in PBS) for 30 min at room temperature and incubated for 18 h at 4°C with a 1:50 dilution of monoclonal FITC-anti-BrdU (BD Pharmingen, Franklin Lakes, NJ), washed, counterstained with propidium iodide (PI, 20 μg/ml) for 10 min and mounted with Vectashield mounting medium. The number of BrdU positive tumor cells were counted in 5 random fields of high cellular density and without necrosis at X200 magnification on two independent sections per tumor. The data is presented as the percent of BrdU positive cells relative to the total number of PI positive cells per field.
The terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay was performed using an apoptosis detection kit (Roche, Indianapolis, IN). The number of TUNEL positive tumor cells were counted in 5 random fields of high cellular density and without necrosis at X200 magnification on two independent sections per tumor. The data is presented as the average number of TUNEL positive cells per field.
Tumor vascularity was evaluated with CD31 immunostaining using sections prepared as described above. Slides were incubated for 18 h at 4°C with a 1:200 dilution antibody (BD Pharmingen) in 2% BSA in PBS, washed in PBS and incubated for 60 min at room temperature with a 1:100 dilution of Texas Red-conjugated donkey anti-rat secondary antibody, protected from light. Sections were washed and mounted as described above. Individual CD31 positive vessels were counted in 10 random fields at X100 magnification in the most vascularized portions of the tumors on two independent sections per tumor. The data is presented as the average number of CD31 positive vessels per field.
Statistics
The survival fractions in the clonogenic assay were calculated with a linear quadratic model for the log (Survival Fraction) as a function of dose as described (25). For tumor growth delay, the mean tumor volumes and their standard errors for each treatment were compared using a two-sided Student's t test for multiple comparison with Bonferroni correction. The combined effect of noscapine and radiation versus radiation alone on proliferation, apoptosis and tumor vessel density was determined by two-sided Student's t test. All values are given as mean ± standard deviation (SD). Statistical significance was indicated by values of p < 0.05. Analyses were conducted using Prism 4 (GraphPad Software, San Diego, CA).
Results
In vitro effect of noscapine and radiation
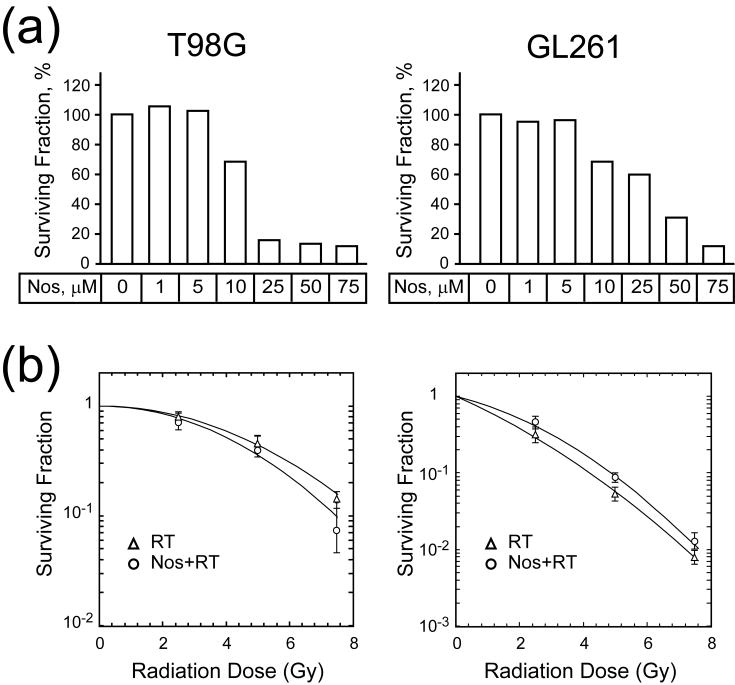
The human glioma T98G and murine glioma GL261 cell lines were exposed to increasing doses of noscapine (0-75 μM) for 24 h and assessed for growth potential in the clonogenic assay (Fig. 1a). For the human glioma T98G cell line, the surviving fraction of noscapine-treated cells was 68% at 10 μM but dropped to less than 15% at doses above 25 μM. For the murine glioma GL261 cell line, the surviving fraction of noscapine-treated cells was 60% at 25 μM, but dropped to less than 40% at 50 μM dose. Based on this data, the doses of noscapine selected for the combination treatment with radiation were 10 μM for T98G and 50 μM for GL261 cell lines, respectively.
