Abstract
Several neurodevelopmental disorders, including schizophrenia, autism, ADD/ADHD and dyslexia are believed to originate during gestation and involve white matter abnormalities. Modulation of glutamate environments and glutamate receptors has also been implicated in alteration of oligodendrocytes, the myelin forming cells of the CNS. To begin to understand how modulation of the glutamate system affects the maturation of oligodendrocytes, developing rats were subjected to prenatal blockade of the NMDA receptor with phencyclidine (PCP). Oligodendrocyte development and differentiation were then examined postnatally by measuring markers for early, middle and late stage cells. The results indicate that, while the level of marker proteins for neurons and astrocytes remains the same, early oligodendrocyte progenitor cell markers are decreased in rat brains prenatally exposed to PCP. Labeling of cells of intermediate, immature cell stages is elevated. Late stage markers for myelinating oligodendrocytes are subsequently decreased. These data suggest that prenatal NMDA receptor blockade reduces the level of progenitors and that the surviving cells are arrested at an immature stage. This premature arrest appears to result in fewer fully differentiated, mature oligodendrocytes that are capable of producing myelin. These results have interesting implications for the role of glutamate and glutamate receptors in white matter abnormalities in neurodevelopmental disorders.
Keywords: NMDA receptor, neurodevelopment, glutamate excitotoxicity, myelination
1. INTRODUCTION
Myelin integrity is important for functional neurocircuitry. Perturbations in myelin, either during development or after its formation, lead to neurological consequences [18]. Oligodendrocytes are the myelinating cells of the central nervous system. Oligodendrocytes arise from pre-progenitor and progenitor (OP) cells through a series of developmental stages, each of which can be distinguished by stage-specific antibodies, cell morphology and proliferative capacity [48]. OP cells are uncommitted, highly proliferative and rapidly mobile. They can be identified by the presence of the proteoglycan NG2 on the surface. Progression of OP cells to immature oligodendrocytes is marked by the appearance of galactocerebroside, a myelin specific lipid, and CNPase (2’,3’-cyclic nucleotide 3’-phosphodiesterase). Mature oligodendrocyte phenotypes are marked by the appearance of myelin basic protein (MBP) and proteolipid protein (PLP).
Myelin production is one of the major biological processes occurring in the mammalian brain during growth and development. Myelin is an extension of the membrane of the mature oligodendrocyte that forms a multilamellar structure surrounding an axon. This lipid-rich structure allows for more efficient conduction of nerve impulses along the axon. The presence of myelin increases the speed of signal transmission without requiring a concomitant increase in the diameter of the axon. The energy required for conduction is also lowered due to the smaller size of the axon. Myelination in humans begins in late prenatal or early perinatal life [2,36] and continues into late adolescence/early adulthood.
Many neurodevelopmental disorders are thought to originate during gestation as a consequence of prenatal stress or in utero insults that jeopardize normal neurological development and alter brain function. Glutamate and glutamate receptors are implicated in the neuropathogenesis of these disorders. Glutamate’s role in modulating cellular development, differentiation, migration and pruning is well established [8,9,46,47]. A variety of glutamate receptors are found on many different types of proliferating and differentiating CNS cells including oligodendrocytes. One well established way to model neurodevelopmental psychiatric diseases, such as schizophrenia, is the administration of phencyclidine (PCP). PCP is a dissociative anesthetic that functions as an antagonist of the glutamate NMDA receptors [28]. In humans, a single exposure to PCP produces a behavioral syndrome that resembles the endogenous symptoms of schizophrenia [5] Several laboratories have generated evidence demonstrating abnormally high glutamate release in rat frontal cortex and corticolimbic regions following NMDA receptor antagonism by ketamine and PCP [38]. During early brain development, NMDA receptors undergo a complicated rearrangement of protein subunits [58]. Consequently, the developing brain is highly likely to be vulnerable to stressful environments that manipulate NMDA receptor function during gestation.
Oligodendrocyte lineage cells are known to express different glutamate receptors depending on their stage of differentiation [7,12,13,19–21,45,54,59]. For example, OP cells contain functional NMDA receptors, while immature or fully differentiated oligodendrocytes express AMPA and Kainate receptor subtypes [19,45]. Additionally, it has recently been demonstrated that myelin from adult animals contains NR2 subunits of NMDA receptors [37]. Káradóttir et al. [30] also demonstrated that these receptors elicit functional current. It is believed that OP cells and immature oligodendrocytes are sensitive to glutamate-mediated over-stimulation through an influx of Ca2+ [10,25,27] which ultimately inhibits OP proliferation [45] and delays differentiation in vitro [20].
Given the intimate relationship between development of neurons, oligodendrocytes and myelination, alterations to glutamatergic function during embryonic brain development may seriously alter downstream mechanisms that signal neurodevelopmental events and ultimately set the stage for global brain dysfunction. Here, we examined the effects of in utero PCP administration on subsequent postnatal oligodendrocyte maturation and myelination. Rat embryonic brains were subjected to PCP throughout gestation and then examined for changes in oligodendrocyte maturation markers by immunohistochemistry and western blot analysis. Results suggest that chronic in utero PCP exposure arrests oligodendrocyte differentiation and severely reduces myelin deposition within the postnatal frontal cortex.
2. RESULTS
The level of neuronal and astrocyte markers are not affected by prenatal PCP exposure
Given glutamate’s critical role in modulating early brain development, we performed a series of experiments to determine the effect of NMDA receptor blockade on oligodendrocyte development and differentiation. For this, PCP was chosen as the NMDA receptor antagonist. PCP is a non-competitive NMDA receptor antagonist that binds within the receptor’s calcium channel at picomolar affinities to block ion transport [11,15,29,39]. Prenatal PCP exposure did not affect maternal weight gain, prenatal weight gained per pup, length of pregnancy (21–22 days), nursing ability of the offspring, postnatal pup weight gain or phenotypic milestone development (i.e. hair development, eye opening; data not shown). The only demographic measurement significantly altered by prenatal PCP exposure was number of live pups delivered. Dams receiving daily PCP averaged 10 live births per litter compared to 14 live births per litter for control females.
