Summary
In animal models, serotonin (5-HT) activity contributes to stress-induced changes in behavior. Syrian hamsters (Mesocricetus auratus) exhibit a stress-induced change in behavior in which social defeat results in increased submissive and defensive behavior and a complete loss of normal territorial aggression directed toward a novel, non-aggressive opponent. We refer to this defeat-induced change in agonistic behavior as conditioned defeat. In this study we tested the hypothesis that 5-HT activity in the dorsal raphe nucleus (DRN) contributes to the acquisition and expression of conditioned defeat. We investigated whether injection of the selective 5-HT1A agonist flesinoxan (200 ng, 400 ng, 800 ng in 200 nl saline) into the DRN would reduce the acquisition and expression of conditioned defeat. Additionally, we investigated whether injection of the selective 5-HT1A antagonist WAY 100635 (400 ng in 200 nl saline) into the DRN would enhance the acquisition and expression of conditioned defeat following a sub-optimal social defeat experience. We found that injection of flesinoxan into the DRN before exposure to a 15-min social defeat reduced the amount of submissive and defensive behavior shown at testing. We also found that injection of flesinoxan into the DRN before testing similarly reduced submissive and defensive behavior. In addition, we found that WAY 100635 enhanced conditioned defeat when injected either before social defeat or before testing. These data support the hypothesis that the activity of 5-HT cells in the DRN, as regulated by 5-HT1A autoreceptors, contributes to the formation and display of conditioned defeat. Further, our results suggest that 5-HT release in DRN projection regions augments defeat-induced changes in behavior.
Keywords: social defeat, conditioned defeat, stress, aggression, anxiety, fear, defensive behavior
1. Introduction
Stress is one of the foremost causal factors in the etiology of several affective disorders, including depression and anxiety (Kendler et al., 1999; Agid et al., 2000; Caspi et al., 2003). In animal models of depression and anxiety, serotonin (5-HT) is a key neurotransmitter contributing to stress-related changes in behavior (Graeff et al., 1996; Lucki, 1998; Neumaier et al., 2002; Maier and Watkins, 2005). Further, the central serotonergic system is an important target for pharmacological treatment of affective disorders (Owens and Nemeroff, 1994; Anderson and Mortimore, 1999; Blier and de Montigny, 1999). The direction of the correlation between serotonergic tone and stress-related and anxiety-like behavior, however, is unclear. The therapeutic efficacy of serotonin-selective reuptake inhibitors suggests that 5-HT reduces symptoms of anxiety and depression (Bergqvist et al., 1999; Blier and de Montigny, 1999; Vieweg et al., 2006). In contrast, data from some animal models indicate that acute stress is associated with increased 5-HT release and turnover (Matsuo et al., 1996; Rueter and Jacobs, 1996; Amat et al., 1998a), and that 5-HT enhances stress-related (Maier et al., 1995; Groenink et al., 2000) and anxiety-like behavior (File et al., 1996; Graeff et al., 1996).
The dorsal raphe nucleus (DRN) gives rise to the vast majority of 5-HT neurons innervating forebrain structures. 5-HT1A receptors are located on the soma and dendrites of DRN neurons where they function as inhibitory autoreceptors. In fact, nearly all 5-HT1A receptors in the general region of the DRN occur on the soma and dendrites of serotonergic neurons (Miquel et al., 1992). 5-HT1A receptor agonists administered into the DRN have been shown to inhibit DRN electrical activity (Sprouse and Aghajanian, 1987), 5-HT synthesis (Hamon et al., 1988), and 5-HT release in DRN projection regions (Sharp et al., 1989). However, 5-HT1A receptors are also located post-synaptically in limbic regions such as the hippocampus, amygdala, and bed nucleus of the stria terminalis where they function as heteroreceptors on non-serotonergic neurons and inhibit the release of other neurotransmitters (Barnes and Sharp, 1999). Consequently, pharmacological treatments that target 5-HT1A autoreceptors require site-specific microinjection into the DRN itself. Injection of a 5-HT1A agonist into the DRN has been reported to diminish anxiety-like behavior on the elevated plus maze (File and Gonzalez, 1996), and to block the enhanced fear conditioning and impaired escape behavior associated with learned helplessness (Maier et al., 1995). The effectiveness of 5-HT1A agonism at both training and testing indicates that elevated 5-HT activity contributes to both the formation and display of learned helplessness.