Fig. 1. In vitro effect of noscapine and radiation.
(a), Survival fraction of T98G and GL261 cell lines at escalating doses of noscapine. (b), Survival curve for T98G and GL261 cell lines after radiation (single dose = 2.5, 5.0 and 7.5 Gy) or radiation and noscapine (10 μM for T98G and 50 μM for GL261).
An additional clonogenic survival assay was performed to examine the effect of noscapine on radiation response of the glioma cell lines. Cells were treated with a constant dose of noscapine (10 μM for T98G and 50 μM for GL261) for 24 h and irradiated with increasing doses of radiation (0, 2.5, 5.0, 7.5 Gy) (Fig. 1b). Radiation alone caused a dose-dependent decrease in cell survival in both T98G and GL261 cell lines. Adding noscapine had no effects on survival of either T98G or GL261 cell lines.
In vivo effect of noscapine: enhancement of radioresponse
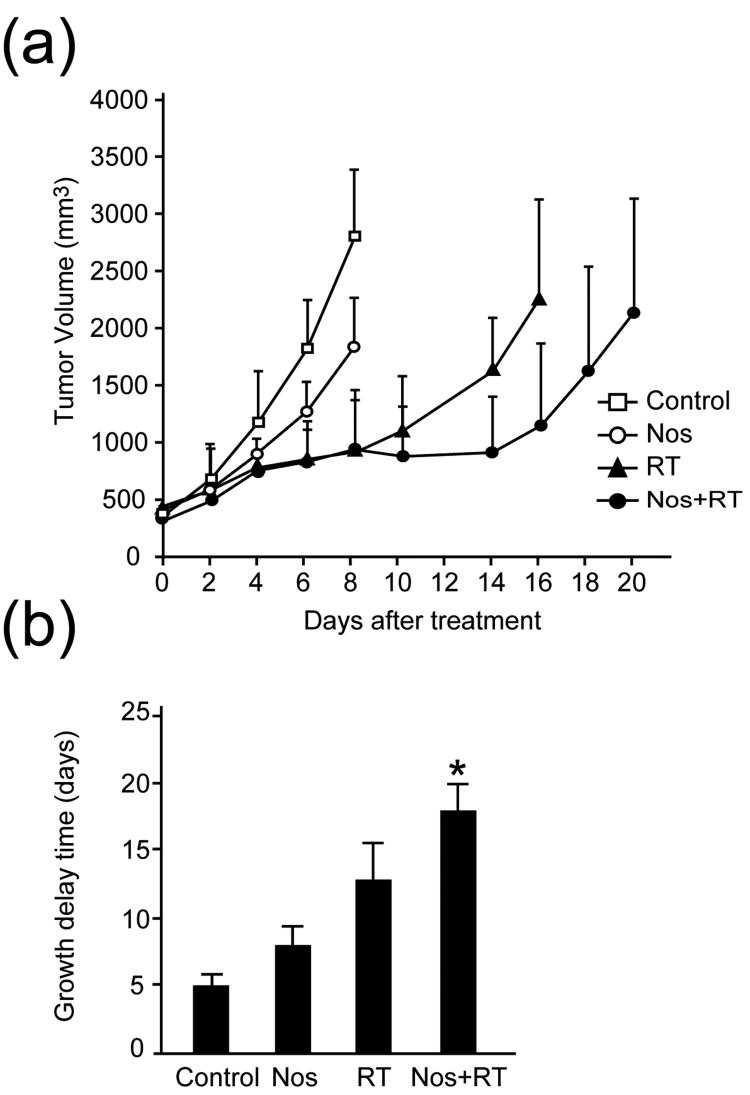
To determine whether noscapine enhances the response of GL261 tumors to radiation, mice with established hind limb tumors of 7-8 mm-diameter in size were randomly assigned to four treatment groups: 1) sham treatment; 2) noscapine (150 mg/kg by gavage once daily); 3) a single fraction of radiation (25 Gy); 4) the combination of radiation and noscapine. The experiment was repeated twice with similar results: the combined results are shown in Fig. 2. There was a difference in the growth rate of GL261 tumors between control and noscapine treatment groups, with tumors showing a mean volume of 2788 ± 587 mm3 in controls and 1818 ± 425 mm3 in noscapine-treated mice, on day 8. There was no significant difference between radiation treatment alone and the combined treatment groups, with tumors showing a mean volume of 889 ± 458 mm3 and 913 ± 362 mm3, respectively, on day 8. Using a two-sided t-test for multiple comparison with Bonferroni correction, on day 8 there was a statistically significant difference between the control group and either the radiation alone or combined treatment group (p < 0.05). The difference between the control group and the noscapine treatment group was also significant (p < 0.05).