We first determined the effect of prenatal exposure to PCP on the three major cell types in the developing brain, namely neurons, astrocytes and oligodendrocytes. To test whether prolonged prenatal stress through chronic PCP exposure affected astrocytes, GFAP levels were measured using western blotting. GFAP is an intermediate filament protein specifically found in astroglia [17]. Animals were exposed to PCP or saline prenatally as described in Experimental Methods. It can be seen that the GFAP levels were increased in the PCP-treated animals at PND2 (Fig. 1). However, the level of GFAP recovers and there are no significant differences in the levels of this marker between PCP-treated and control animals at PND9, 16 or 22 (Fig. 1).
Fig. 1. GFAP levels do not decrease in animals exposed prenatally to PCP.

A: Equal amounts of protein from the frontal cortex of control (Ctrl) or PCP-treated (PCP) animals were subjected to western blotting for GFAP (top panels). GAPDH (lower panels) was used as a loading control. The age of the animal from which the protein was extracted is indicated below each pair of lanes. B: Densitometry was performed on the western blots. The integrated densities were normalized to GAPDH levels. Results are presented as Integrated Density ± SEM. *Denotes statistical significance at the p<0.05 level.
NeuN, a nuclear protein found specifically in mature vertebrate neurons, was measured by western blotting as a means to determine if there were gross changes in neuronal numbers in PCP-treated animals. Western blotting for NeuN showed no difference in levels between PCP-treated and control animals at PND2, 16 or 22 (Fig. 2).
Fig. 2. The levels of NeuN do not change with prenatal exposure to PCP.

A: Equal amounts of protein from the frontal cortex of control (Ctrl) or PCP-treated (PCP) animals were subjected to western blotting for NeuN (top panels). GAPDH (lower panels) was used as a loading control. The age of the animal from which the protein was extracted is indicated below each pair of lanes. B: Densitometry was performed on the western blots. The integrated densities were normalized to GAPDH levels. Results are presented as Integrated Density ± SEM.
The level of the oligodendrocyte progenitor marker NG2 is decreased after prenatal exposure to PCP
To determine the effects of prenatal PCP exposure on oligodendroglial progenitors (OP), we assessed NG2 levels by immunohistochemistry in PND2 rat frontal cortex. NG2 is a chondroitin sulfate proteoglycan that marks OP cells in young animals [41,42,55]. It is normally found in high levels throughout the rat brain from birth through PND6 [41]. Interestingly, NG2 immunolabeling was reduced in the frontal cortex of 2 day old neonatal rat brains from PCP-treated animals as compared with controls (Fig.3A). When the label intensities were averaged over several sections the decrease in NG2 labeling was found to be statistically significant (Fig. 3B).
Fig. 3. Prenatal exposure to PCP decreases NG2 levels.
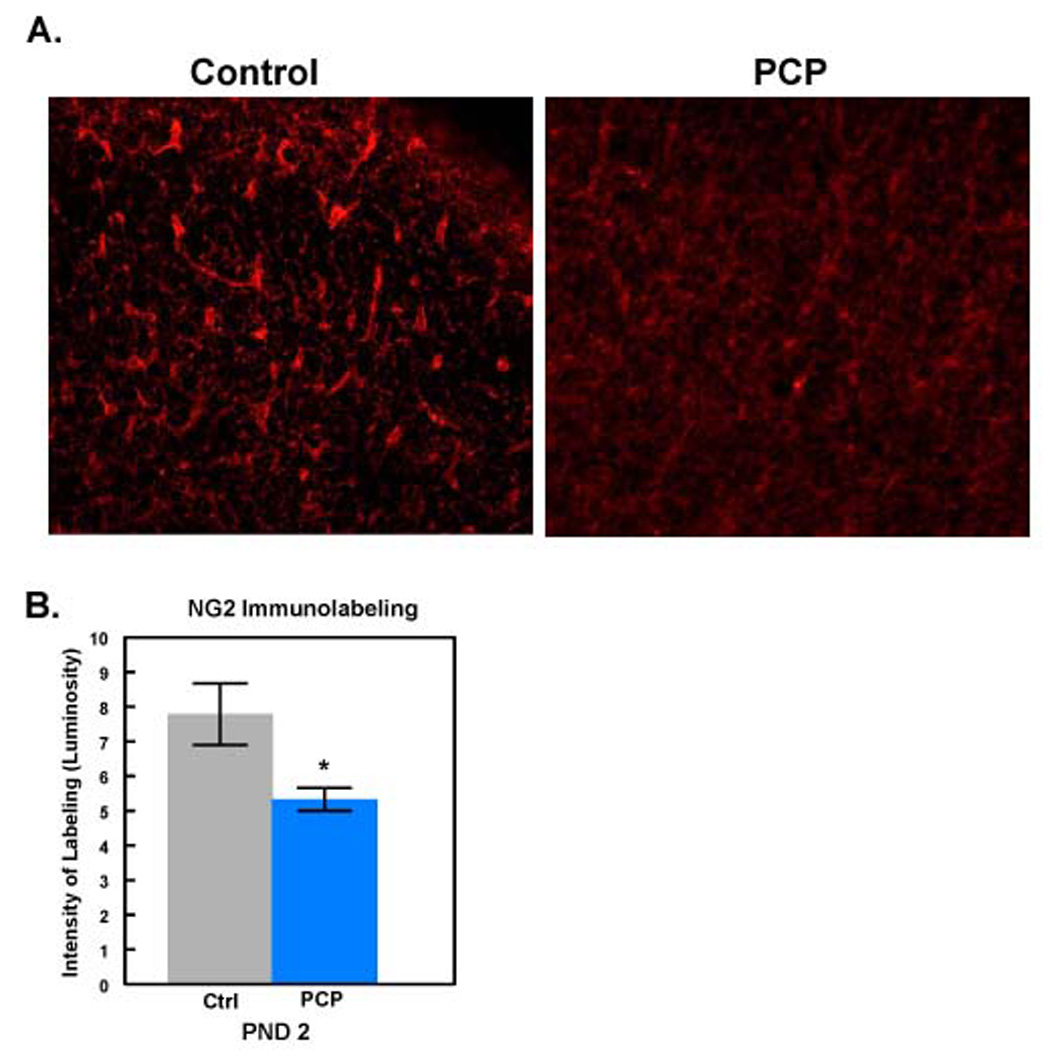
A: Frontal cortex from PND2 control animals (n=6; left panel) or animals exposed prenatally to PCP (n=6; right panel) was labeled with antibody to NG2. Scale bar = 100µm. B: Label intensities from several sections were averaged and are reported as Immunolabeling ± SEM. *Denotes statistical significance at the p<0.05 level.
