Abstract
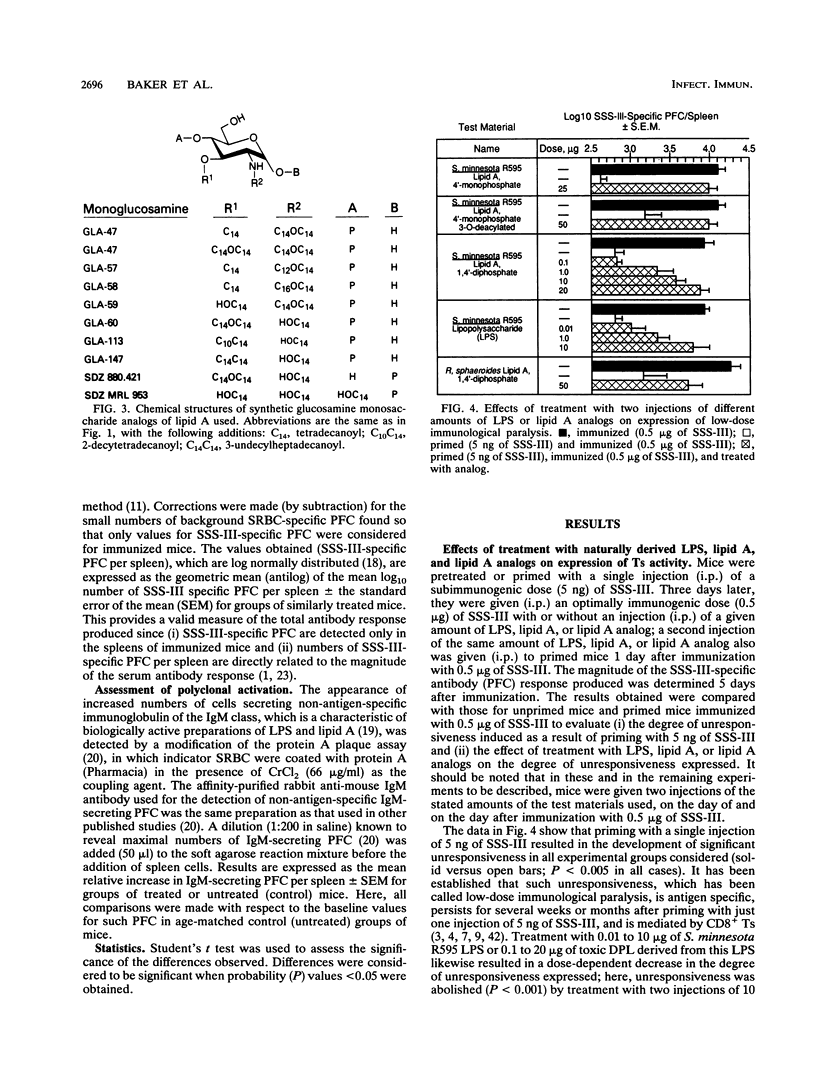
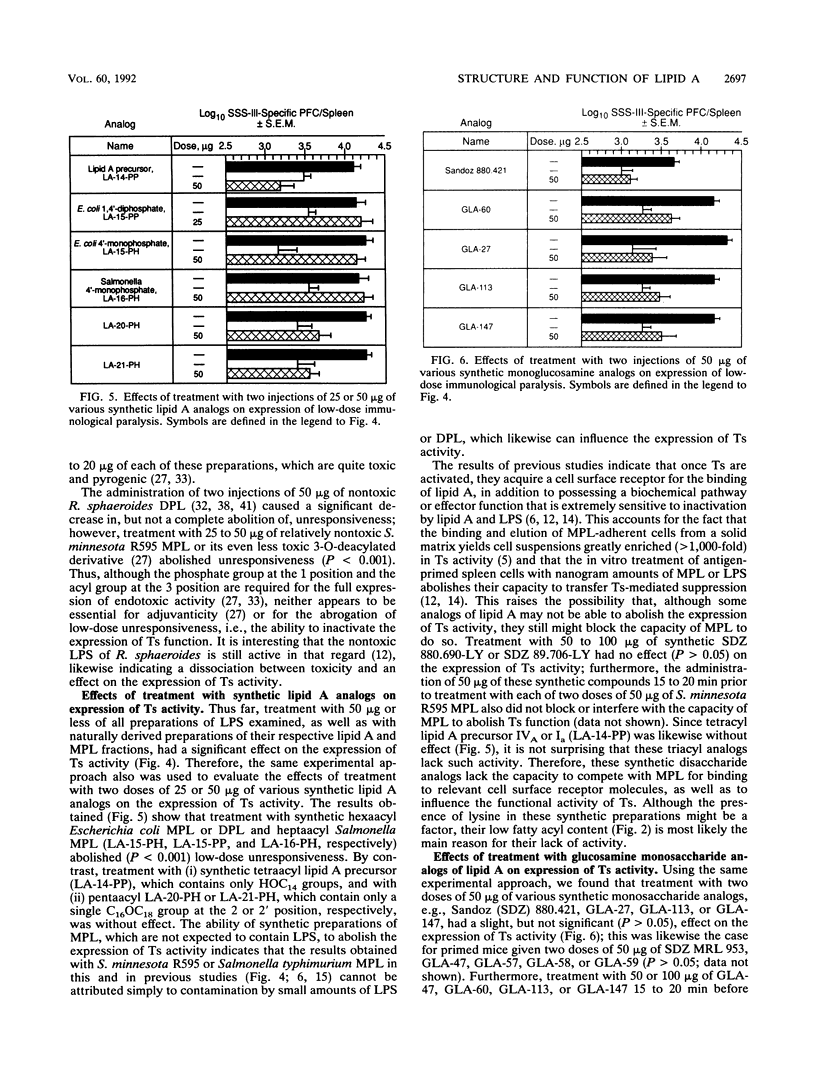
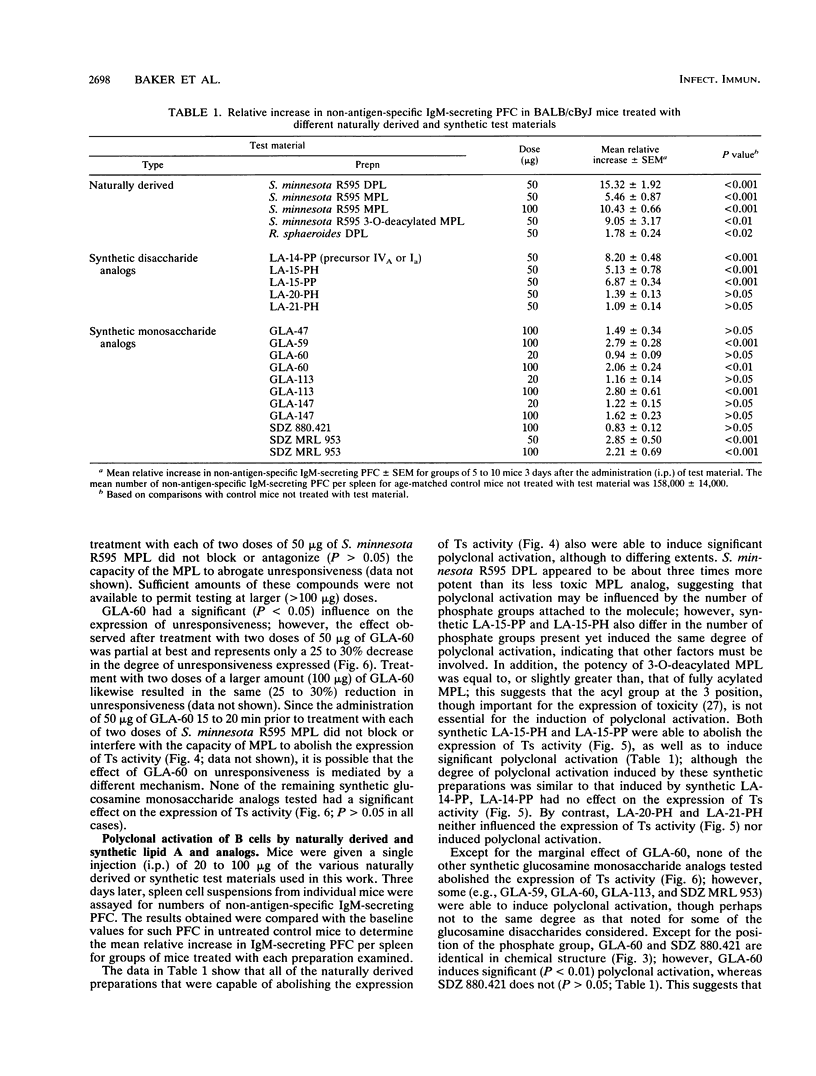
Lipid A preparations derived from the lipopolysaccharides of several gram-negative bacteria, as well as chemically defined synthetic lipid A's and their analogs (both glucosamine mono- and disaccharides), were used to establish the chemical structures required for (i) abolishing the expression of suppressor T cell (Ts) function and (ii) inducing polyclonal activation of B cells. Salmonella minnesota R595 lipid A (diphosphoryl lipid A) possesses both of these activities. Decreasing the number of phosphate groups in lipid A from two to one (monophosphoryl lipid A) as well as decreasing the fatty acyl content, primarily by removing the residue at the 3 position, resulted in a progressive reduction in toxicity; however, these structural modifications did not influence its ability to abolish the expression of Ts function. Reducing the fatty acyl content from five to four (lipid A precursor IVA or Ia) eliminated the capacity to influence Ts function but not to induce polyclonal activation of B cells. None of the monosaccharide analogs of lipid A examined influenced the expression of Ts activity, although some were able to activate B cells polyclonally. Thus, in order to be able to abolish the expression of Ts function, lipid A (i) must be a glucosamine disaccharide, (ii) may have either one or two phosphate groups, and (iii) must have at least five fatty acyl groups. Also, the chain length of the nonhydroxylated fatty acid, as well as the location of acyloxyacyl groups (2' versus 3' position), may play an important role. These findings indicate that the chemical structures responsible for the toxicity of lipid A differ from those that influence its capacity to abolish the expression of Ts function and to induce polyclonal activation of B cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsbaugh D. F., Prescott B., Baker P. J. Effect of splenectomy on the expression of regulatory T cell activity. J Immunol. 1978 Oct;121(4):1483–1485. [PubMed] [Google Scholar]