Fig. 2. In vivo effect of noscapine: enhancement of radioresponse.
(a), Tumor growth delay assay. (b), Time in days for tumors to grow four-fold. Tumor volumes are shown for each treatment group at the indicated time points. *A statistically significant difference was found between the control and noscapine treated groups (p < 0.001).
To further analyze the antitumor effects of the different treatments, the time in days for tumors to grow to four-fold their initial volume was determined (Fig. 2b). This time was 5.1 ± 0.6 days for the control, 8.0 ± 1.4 days for the noscapine, 12.7 ± 2.8 days for the radiation, and 18.0 ± 2.1 days for the noscapine plus radiation group. Using the Kruskal-Wallis test, there was a statistically significant difference among the four groups (p <0.001), with all pairwise comparisons significantly different. The difference in size between the control group and the group treated with radiation and noscapine was also statistically significant (p <0.05).
Noscapine with radiation treatment decreases proliferation and increases the apoptotic index in vivo
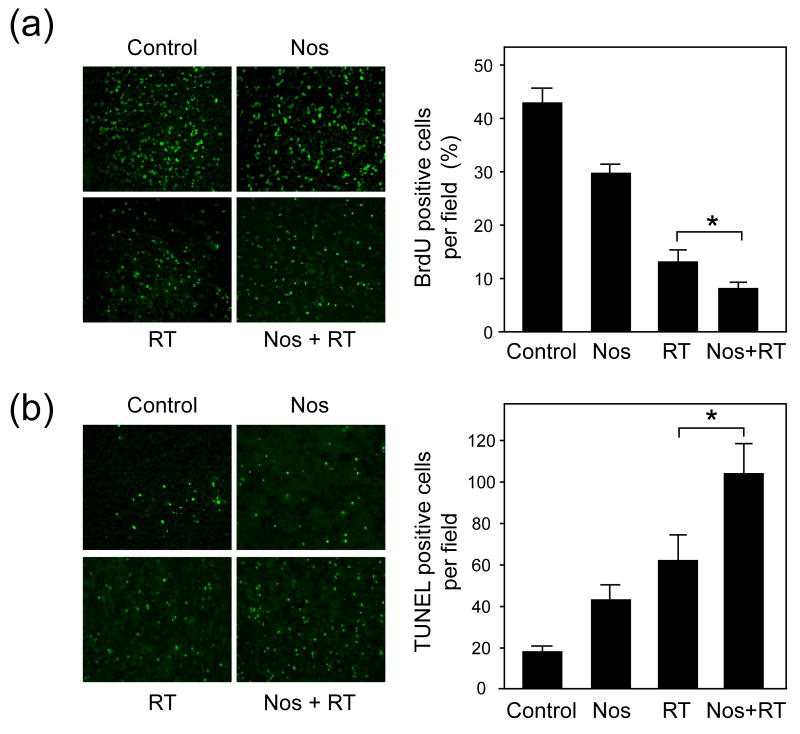
To determine whether the tumor growth delay from the combined treatment results from decreased tumor cell proliferation and/or increased apoptosis, we used BrdU and TUNEL staining on tissue sections. In a separate experiment, mice were treated similarly to those in the tumor growth delay study (Fig. 2a), and 7 days after radiation tumors were resected and frozen for immunohistochemistry studies. Representative images of tumor tissue sections from each treatment group are shown for BrdU labeling (Fig. 3a) and TUNEL staining (Fig. 3b). Tumor cell proliferation was lowest in the combination treatment group. The average percentage of BrdU positive cells was 43.1 ± 2.5 for the control group, 29.4 ± 2.1 for the noscapine group, 12.9 ± 2.3 for the radiation group, and 7.8 ± 1.4 for the noscapine plus radiation group. Radiation treatment alone resulted in a 3.3-fold decrease in the number of proliferating cells compared with the combined treatment group that showed a 5.6-fold reduction (p < 0.005).