Intermediate stage oligodendrocyte markers are elevated after prenatal NMDA receptor blockade
The above results suggest that the decrease in NG2 labeling of OPs in the PCP-treated animals at PND2 results, at least in part, from a direct effect of PCP on NMDA receptors present in oligodendrocytes prenatally. To further define the effects on the development of these cells, later stage markers of oligodendrocyte development were measured. To determine the progression of OPs to the next stage sections from PND9 animals were stained using the O4 antibody. O4 recognizes a sulfated surface antigen on early oligodendroblast cells. Surprisingly, the level of O4 staining was increased in PCP-treated animals (Fig. 4). These results indicate that, either the surviving OP cells recover and repopulate the frontal cortex to levels similar to control cells, or that surviving OP cells arrest at an immature stage of differentiation.
Fig. 4. O4 levels are increased in frontal cortex of animals prenatally exposed to PCP.
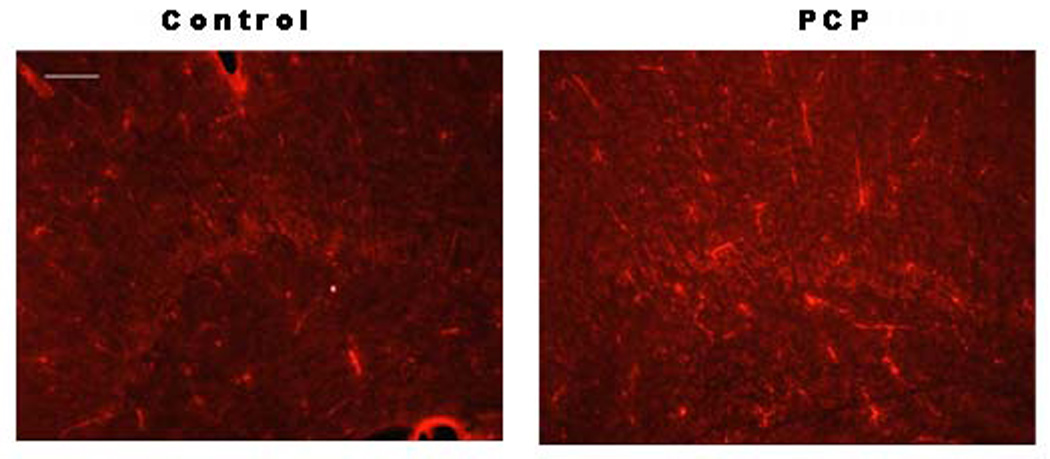
Frontal cortex from PND9 control animals (n=6; left panel) or animals exposed prenatally to PCP (n=6; right panel) was labeled with antibody to O4. Scale bar = 100µm.
To determine whether oligodendrocytes in the PCP-treated animals were arresting or were capable of progressing to myelination at levels similar to control animals, the levels of a marker of immature oligodendrocytes, CNPase, was measured by both immunocytochemistry (Fig. 5A) and western blot analysis (Fig. 5B). At PND16, close to the peak of myelination in the rat, CNPase levels were found to be elevated in neonatal rat brains exposed to PCP during embryonic development (Fig. 5). Western blot analysis of PND2-PND60 brains showed a greater increase in CNPase from PCP treated brains at PND16, 22 and 60 than from control brains at the same time points, confirming the increase noted in immunohistochemical images (Fig. 5). Quantitation of the western blot data showed a statistically significant increase in CNPase levels at later ages in the PCP-treated animals. These results can be explained either by a recovery of the surviving NG2-positive cells to repopulate the oligodendrocytes or a developmental block of these cells at an immature stage.
Fig. 5. CNPase levels are increased by prenatal exposure to PCP.

A: Frontal cortex from PND16 control animals (n=6; left panel) or animals exposed prenatally to PCP (n=6; right panel) was labeled with antibody to CNPase. Scale bar = 100µm. B: Equal amounts of protein from control (Ctrl) or PCP-treated (PCP) animals were subjected to western blotting for CNPase. The age of the animal from which the protein was extracted is indicated above each lane. The arrowhead indicates the position of GAPDH which was used as a loading control. C: Densitometry was performed on the western blots. The integrated densities were normalized to GAPDH levels. Results are presented as Integrated Density ± SEM. *Denotes statistical significance at the p <0.05 level.
Animals exposed to PCP prenatally show a decrease in myelin basic protein levels
It was therefore of particular interest to determine the effect of prenatal PCP exposure on myelination. To do this, myelin basic protein (MBP) levels were examined in frontal cortex from control and prenatally-PCP treated neonatal brains by immunohistochemistry (Fig. 6) and Western blot analyses (Fig. 7). At PND9, frontal cortex MBP levels were remarkably reduced following chronic NMDA receptor antagonism (Fig. 6A, Fig. 7A). At PND16 (Fig. 6B, Fig. 7A) and PND22 (Fig. 7A), myelination remained compromised throughout the frontal cortex. Most striking was the persistent effect of prenatal PCP exposure on adult MBP levels. Not only were MBP levels reduced during the early postnatal period compared to controls, they remained depressed well into adulthood (PND60). Importantly, MBP levels in PCP-treated animals increased at a rate similar to controls, albeit at a consistently lower level (Fig. 7B).