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Direct evidence for the involvement of T suppressor cells in the expression of low-dose paralysis to type III pneumococcal polysaccharide. J Immunol. 1982 Mar;128(3):1059–1062. [PubMed] [Google Scholar]
- Baker P. J., Haslov K. R., Fauntleroy M. B., Stashak P. W., Myers K., Ulrich J. T. Enrichment of suppressor T cells by means of binding to monophosphoryl lipid A. Infect Immun. 1990 Mar;58(3):726–731. doi: 10.1128/iai.58.3.726-731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Prescott B., Cantrell J. L., Rudbach J. A. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988 May;56(5):1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J. Homeostatic control of antibody responses: a model based on the recognition of cell-associated antibody by regulatory T cells. Transplant Rev. 1975;26:3–20. doi: 10.1111/j.1600-065x.1975.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Baker P. J. Regulation of magnitude of antibody response to bacterial polysaccharide antigens by thymus-derived lymphocytes. Infect Immun. 1990 Nov;58(11):3465–3468. doi: 10.1128/iai.58.11.3465-3468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. II. Studies on the relative rate of antibody synthesis and release by antibody-producing cells. Immunology. 1971 Apr;20(4):481–492. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Taylor C. E., Stashak P. W., Fauntleroy M. B., Hasløv K., Qureshi N., Takayama K. Inactivation of suppressor T cell activity by the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides. Infect Immun. 1990 Sep;58(9):2862–2868. doi: 10.1128/iai.58.9.2862-2868.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwunife F. S., Taylor C. E., Fauntleroy M. B., Stashak P. W., Baker P. J. Differential effects of monophosphoryl lipid A on expression of suppressor T cell activity in lipopolysaccharide-responsive and lipopolysaccharide-defective strains of C3H mice. Infect Immun. 1991 Jun;59(6):2192–2194. doi: 10.1128/iai.59.6.2192-2194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquivel F., Taylor C. E., Baker P. J. Differential sensitivity of CD8+ suppressor and cytotoxic T lymphocyte activity to bacterial monophosphoryl lipid A. Infect Immun. 1991 Sep;59(9):2994–2998. doi: 10.1128/iai.59.9.2994-2998.