Fig. 3. Effect of noscapine and radiation on tumor cell proliferation and apoptosis.
(a), Left panel: Representative images of BrdU staining from each group (X200). Right panel: Percentage of BrdU positive cells per microscopic. Columns and error bars: mean ± SD. *A statistically significant difference was found between radiation treatment alone and the combined treatment group (p < 0.005). (b), Left panel: Representative images of TUNEL staining from each group (X200). Average number of TUNEL positive cells per microscopic field. Columns and error bars: mean ± SD. *A statistically significant difference was found between radiation treatment alone and the combined treatment group (p < 0.04).
TUNEL staining was highest in the combination treatment group. The average number of TUNEL positive cells per field was 16.2 ± 2.4 for the control group, 41.0 ± 7.4 for the noscapine group, 60.7 ± 12.6 for the radiation group, and 103.3 ± 14.9 for the noscapine plus radiation group. Radiation treatment alone resulted in a 3.7-fold increase in the number of apoptotic cells compared with the combined treatment group that showed a 6.4-fold increase (p < 0.04).
Noscapine alone and combined with radiation inhibits blood vessel formation in vitro and in vivo
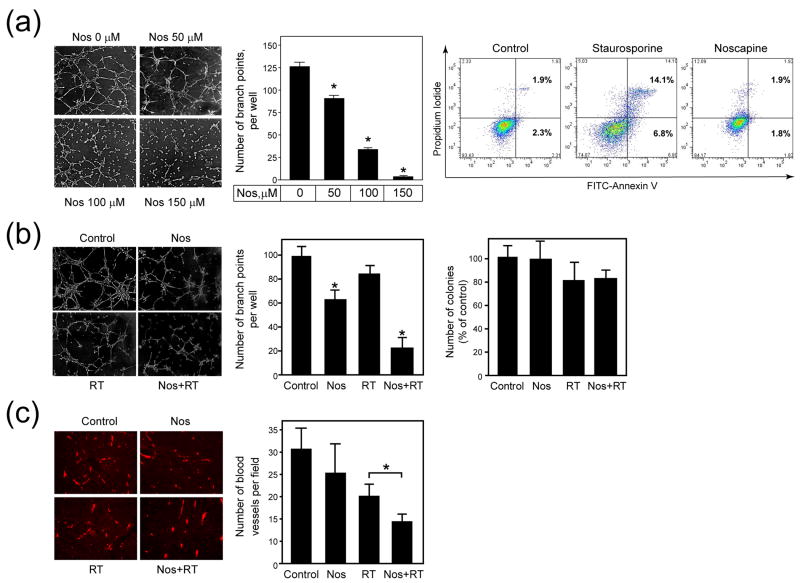
The 2H11 endothelial cells were seeded on Matrigel-coated wells with different doses of noscapine (0-150 μM). The results of one representative tubule formation assay together with quantitative data pooled from three independent experiments are shown (Fig. 4a). In the presence of noscapine, tubule formation was inhibited in a dose-dependent manner, with a 28% reduction observed at 50 μM, 73% at 100 μM, and 97% at 150 μM, respectively. Noscapine treatment of 2H11 endothelial cells significantly reduced the number of branched tubules at all drug doses compared with untreated cultures. Using analysis of variance test, there was a statistically significant difference among the four groups (p <0.0001).
Fig. 4. Effect of noscapine alone and combined with radiation on blood vessel formation in vitro and in vivo.
(a), Left panel: Representative images of tubule formation at 6 h (X40). Middle panel: Number of branch points per well. Columns and error bars: mean ± SD. Right panel: Representative histograms for cells analyzed by flow cytometry for the number of Annexin V positive cells. *A statistically significant difference was found between the control and noscapine treated groups (p < 0.0001). (b), Left panel: Representative images of tubule formation at 6 h (X40). Middle panel: Number of branch points per well. Columns and error bars: mean ± SD. Number of branch points per well (middle). Columns and error bars: mean ± SD. Right panel: Number of colonies in the clonogenic survival assay per treatment group as percent of control. Columns and error bars: mean ± SD. *A statistically significant difference was found between the control and noscapine treated groups (p < 0.001). (c), Left panel: Representative images of sections stained for CD31 (X100). Right panel: Number of blood vessels per field. Columns and error bars: mean ± SD. *A statistically significant difference was found between radiation treatment alone and the combined treatment group (p < 0.001).