Fig. 6. Prenatal exposure to PCP results in a decrease in MBP levels.
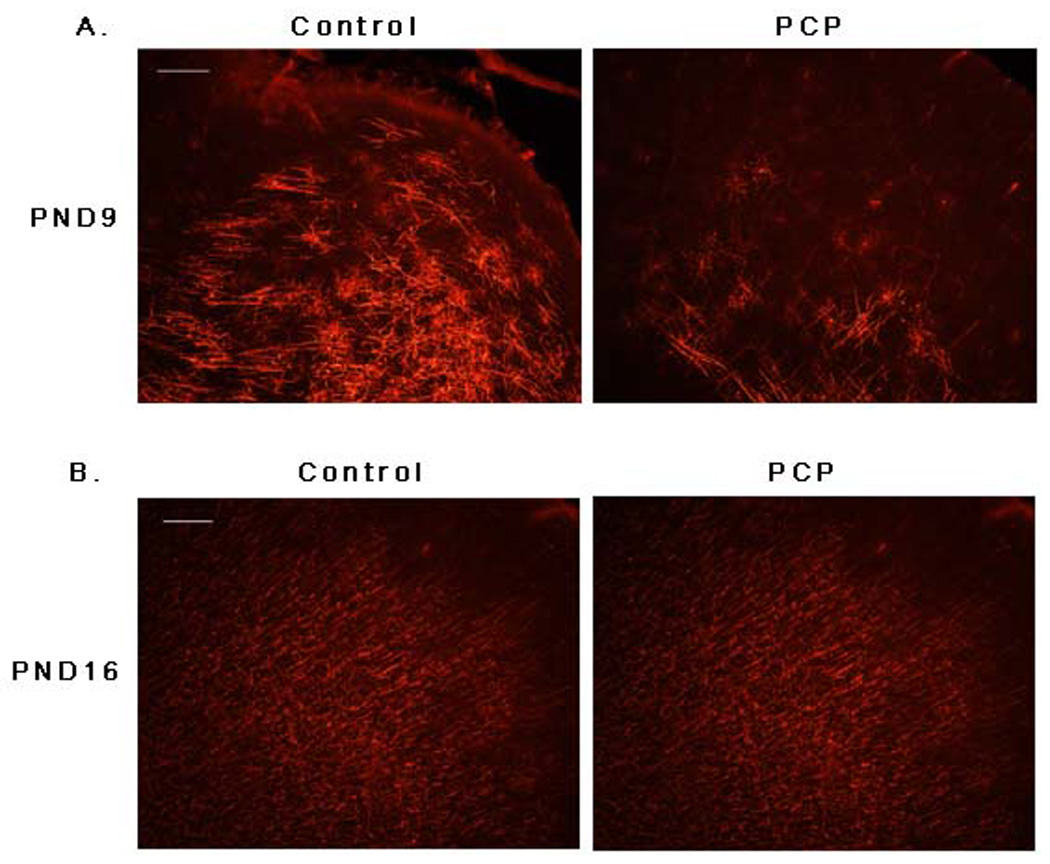
Frontal cortex from PND9 (A) or PND16 (B) control animals (n=6; left panel) or animals exposed prenatally to PCP (n=6; right panel) was labeled with antibody to MBP. Scale bar = 100µm.
Fig. 7. MBP levels are persistently decreased by PCP exposure.

A: Equal amounts of protein from control (Ctrl) or PCP-treated (PCP) animals were subjected to western blotting for MBP. The 18 kDa isoform is indicated by an arrow on the left. The age of the animal from which the protein was extracted is indicated below each pair of samples. Actin was blotted as a loading control. B: Densitometry was performed on the MBP western blots and the densities were normalized to actin. Results are presented as Integrated Density ± SEM. *Denotes statistical significance at the p<0.05 level.
Since the intermediate stage markers were elevated, but MBP levels were decreased we determined the level of a second late stage marker, MOG (myelin oligodendrocyte glycoprotein). MOG appears later in oligodendrocyte development than MBP and correlates closely with myelin formation [53]. MOG levels were assessed by immunocytochemistry and western blot analysis (Fig. 8). Both measures showed a decrease in MOG levels in brains of rats prenatally exposed to PCP.
Fig. 8. PCP exposure leads to decreased MOG levels.