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Bacterial lipopolysaccharides: structure, metabolism and mechanisms of action. Int Rev Immunol. 1990;6(4):207–221. doi: 10.3109/08830189009056632. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985 Apr 1;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb C. F. Application of transformations to normalize the distribution of plaque-forming cells. J Immunol. 1974 Jul;113(1):51–57. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hiernaux J. R., Stashak P. W., Cantrell J. L., Rudbach J. A., Baker P. J. Immunomodulatory activity of monophosphoryl lipid A in C3H/HeJ and C3H/HeSnJ mice. Infect Immun. 1989 May;57(5):1483–1490. doi: 10.1128/iai.57.5.1483-1490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON A. G., GAINES S., LANDY M. Studies on the O antigen of Salmonella typhosa. V. Enhancement of antibody response to protein antigens by the purified lipopolysaccharide. J Exp Med. 1956 Feb 1;103(2):225–246. doi: 10.1084/jem.103.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Stashak P. W., Prescott B., Baker P. J., Alling D. W. Kinetics of the antibody response to type III pneumococcal polysaccharide. I. Evidence that suppressor cells function by inhibiting the recruitment and proliferation of antibody-producing cells. J Immunol. 1976 Mar;116(3):647–656. [PubMed] [Google Scholar]
- McGhee J. R., Farrar J. J., Michalek S. M., Mergenhagen S. E., Rosenstreich D. L. Cellular requirements for lipopolysaccharide adjuvanticity. A role for both T lymphocytes and macrophages for in vitro responses to particulate antigens. J Exp Med. 1979 Apr 1;149(4):793–807. doi: 10.1084/jem.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- NOWOTNY A. M., THOMAS S., DURON O. S., NOWOTNY A. Relation of structure to function in bacterial O antigens. I. Isolation methods. J Bacteriol. 1963 Feb;85:418–426. doi: 10.1128/jb.85.2.418-426.