To determine whether noscapine inhibition of tubule formation was associated with induction of apoptosis or inhibition of cell proliferation, 2H11 cells were incubated for 6 h with different doses of noscapine (1-150 μM). The drug staurosporine (400 nM), was used as a positive control for induction of apoptosis. After 6 h of treatment the cells were harvested and stained with Annexin V/PI and analyzed by flow cytometry to detect apoptotic cells. Aliquots from the cell same suspension were plated in a clonogenic survival assay. Staurosporine treatment for 6 h increased the number of Annexin V positive cells to 4.7% compared with 1.1% in untreated control cultures. Noscapine treatment of 2H11 cells for 6 h, even at the highest concentration of 150 μM, did not increase the number of Annexin V positive cells compared to the control (Fig. 4a). Similar results were obtained in the clonogenic survival assay (data not shown). Whereas staurosporine treatment decreased colony formation, none of noscapine concentrations (50, 100, 150 μM) significantly decreased the number of colonies compared with the control.
To determine whether noscapine sensitizes 2H11 cells to radiation treatment the 2H11 endothelial cells were untreated, treated with a single dose of radiation (3 Gy), a single dose of noscapine (100 μM) or the combination. After 6 h following treatment, 2H11 cells were seeded on Matrigel-coated wells in the absence of noscapine. The results of one representative tubule formation assay (Fig. 4b), as well as the quantitative display of the data pooled from two consecutive experiments, are shown. After incubation for 6 h, the cords fuse into continuous tubules to form capillary-like structures. An average of 98.3 ± 9.0 branch points per well was found in the controls, 85.0 ± 6.0 after radiation, 62 ± 7.8 after noscapine, and 21 ± 9.4 after noscapine and radiation. Noscapine treatment and the combination treatment groups had a significant decrease in the number of branch points compared with the control group (p < 0.001). To determine whether the inhibition of tubule formation by 2H11 endothelial cells was due to inhibition of proliferation, aliquots of the same cell suspensions used for the tubule formation assay were plated in a clonogenic survival assay. The noscapine group alone had 98 ± 15.6 colonies, radiation alone had 80.1 ± 16.6 colonies, and the combination of noscapine plus radiation had 82.1 ± 7.6 colonies. Although radiation treatments, alone or in combination with noscapine, decreased the number of colonies by approximately 20% compared with the control, the results were not statistically significant (Fig. 4b).
We next investigated whether noscapine in combination with radiation treatment affected the tumor vasculature in vivo. Mice were treated with noscapine and radiation and 7 days after radiation tumors were resected for immunohistochemistry studies with the CD31 antibody to detect tumor blood vessel density (Fig. 4c). The average number of blood vessels per microscope field was determined for each treatment group. The control tumors had an average of 30.6 ± 5.7 vessels per microscopic field, noscapine alone had 24.5 ± 8.3 vessels, radiation alone had 20.5 ± 2.9 vessels, and the combination of noscapine plus radiation had 13.6 ± 2.3 vessels. Radiation treatment alone achieved a 1.5-fold decrease in the number of vessels compared with the combined treatment that showed a 2.3-fold decrease (p < 0.001).
Discussion
In the current study, we present evidence that noscapine enhanced the response of GL261 tumors to radiation. To our knowledge, this is the first study to explore the combination of noscapine and radiation in an in vivo model.
Noscapine significantly increased tumor growth delay induced by radiation, while having a small effect when used alone (Fig. 2a). When a four-fold increase in tumor volume was used for comparison, noscapine and radiation induced a tumor growth delay of approximately 18 days compared with 13 days for radiation and 8 days for noscapine alone (Fig. 2b). To explore possible mechanisms of tumor growth delay, we analyzed proliferation and apoptosis within the tumors on day 7 after treatment. This time interval was selected to better assess the response of a tissue to radiation which involves several mechanisms including repair of tumor cells from radiation damage, tumor cell repopulation and reoxygenation of hypoxic cells (26, 27). There was a marked decrease in BrdU incorporation in mice that received combination treatment compared with either treatment alone. Similarly, the number of TUNEL positive cells was significantly increased in the combination treatment group (Fig. 3). Taken together, these results suggest that the mechanism of tumor growth delay was associated with both decreased proliferation and increased apoptosis of GL261 tumor cells.