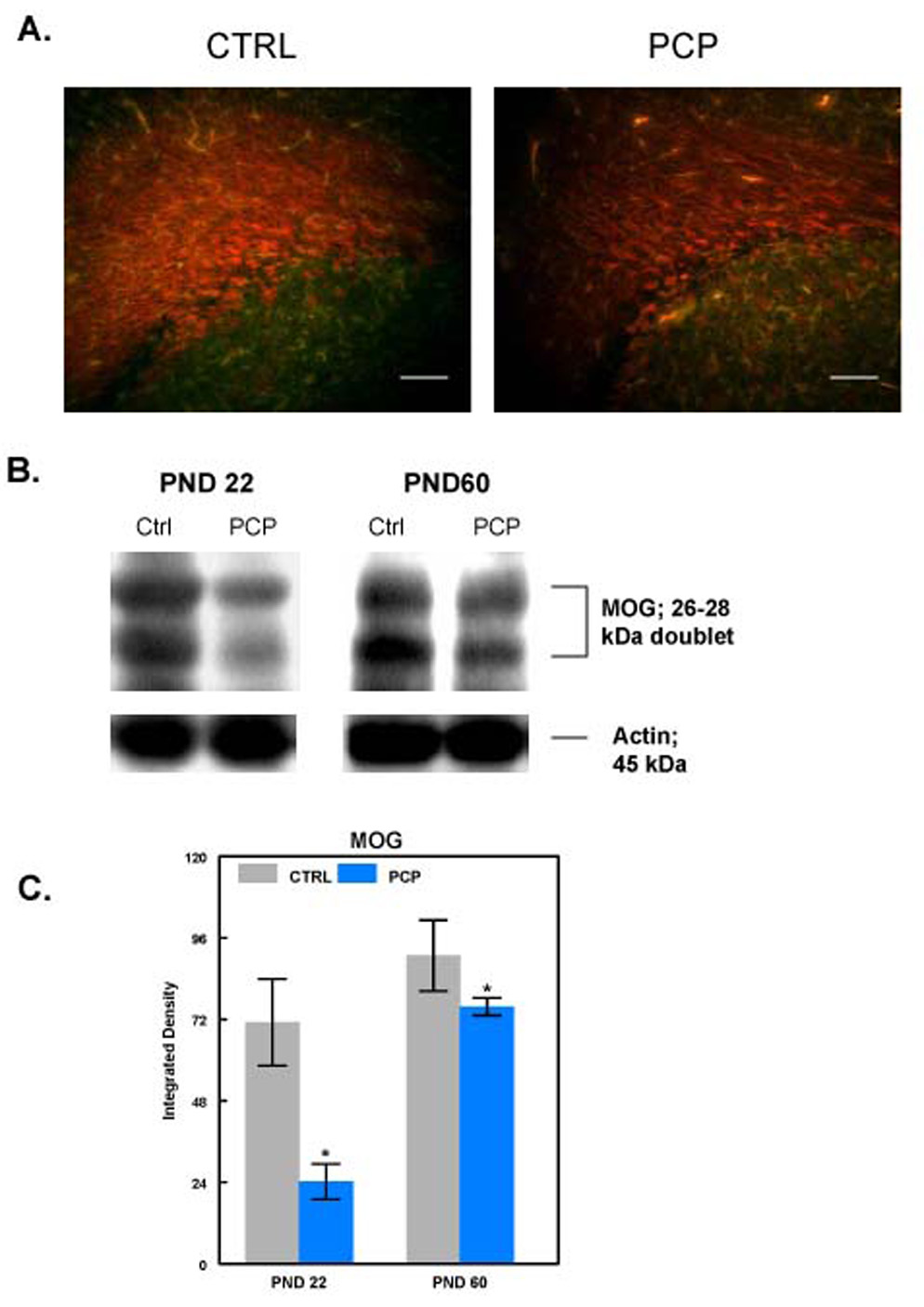
A: Sections from PND22 control animals (n=6; left panel) or animals exposed prenatally to PCP (n=6; right panel) were labeled with antibody to MOG. Scale bar = 100µm. B: Equal amounts of protein from control (Ctrl) or PCP-treated (PCP) animals were subjected to western blotting for MOG. The age of the animal from which the protein was extracted is indicated above each pair of samples. Actin was blotted as a loading control. C: Densitometry was performed on the MOG western blots and the densities were normalized to actin. Results are presented as Integrated Density ± SEM. *Denotes statistical significance at the p<0.05 level.
These results suggest that, even if the surviving OP cells proliferate at a more rapid rate and replenish the depleted pools of oligodendrocytes, these cells arrest at an immature stage of differentiation and are incapable of complete recovery of myelin deposition.
3. DISCUSSION
The results presented here suggest that PCP exposure during prenatal development of the brain leads to significant changes in the pattern of development of oligodendrocytes in the frontal cortex. Indeed, it appears that OPs are the cell type most affected by prenatal PCP exposure. While there appears to be an early decrease in astrocyte markers, reflected in lowered GFAP levels, these cells appear to recover and reach the same levels as control animals by PND9. A marker of mature neurons also does not appear to be decreased by prenatal exposure to PCP. This is surprising in that others have reported that brief exposure of late fetal or early postnatal rats to NMDA receptor blockade results in increased neuronal apoptosis in the developing brain which might be expected to result in decreased numbers of mature neurons [26]. Indeed, in a recent publication, Abekawa et al. [1], treated animals prenatally with MK-801, another NMDA receptor antagonist. This resulted in a decrease in parvalbumin positive GABA neurons in the prefrontal cortex. However, the paradigm used here involved PCP exposure throughout the gestation period while Abekawa et al. treated the animals only between E15 and E18. Thus, it is possible that, with continued exposure to PCP, the neurons adapt or astrocytes increase their levels of glutamate uptake resulting in a rescue of neuronal number.
Our results, indicating a decrease in OP cells as evidenced by a decrease in NG2 labeling, suggest that prenatal PCP exposure leads either to the death of cells destined to become oligodendrocytes or a change in the cell fate of these cells. While glutamatergic pathways are most commonly associated with excitatory responses, the NMDA receptor hypofunction theory postulates that disruption of NMDA receptor function stimulates abnormally high levels of glutamate release within the synapse. High synaptic glutamate then feeds back to disinhibit GABA-controlled pathways which further results in unregulated excitotoxicity and neuronal degeneration [24,32,43,44]. Thus, while PCP can act as an antagonist of other receptor types, such as sigma receptors, the results here are most likely due to NMDA receptor antagonism.
Cells of the oligodendrocyte lineage are known to express both ligand- and voltage-gated ion channels [7,12,13,19,20,45]. OP cells have been shown to contain functional NMDA receptors that are responsible for the expression of PSA-NCAM and hence, assist in directing cell migration [57]. NMDA receptors have not been demonstrated on immature or fully differentiated oligodendrocytes [19,45]. However, it has recently been demonstrated that myelin from adult animals does contain NR1 and NR2 subunits of NMDA receptors [37]. Káradóttir et al. [30] also demonstrated that these receptors elicit functional current. The presence of NMDA receptors in myelin suggests that PCP can act directly on cells of the oligodendrocyte lineage. Indeed the presence of the NR1 and NR2B subunits further supports this as each contributes to the PCP binding site. Therefore, we would expect that PCP would affect oligodendrocytes at an early stage of development with consequences that, as in the case of neuronal cell death [23], would be manifest throughout the life of the animal. While oligodendrocytes at many stages of development can be killed by excitatory amino acids, oligodendrocyte progenitors and immature oligodendrocytes are particularly vulnerable to excitotoxic cell death [35,49,56]. Indeed, in culture, MBP positive oligodendrocytes were shown to be resistant to kainate-induced cell death while cells at earlier stages of development were vulnerable [50]. Thus the decrease in NG2 staining observed in the early postnatal period is consistent with death of OP cells due to excitotoxicity. Some of this death may now be directly attributed to the presence of NMDA receptors in oligodendrocytes.