Social stress, in the form of acute social defeat or chronic subordination, activates the hypothalamic-pituitary-adrenal axis (Blanchard et al., 1995; Koolhaas et al., 1997) and produces marked behavioral changes including reduced locomotor activity (Meerlo et al., 1996; Berton et al., 1998; Rygula et al., 2005), increased depression-like and anxiety-like behavior (Heinrichs et al., 1992; Rodgers and Cole, 1993; Berton et al., 1998; Keeney et al., 2006), disrupted circadian and sleep rhythms (Harper et al., 1996; Meerlo et al., 2002), and altered feeding (Bartolomucci et al., 2004; Foster et al., 2006; Solomon et al., 2007). Social stress affects the 5-HT system as well. Chronic subordination has been shown to increase the binding capacity of 5-HT2A receptors in frontal cortex (McKittrick et al., 1995; Berton et al., 1998), whereas it down-regulates 5-HT1A receptors in the hippocampus (McKittrick et al., 1995; Flugge et al., 1998). Further, antidepressant treatment dampens the behavioral consequences of social defeat (Fuchs et al., 1996; Berton et al., 1999). 5-HT neurotransmission within the DRN may also contribute to the effects of social stress. For example, the firing rate of DRN neurons is briefly increased in tree shews producing defensive behavior during aggressive encounters (Walletschek and Raab, 1982). Further, social defeat has been shown to increase c-fos mRNA levels in the DRN (Kollack-Walker et al., 1999) and, more recently, to increase c-Fos immunoreactivity in 5-HT cells within selective subregions of the DRN (Gardner et al., 2005).
In the present study, we used a social defeat model in Syrian hamsters called conditioned defeat (Potegal et al., 1993; Huhman et al., 2003). In this model, hamsters are exposed to a single social defeat in the home cage of a larger, more aggressive individual. Later, hamsters are tested in their own home cage with a smaller, non-aggressive opponent where they display an array of submissive behaviors and defensive postures instead of their normal territorial aggression. The aim of the present study was to determine whether 5-HT activity in the DRN contributes to either the formation or the display of conditioned defeat. We approached this question by using microinjections of selective 5-HT1A agonists and antagonists into the DRN either before initial social defeat training or before conditioned defeat testing.
2. Methods
2.1. Subjects
We used male Syrian hamsters (Mesocricetus auratus) that weighed 120–140 g (3–4 months) at the start of the study, and individually housed them for 10–14 days prior to testing. Older hamsters that weighed 160–180 g (> 6 months) were housed individually and used as resident aggressors for social defeat training. Younger hamsters that weighed 90–110 g (~ 2 months) were group-housed (5 per cage) and used as non-aggressive opponents for conditioned defeat testing. All animals were housed in polycarbonate cages (20 × 40 × 20 cm) with corncob bedding, cotton nesting materials, and wire mesh tops. Animal cages were not changed for one week prior to testing to allow individuals to scent mark their territory. Animals were housed in a temperature-controlled colony room (20 ± 2 °C) and maintained on a 14:10 h light-dark cycle with food and water available ad libitum. All procedures were approved by the Georgia State University Animal Care and Use Committee, and are in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Stereotaxic Surgery
Hamsters were anesthetized with sodium pentobarbital (90 mg/kg) and stereotaxically implanted with a 26-gauge guide cannula aimed at the DRN. Lambda and bregma were leveled prior to guide cannula implantation. The angle of approach was 20° from vertical to avoid penetrating the 4th ventricle, and the stereotaxic coordinates were 4.5 mm posterior to bregma, 1.8 mm lateral to bregma, and 2.3 mm below dura. These coordinates aimed the guide cannula toward the midpoint of the rostral-caudal extent of the DRN. Also, the tip of the guide cannula was dorsal to the DRN to avoid damaging the nucleus and lateral to the DRN to avoid penetrating the 4th ventricle. Later, a 33-gauge injection needle was inserted that projected 3 mm below the guide cannula for a final depth of 5.3 mm below dura. After surgery, dummy stylets were placed in the guide cannula to help prevent clogging. All animals were given 10–14 days to recover from surgery before behavioral experiments. Hamsters were handled daily following surgery by gently restraining them and removing and replacing the dummy stylet in order to habituate them to the experimental procedure.