Interestingly, in vitro experiments failed to show a similar enhancement by the combination of noscapine and radiation, suggesting that the effect seen in vivo is mediated by the tumor microenvironment. Our in vitro findings differ from those reported for the rat C6 glioma cell line where noscapine combined with radiation enhanced clonogenic cell death (28).
Based on our previous studies with HUVECs showing noscapine's antiangiogenic activity in vitro, we tested its effects on the tumor vasculature when combined with radiation (24). First we studied the effects on the process of tubule formation in vitro, in murine endothelial 2H11 cells, specifically developed to serve as a model of mouse tumor vasculature for screening antiangiogenic agents (29). The combined treatment resulted in a marked decrease in tubule formation in cultures that received the combination treatment. Similar results were reported with HUVECs treated with another VEGF targeting therapy, the VEGFR2 inhibitor AZD2171 (30).
We further explored this effect by evaluating tumor vessel density using CD31 immunostaining (Fig. 4). Combined therapy significantly decreased tumor vessel density compared with radiation treatment alone. The data presented suggest that tumor growth delay observed with combination treatment is likely due to targeting two compartments, the tumor as well as the tumor vasculature.
As noted more than a decade ago, combining antiangiogenic agents with radiotherapy improved tumor oxygenation and increased antitumor activity (4). This effect, described as “vascular normalization” is associated with reduced size and length of blood vessels, increased permeability and decreased interstitial fluid pressure, resulting in increased chemo- and radiosensitivity (31, 32). Many studies combining VEGF inhibitors and radiation have since confirmed this model (13, 14, 35-39, and reviewed in 16, 33, 34).
Optimal timing of radiotherapy and antiangiogenic agents can improve the response of tumors to radiotherapy (40). Two inhibitors of angiogenesis, anginex and bevacizumab, were tested for their ability to alter tumor oxygenation levels in three different solid tumor models (a human ovarian carcinoma, a murine melanoma and a murine breast carcinoma) and the response to different schedules of radiation was monitored by tumor growth delay (40). Oxygen levels within the tumors increased within the first 4 days following treatment with a rapid decline from day 5 onward. When radiotherapy was administered during this interval, tumor growth delay was significantly enhanced compared with treatment given before or after this critical time interval. Similarly, antiangiogenic agents, such as thalidomide and a VEGFR2 inhibitor, also induced “vascular normalization” within the first few days of treatment (41, 42).
The data presented is clinically relevant since angiogenesis in high grade gliomas is a key event driving tumor progression (43, 44, 45).
In summary, in addition to the classical cytotoxic mechanism of noscapine, its antiangiogenic effect is likely to underlie the enhanced tumor radioresponse observed in the preclinical GL261 glioma model. Noscapine is a natural product, a non-narcotic alkaloid derived from opium, and has traditionally been used as an oral cough suppressant with high tolerability (21). Given its very favorable toxicity profile, oral bioavailability and radiosensitizing properties, noscapine should be tested in clinical trials in combination with radiotherapy.
Acknowledgments
Supported in part by the National Institutes of Health research grant NS057829-02 (E.W.N.), the Long Island League to Abolish Cancer (E.W.N.), Department of Defense Center of Excellence Grant BC030282 (S.C.F.), and by a grant from the Radiological Society of North America Research and Education Foundation (M.A-B.).
Footnotes
Presented at the 49th Annual Meeting of the American Society for Therapeutic Radiology and Oncology (ASTRO), October 28-November 1, 2007, Los Angeles, CA.