The increase in O4+ and CNPase+ cells further suggests that those OP cells that are not killed by increased glutamate levels during development can further proliferate and repopulate the pool of oligodendrocytes. However, they appear to arrest at an immature stage. A similar block in differentiation was seen in vitro using purified cortical oligodendrocytes. Activation of AMPA glutamate receptors prevented lineage progression from OP stage to the prooligodendroblast stage [20].
In addition, the decrease seen in both late stage markers of myelination, MBP and MOG following in utero PCP exposure, suggest that few of the cells advance beyond this block in differentiation. The decreased levels of MBP and MOG indicate a significant decrease in the degree of myelination present in the PCP-treated animals. Indeed, MOG levels correlate strongly with myelination levels [53]. While the exact extent and effect of the myelination deficit remains to be determined, it is likely that this decrease will have significant effects on neurocircuitry and subsequent nervous system function.
With cortical connectivity implicated as a central abnormality in many neurodevelopmental disorders, and oligodendroglial function and myelination known to affect neuronal connections, it is highly plausible that the relationship between NMDA receptors and differentiating oligodendrocytes plays an important role in the pathogenesis of neurodevelopmental disorders. For example, white matter anomalies have been documented in schizophrenic brain using several techniques, including MRI [4,52] and diffusion tensor imaging (DTI) [3,14,31,33] in the frontal cortex. Additional evidence implicating oligodendrocyte development and myelination in the pathophysiology of certain neurodevelopmental disorders comes from microarray analysis of post-mortem human schizophrenic brain. In one such analysis of prefrontal cortex tissue, only 7 of 6500 screened genes were decreased in schizophrenic brains. Six of the 7 were myelin-related genes [22]. MRI examinations of brains from autistic children also showed patterns of white matter abnormalities [16]. Most interesting is the finding that myelinated corpus callosum tracts responsible for inter-hemisphere integration of emotion, recognition and appropriate behavioral response in social settings are consistently reduced throughout the average autistic individual’s lifespan [6]. The data presented here paint a compelling picture suggesting an important relationship between functional NMDA receptors, oligodendrocyte development and white matter integrity during embryonic brain development. Currently, this relationship is poorly understood. Further testing of animals subjected to prenatal antagonism of the NMDA receptor needs to be done to determine if the changes in myelination observed lead to behavioral and/or cognitive consequences.
4. EXPERIMENTAL PROCEDURES
Administration of Phencyclidine and Brain Tissue Preparation
Adult female Sprague Dawley rats (n=8; the entire litter constitutes a single n; Charles River Laboratories) were monitored for conception by vaginal plug release. Twenty-four hours later, pregnant females began receiving daily intraperitoneal (IP) injections of phencyclidine (PCP; 10mg/kg in 0.5ml of 0.9% saline) through E20. The PCP dose chosen has been shown to elicit stereotypic behaviors in rats without producing lethal toxicity [40,51]. Intraperitoneal injections were chosen as the preferred delivery method based on PCP’s lipid solubility characteristics, the drug’s ability to easily cross blood:brain/blood:placental barriers and the ease of modifying dosage volumes based on maternal weight. Age matched, pregnant control rats (n=8) received daily IP saline injections. Maternal PCP injections were discontinued 24–48 hours prior to parturition (E22). Following birth, pups were cross-fostered to surrogate females to eliminate postnatal PCP exposure while nursing and to control for nutritional opportunity by equally distributing pups among foster mothers. Pups were weaned on postnatal day 21. Two pups from each litter were sacrificed by decapitation on postnatal days PND2, 9, 16, 22 and 60, and tissues were harvested. Brains destined for protein analysis were snap frozen in liquid nitrogen prior to processing. For immunohistochemistry, brain samples from PND2 and PND9 were directly post-fixed in 1% paraformaldehyde, transferred to 25% sucrose, gelatin embedded and stored until cryosectioning. PND16 and 22 animals were anesthetized with sodium pentobarbital (60mg/kg), transcardially perfused with 0.9% saline followed by cold 1% paraformaldehyde prior to decapitation. Protocols involving the use of animals complied with the guidelines of the NIH and the Institutional Animal Care and Use Committee.
Immunohistochemistry and Imaging Analysis
Tissue was sectioned at 30µm in the coronal plane and mounted serially on coated slides, rostral to caudal. Antigen retrieval was performed for CNPase, MBP and MOG antibodies by heating affixed sections in 0.05% citric anhydride at 90°C for 45 min. Tissues were bench cooled and rinsed with copious amount of deionized water. Immunocytochemistry was performed as previously described [34]. Briefly, tissues were blocked in 4% non-fat dry milk (NFDM), rinsed in 0.1M phosphate buffer containing 0.2% Tween-20 followed by incubation with primary antibodies diluted in 3.0% normal goat serum and 0.6% TritonX-100.
Antibodies used include anti-NG2, a mouse monoclonal antibody to the proteoglycan, a generous gift from William Stallcup, Ph.D. (Burnham Institute), anti-MOG mouse monoclonal antibody, a generous gift from Minnetta Gardinier, Ph.D. (University of Iowa) and mouse anti-O4, which was produced in our laboratory. Mouse anti-CNPase antibody (MAB326) and anti-MBP (MAB 384) were from Chemicon (Temeculah, CA). Secondary antibodies were species specific Cy3-conjugated IgG’s (Jackson ImmunoResearch, West Grove, PA) diluted 1:200 in PBS containing 3.0% normal goat serum and 0.6% Triton X-100.