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N., Mascagni P., Ribi E., Takayama K. Monophosphoryl lipid A obtained from lipopolysaccharides of Salmonella minnesota R595. Purification of the dimethyl derivative by high performance liquid chromatography and complete structural determination. J Biol Chem. 1985 May 10;260(9):5271–5278. [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Meyer K. C., Kirkland T. N., Bush C. A., Chen L., Wang R., Cotter R. J. Chemical reduction of 3-oxo and unsaturated groups in fatty acids of diphosphoryl lipid A from the lipopolysaccharide of Rhodopseudomonas sphaeroides. Comparison of biological properties before and after reduction. J Biol Chem. 1991 Apr 5;266(10):6532–6538. [PubMed] [Google Scholar]
- Ribi E. Beneficial modification of the endotoxin molecule. J Biol Response Mod. 1984;3(1):1–9. [PubMed] [Google Scholar]
- Strain S. M., Armitage I. M., Anderson L., Takayama K., Qureshi N., Raetz C. R. Location of polar substituents and fatty acyl chains on lipid A precursors from a 3-deoxy-D-manno-octulosonic acid-deficient mutant of Salmonella typhimurium. Studies by 1H, 13C, and 31P nuclear magnetic resonance. J Biol Chem. 1985 Dec 25;260(30):16089–16098. [PubMed] [Google Scholar]
- Strittmatter W., Weckesser J., Salimath P. V., Galanos C. Nontoxic lipopolysaccharide from Rhodopseudomonas sphaeroides ATCC 17023. J Bacteriol. 1983 Jul;155(1):153–158. doi: 10.1128/jb.155.1.153-158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Kotani S. Structural requirements of lipid A for endotoxicity and other biological activities. Crit Rev Microbiol. 1989;16(6):477–523. doi: 10.3109/10408418909104475. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Beutler B., Kirkland T. N. Diphosphoryl lipid A from Rhodopseudomonas sphaeroides ATCC 17023 blocks induction of cachectin in macrophages by lipopolysaccharide. Infect Immun. 1989 Apr;57(4):1336–1338. doi: 10.1128/iai.57.4.1336-1338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. E., Stashak P. W., Caldes G., Prescott B., Chused T. E., Brooks A., Baker P. J. Activation of antigen-specific suppressor T cells by B cells from mice immunized with type III pneumococcal polysaccharide. J Exp Med. 1983 Sep 1;158(3):703–717. doi: 10.1084/jem.158.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomai M. A., Solem L. E., Johnson A. G., Ribi E. The adjuvant properties of a nontoxic monophosphoryl lipid A in hyporesponsive and aging mice. J Biol Response Mod. 1987 Apr;6(2):99–107. [PubMed] [Google Scholar]