Conflict of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brem S, Brem H, Folkman J, et al. Prolonged tumor dormancy by prevention of neovascularization in the vitreous. Cancer Res. 1976;36:2807–12. [PubMed] [Google Scholar]
- 2.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 3.Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma: Rationale and potential role of targeted agents. Oncologist. 2006;11:152–64. doi: 10.1634/theoncologist.11-2-152. [DOI] [PubMed] [Google Scholar]
- 4.Teicher BA, Dupuis N, Kusomoto T, et al. Antiangiogenic agents can increase tumor oxygenation and response to radiation therapy. Rad Oncol Invest. 1995;2:269–76. [Google Scholar]
- 5.Teicher BA. A systems approach to cancer therapy. (Antioncogenics + standard cytotoxics-->mechanism(s) of interaction) Cancer Metastasis Rev. 1996a;15:247–72. doi: 10.1007/BF00437479. [DOI] [PubMed] [Google Scholar]
- 6.Mauceri HJ, Hanna NN, Beckett MA, et al. Combined effect of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287–91. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]
- 7.Gorski DH, Beckett MA, Jaskoowiak NT, et al. Blockade of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–8. [PubMed] [Google Scholar]
- 8.Lee CG, Heijn D, Tomaso E, et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60:5565–70. [PubMed] [Google Scholar]
- 9.Geng L, Donnelly E, McMahon G, et al. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res. 2001;61:2413–9. [PubMed] [Google Scholar]
- 10.Hess C, Vuong V, Hegyi I, et al. Effect of VEGF receptor inhibitor PTK787/ZK222584 combined with ionizing radiation on endothelial cells and tumour growth. Br J Cancer. 2001;85:2010–16. doi: 10.1054/bjoc.2001.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu B, Geng L, Musiek A, et al. Broad spectrum receptor tyrosine kinase inhibitor, SU6668, sensitizes radiation via targeting survival pathway of vascular endothelium. Int J Radiat Oncol Biol Phys. 2004;58:844–50. doi: 10.1016/j.ijrobp.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 12.Schuuring J, Bussink J, Bernsen HJ, et al. Radiation combined with SU5416: Microvascular changes and growth delay in a human xenograft glioblastoma tumor line. Int J Radiat Oncol Biol Phys. 2005;61:529–34. doi: 10.1016/j.ijrobp.2004.09.063. [DOI] [PubMed] [Google Scholar]
- 13.Itasaka S, Komaki R, Herbst RS, et al. Endostatin improves radioresponse and blocks tumor revascularization after radiation therapy for A431 xenografts in mice. Int J Radiat Oncol Biol Phys. 2007;67:870–8. doi: 10.1016/j.ijrobp.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wachsberger PR, Burd R, Cardi C, et al. VEGF trap in combination with radiotherapy improves tumor control in U87 glioblastoma. Int J Radiat Oncol Biol Phys. 2007;67:1526–37. doi: 10.1016/j.ijrobp.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Kim DW, Huamani J, Fu A, et al. Molecular strategies targeting the host component of cancer to enhance tumor response to radiation therapy. Int J Radiat Oncol Biol Phys. 2006;64:38–46. doi: 10.1016/j.ijrobp.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Nider C, Wiedenmann N, Andratschke N, et al. Current status of angiogenesis inhibitors combined with radiation therapy. Cancer Treat Rev. 2006;32:348–64. doi: 10.1016/j.ctrv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 18.Belotti D, Vergani V, Drudis T, et al. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res. 1996;2:1843–9. [PubMed] [Google Scholar]
- 19.Grant DS, Williams TL, Zahaczewsky M, et al. Comparison of antiangiogenic activities using paclitaxel (Taxol) and docetaxel (Taxotere) Int J Cancer. 2003;104:121–9. doi: 10.1002/ijc.10907. [DOI] [PubMed] [Google Scholar]
- 20.Dicker AP, Williams TL, Iliakis G, et al. Targeting angiogenic processes by combination low-dose paclitaxel and radiation therapy. Am J Clin Oncol. 2003;26:e45–53. doi: 10.1097/01.COC.0000072504.22544.3C. [DOI] [PubMed] [Google Scholar]
- 21.Ye K, Ke Y, Keshava N, et al. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci U S A. 1998;95:1601–6. doi: 10.1073/pnas.95.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aneja R, Lopus M, Zhou J, et al. Rational design of the microtubule-targeting anti-breast cancer drug EM015. Cancer Res. 2006;66:3782–91. doi: 10.1158/0008-5472.CAN-05-2962. [DOI] [PubMed] [Google Scholar]