Frontal cortex was analyzed from approximately 2.2mm rostral to bregma, to −0.40mm caudal according to Paxinos and Watson (to approximately the level of the striatum). Four to six serial sections of frontal cortex were chosen and regionally matched for each brain analyzed. Six separate images of each section were analyzed from consistent regions in each hemisphere. A uniform sized square of the same magnification was used as the window of analysis located a fixed distance from the cortical surface. Digital images of immunoreactive tissue sections were captured by epifluorescence on a Zeiss Axioskop 2 Mot microscope camera with an Axiocam high-resolution camera (Zeiss) and imported for computer-assisted quantitation of luminosity using Adobe Photoshop software. Staining variation was controlled for by subtracting background from each image prior to collapsing the data. Computer generated luminosity levels were averaged from all field images per region, per section, per brain, and standard deviations of the pixels were calculated as in [34]. StatView software was used for statistical analysis using ANOVA to determine significance levels. All analyses were performed double-blinded to the experimental group from which the tissues were obtained.
Western Blot Analysis
Proteins were extracted from prefrontal cortex tissue according to our published methods. While each litter represented an independent n, three SDS-PAGE runs on each sample from three independent litters constituted a repeated measure of analysis. PCP treated brain samples and control samples were paired and electrophoretically separated on the same gel to control for inter-gel differences. Proteins were transferred to PVDF membrane using a semidry transfer apparatus and blocked with 5% NFDM in PBS for one hour. Anti-MBP (1:500), anti-CNPase (1:500), anti-glial fibrillary acidic protein (GFAP, MAB360, 1:500), anti NR2B (AB1557P, 1:500) and anti-neuron-specific nuclear antigen (NeuN, MAB377) were from Chemicon. Anti NR1 (556308, 1:250) was from BD Pharmingen (San Jose, CA). Anti-MOG was used at a 1:30 dilution. Host-appropriate HRP-conjugated secondary antibodies were from Zymed Laboratories (South San Francisco, CA). Proteins were detected using the ECL-chemiluminescence system (Amersham Pharmacia, Piscataway, NJ). Membranes were stripped using an acid glycine/Tween-20/SDS solution. Blots were then probed for α-actin (MAB1501R, Chemicon, 1:500) or glyceraldehyde-phosphate-dehydrogenase (GAPDH; MAB374, Chemicon, 1:500) to verify protein loading accuracy and for the normalization of detected proteins. Immunoreactive signals were captured on Kodak hyperfilm (Eastman Kodak, Rochester, NY), quantified by computer assisted densitometry (ChemiImager software) and normalized to loading control protein levels prior to averaging. StatView software was used for statistical analysis by a paired t-test.
Acknowledgments
The authors thank William Stallcup for generously supplying anti-NG2 antibody and Minnetta Gardinier for a generous supply of anti-MOG.
This work was supported by NIH grant MH64552 (J.S.L.), National Multiple Sclerosis grant #RG3293-B-7 (R.M.) and by NIH Grant Number P20 RR-015567 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abekawa T, Ito K, Nakagawa S, Koyama T. Prenatal exposure to an NMDA receptor antagonist, MK-801 reduces density of parvalbumin-immunoreactive GABAergic neurons in the medial prefrontal cortex and enhances phencyclidine-induced hyperlocomotion but not behavioral sensitization to methamphetamine in postpubertal rats. Psychopharmacology (Berl) 2007;192:303–316. doi: 10.1007/s00213-007-0729-8. [DOI] [PubMed] [Google Scholar]
- 2.Adams CW, Liebowitz S. Morphologocal aspects of myelin and demyelination. In: Adams CWM, editor. Research in Multiple Sclerosis. Springfield, IL: Thomas; 1972. pp. 19–60. [Google Scholar]
- 3.Agartz I, Andersson JL, Skare S. Abnormal brain white matter in schizophrenia: a diffusion tensor imaging study. Neuroreport. 2001;12:2251–2254. doi: 10.1097/00001756-200107200-00041. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V, 2nd, O'leary DS, Ehrhardt JC, Yuh WT. Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. Jama. 1994;272:1763–1769. [PubMed] [Google Scholar]
- 5.Bakker CB, Amini FB. Observations on the psychotomimetic effects of Sernyl. Compr Psychiatry. 1961;2:269–280. doi: 10.1016/s0010-440x(61)80033-3. [DOI] [PubMed] [Google Scholar]
- 6.Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Barres BA, Koroshetz WJ, Swartz KJ, Chun LL, Corey DP. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990;4:507–524. doi: 10.1016/0896-6273(90)90109-s. [DOI] [PubMed] [Google Scholar]
- 8.Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31:251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 9.Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- 10.Borges K, Ohlemeyer C, Trotter J, Kettenmann H. AMPA/kainate receptor activation in murine oligodendrocyte precursor cells leads to activation of a cation conductance, calcium influx and blockade of delayed rectifying K+ channels. Neuroscience. 1994;63:135–149. doi: 10.1016/0306-4522(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 11.Bortolotto ZA, Lauri S, Isaac JT, Collingridge GL. Kainate receptors and the induction of mossy fibre long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:657–666. doi: 10.1098/rstb.2002.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand Schieber E, Werner P. (+/−)-Alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid and kainate receptor subunit expression in mouse versus rat spinal cord white matter: similarities in astrocytes but differences in oligodendrocytes. Neurosci Lett. 2003;345:126–130. doi: 10.1016/s0304-3940(03)00469-5. [DOI] [PubMed] [Google Scholar]
- 13.Brand Schieber E, Werner P. AMPA/kainate receptors in mouse spinal cord cell-specific display of receptor subunits by oligodendrocytes and astrocytes and at the nodes of Ranvier. Glia. 2003;42:12–24. doi: 10.1002/glia.10136. [DOI] [PubMed] [Google Scholar]