- 23.Attard G, Greystoke A, Kaye S, et al. Update on tubulin-binding agents. Pathol Biol (Paris) 2006;54:72–84. doi: 10.1016/j.patbio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Newcomb EW, Lukyanov Y, Schnee T, et al. Noscapine inhibits hypoxia-mediated HIF-1α expression and angiogenesis in vitro: a novel function for an old drug. Int J Oncol. 2006;28:1121–30. [PubMed] [Google Scholar]
- 25.Pajonk F, van Ophoven A, Weissenberger C, et al. The proteasome inhibitor MG-132 sensitizes PC-3 prostate cancer cells to ionizing radiation by a DNA-PK-independent mechanism. BMC Cancer. 2005;5:76–87. doi: 10.1186/1471-2407-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milas L, Hunter NR, Mason KA, et al. Role of reoxygenation in induction of enhancement of tumor radioresponse by paclitaxel. Cancer Res. 1995;55:3564–68. [PubMed] [Google Scholar]
- 27.Milross CG, Mason KA, Hunter NA, et al. Enhanced radioresponse of paclitaxel-sensitive and –resistant tumor in vivo. Eur J Cancer. 1997;33:1299–08. doi: 10.1016/s0959-8049(97)00107-x. [DOI] [PubMed] [Google Scholar]
- 28.Altinoz MA, Bilir A, Del Maestro RF, et al. Noscapine and diltiazem augment taxol and radiation-induced S-phase arrest and clonogenic death of C6 glioma in vitro. Surg Neurol. 2006;65:478–85. doi: 10.1016/j.surneu.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Walter-Yohrling J, Morgenbesser S, Rouleau C, et al. Murine endothelial cell lines as models of tumor endothelial cells. Clin Cancer Res. 2004;10:2179–89. doi: 10.1158/1078-0432.ccr-03-1013. [DOI] [PubMed] [Google Scholar]
- 30.Cao C, Albert JM, Geng L, et al. Vascular endothelial growth factor tyrosine kinase inhibitor AZD2171 and fractionated radiotherapy in mouse models of lung cancer. Cancer Res. 2006;66:11409–15. doi: 10.1158/0008-5472.CAN-06-2414. [DOI] [PubMed] [Google Scholar]
- 31.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 32.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: Targets for antiangiogenesis and normalization. Microvasc Res. 2007 doi: 10.1016/j.mvr.2007.05.003. E-Pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wachsberger PR, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res. 2003;9:1957–71. [PubMed] [Google Scholar]
- 34.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Teicher BA, Holden SA, Ara G, et al. Comparison of several antiangiogenic regimens alone and with cytotoxic therapies in the Lewis lung carcinoma. Cancer Chemother Pharmacol. 1996b;38:169–77. doi: 10.1007/s002800050466. [DOI] [PubMed] [Google Scholar]
- 36.Lund EL, Bastholm L, Kristjansen PE. Therapeutic synergy of TNP-470 and ionizing radiation: effects on tumor growth, vessel morphology, and angiogenesis in human glioblastoma multiforme xenografts. Clin Cancer Res. 2000;6:971–8. [PubMed] [Google Scholar]
- 37.Salloum RM, Jaskowiak NT, Mauceri HJ, et al. NM-3, an isocoumarin, increases the antitumor effects of radiotherapy without toxicity. Cancer Res. 2000;60:6958–63. [PubMed] [Google Scholar]
- 38.Sakimoto I, Ohta K, Yamazaki T, et al. Alpha-sulfoquinovosylmonoacylglycerol is a novel potent radiosensitizer targeting tumor angiogenesis. Cancer Res. 2006;66:2287–95. doi: 10.1158/0008-5472.CAN-05-2209. [DOI] [PubMed] [Google Scholar]
- 39.Amano M, Suzuki M, Andoh S, et al. Antiangiogenesis therapy using a novel angiogenesis inhibitor, anginex, following radiation cause tumor growth delay. Int J Clin Oncol. 2007;12:42–7. doi: 10.1007/s10147-006-0625-y. [DOI] [PubMed] [Google Scholar]
- 40.Dings RPM, Loren M, Heun H, et al. Scheduling of radiation with angiogenesis inhibitors anginex and avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res. 2007;13:3395–402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–63. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Ansiaux R, Baudelet C, Jordan BF, et al. Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. Clin Cancer Res. 2005;11:743–50. [PubMed] [Google Scholar]
- 43.Fischer I, Gagner JP, Law M, et al. Angiogenesis in gliomas: Biology and molecular pathophysiology. Brain Pathol. 2005;15:297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gagner JP, Law M, Fischer I, et al. Angiogenesis in gliomas: Imaging and experimental therapeutics. Brain Pathol. 2005;15:342–63. doi: 10.1111/j.1750-3639.2005.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zagzag D, Armirnovin R, Greco MA, et al. Vascular apoptosis and evolution in gliomas precede neovascularization: A novel concept for glioma growth and angiogenesis. Lab Invest. 2000;80:837–49. doi: 10.1038/labinvest.3780088. [DOI] [PubMed] [Google Scholar]