- 14.Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA, Downhill J, Haznedar M, Fallon JH, Atlas SW. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- 15.Chung MK, Dalton KM, Alexander AL, Davidson RJ. Less white matter concentration in autism: 2D voxel-based morphometry. Neuroimage. 2004;23:242–251. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 16.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 17.Debus E, Weber K, Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25:193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 18.Duncan ID, Lunn KF, Holmgren B, Urba-Holmgren R, Brignolo-Holmes L. The taiep rat: a myelin mutant with an associated oligodendrocyte microtubular defect. J Neurocytol. 1992;21:870–884. doi: 10.1007/BF01191684. [DOI] [PubMed] [Google Scholar]
- 19.Gallo V, Patneau DK, Mayer ML, Vaccarino FM. Excitatory amino acid receptors in glial progenitor cells: molecular and functional properties. Glia. 1994;11:94–101. doi: 10.1002/glia.440110204. [DOI] [PubMed] [Google Scholar]
- 20.Gallo V, Zhou JM, Mcbain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Barcina JM, Matute C. Expression of kainate-selective glutamate receptor subunits in glial cells of the adult bovine white matter. Eur J Neurosci. 1996;8:2379–2387. doi: 10.1111/j.1460-9568.1996.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 22.Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris LW, Sharp T, Gartlon J, Jones DN, Harrison PJ. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. Eur J Neurosci. 2003;18:1706–1710. doi: 10.1046/j.1460-9568.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch SR, Das I, Garey LJ, De Belleroche J. A pivotal role for glutamate in the pathogenesis of schizophrenia, and its cognitive dysfunction. Pharmacol Biochem Behav. 1997;56:797–802. doi: 10.1016/s0091-3057(96)00428-5. [DOI] [PubMed] [Google Scholar]
- 25.Holtzclaw LA, Gallo V, Russell JT. AMPA receptors shape Ca2+ responses in cortical oligodendrocyte progenitors and CG-4 cells. J Neurosci Res. 1995;42:124–130. doi: 10.1002/jnr.490420114. [DOI] [PubMed] [Google Scholar]
- 26.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 27.Itoh T, Beesley J, Itoh A, Cohen AS, Kavanaugh B, Coulter DA, Grinspan JB, Pleasure D. AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem. 2002;81:390–402. doi: 10.1046/j.1471-4159.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- 28.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 29.Kamiya H. Kainate receptor-dependent presynaptic modulation and plasticity. Neurosci Res. 2002;42:1–6. doi: 10.1016/s0168-0102(01)00303-0. [DOI] [PubMed] [Google Scholar]
- 30.Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, Mccarley RW, Shenton ME. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 33.Lim KO, Hedehus M, Moseley M, De Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- 34.Lindahl JS, Keifer J. Glutamate Receptor Subunits are Altered in Forebrain and Cerebellum in Rats Chronically Exposed to the NMDA Receptor Antagonist Phencyclidine. Neuropsychopharmacology. 2004 doi: 10.1038/sj.npp.1300485. [DOI] [PubMed] [Google Scholar]
- 35.Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24:224–230. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- 36.Mehler MF, Marmur R, Gross R, Mabie PC, Zang Z, Papavasiliou A, Kessler JA. Cytokines regulate the cellular phenotype of developing neural lineage species. Int J Dev Neurosci. 1995;13:213–240. doi: 10.1016/0736-5748(94)00060-g. [DOI] [PubMed] [Google Scholar]
- 37.Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, Mcrory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 38.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 40.Murray TF, Horita A. Phencyclidine-induced stereotyped behavior in rats: dose response effects and antagonism by neuroleptics. Life Sci. 1979;24:2217–2225. doi: 10.1016/0024-3205(79)90097-3. [DOI] [PubMed] [Google Scholar]
- 41.Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 42.Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996;43:315–330. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 43.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 44.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 45.Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994;12:357–371. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 46.Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 47.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 48.Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- 49.Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg PA, Dai W, Gan XD, Ali S, Fu J, Back SA, Sanchez RM, Segal MM, Follett PL, Jensen FE, Volpe JJ. Mature myelin basic protein-expressing oligodendrocytes are insensitive to kainate toxicity. J Neurosci Res. 2003;71:237–245. doi: 10.1002/jnr.10472. [DOI] [PubMed] [Google Scholar]
- 51.Scalzo FM, Holson RR. The ontogeny of behavioral sensitization to phencyclidine. Neurotoxicol Teratol. 1992;14:7–14. doi: 10.1016/0892-0362(92)90023-4. [DOI] [PubMed] [Google Scholar]
- 52.Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, Toone B. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- 53.Solly SK, Thomas JL, Monge M, Demerens C, Lubetzki C, Gardinier MV, Matthieu JM, Zalc B. Myelin/oligodendrocyte glycoprotein (MOG) expression is associated with myelin deposition. Glia. 1996;18:39–48. doi: 10.1002/(SICI)1098-1136(199609)18:1<39::AID-GLIA4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 54.Sontheimer H, Trotter J, Schachner M, Kettenmann H. Channel expression correlates with differentiation stage during the development of oligodendrocytes from their precursor cells in culture. Neuron. 1989;2:1135–1145. doi: 10.1016/0896-6273(89)90180-3. [DOI] [PubMed] [Google Scholar]
- 55.Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tekkok SB, Goldberg MP. Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21:4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C, Pralong WF, Schulz MF, Rougon G, Aubry JM, Pagliusi S, Robert A, Kiss JZ. Functional N-methyl-D-aspartate receptors in O-2A glial precursor cells: a critical role in regulating polysialic acid-neural cell adhesion molecule expression and cell migration. J Cell Biol. 1996;135:1565–1581. doi: 10.1083/jcb.135.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- 59.Yoshioka A, Hardy M, Younkin DP, Grinspan JB, Stern JL, Pleasure D. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors mediate excitotoxicity in the oligodendroglial lineage. J Neurochem. 1995;64:2442–2448. doi: 10.1046/j.1471-4159.1995.64062442.x. [DOI] [PubMed] [Google Scholar]