2.3. Conditioned Defeat Protocol
Social defeat training consisted of either one 15-min or one 5-min encounter with a resident aggressor in the aggressor’s home-cage. Sub-optimal 5-min aggressive encounters were used to avoid a ceiling effect in experiments in which we expected the treatment to increase conditioned defeat. Resident aggressors reliably attacked and defeated the experimental hamsters. To equalize the duration of social defeat, timing of aggressive encounters began at the first attack by the resident aggressor. Attacks usually occurred within the first 30 s of the encounter. Any hamster bitten such that it bled was removed from the study and examined by a veterinarian, and thus 5 of 290 animals were excluded due to wounding. During social defeat training we recorded the total duration of aggression displayed by the resident aggressor, the number of attacks, and the total duration of submissive and defensive behavior displayed by the experimental subjects. To investigate whether drug treatments affected agonistic behavior in the absence of social defeat experience, we included no defeat control groups that were exposed to a resident aggressor’s empty cage. We performed all training and subsequent testing under dim red light during the first 3 h of the dark phase of the light-dark cycle.
Behavioral testing occurred 24 h after training and consisted of one, 5-min encounter with a novel, non-aggressive opponent in the subject’s home cage. Testing sessions were later scored by a researcher blind to the experimental conditions using behavioral definitions adapted from Albers et al. (2002). We recorded the total duration of four classes of behavior during the 5-min tests: (a) social (attend, approach, investigate, sniff, nose touch, and flank mark); (b) nonsocial (locomotion, exploration, self-groom, nest build, feed, and sleep); (c) submissive and defensive (flight, avoid, tail up, upright and side defense, full submissive posture, stretch-attend, head flag, attempt to escape from cage); and (d) aggressive (upright and side offense, chase, and attack including bite). For a more detailed analysis of the subject’s agonistic behavior, we also recorded the frequency of flight, stretch-attend, and attack. A second researcher separately scored a subset of testing sessions, and inter-rater reliability was 93% with r = .98.
2.4. Drugs
Flesinoxan-hydrochloride (courtesy of Solvay Pharmaceuticals, Weesp, The Netherlands) was dissolved in sterile saline with a final pH of 5.5 (see Groenink et al., 2000). Flesinoxan is a highly selective agonist at the 5-HT1A receptor (Schoeffter and Hoyer, 1988). WAY 100635 (Sigma) was also dissolved in sterile saline with a final pH of 7.4. WAY 100635 is regarded as a highly selective 5-HT1A receptor antagonist (Mos et al., 1997).
2.5. Experiments 1 and 2: 5-HT1A Receptor Agonist
We designed Experiment 1 to test whether injection of a selective 5-HT1A receptor agonist into the DRN would reduce the acquisition of conditioned defeat. We infused flesinoxan (200 ng, 400 ng, or 800 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN 10 min prior to a 15-min social defeat. For no defeat controls, we infused flesinoxan (800 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN 10 min prior to exposure to a resident aggressor’s empty cage. We performed infusions with a 1 µl Hamilton syringe connected to a 33-gauge needle via polyethylene tubing. The syringe was mounted onto a syringe pump (Harvard apparatus PHD 2000, Natick, MA), programmed to infuse 200 nl per min. The needle remained in place for an additional 1 min to allow diffusion of the solution. An air bubble separated water in the tubing from the drug solution, and movement of the air bubble down the tubing indicated a successful injection. Animals were tested for conditioned defeat 24 h later as described above.
We designed Experiment 2 to test whether injection of a selective 5-HT1A receptor agonist into the DRN would reduce the expression of conditioned defeat. Hamsters experienced a 15-min social defeat, and we infused flesinoxan (200 ng, 400 ng, or 800 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN on the next day 10 min prior to conditioned defeat testing. Likewise, no defeat controls received exposure to a resident aggressor’s empty cage instead of social defeat training, and we infused flesinoxan (800 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN on the next day 10 min prior to conditioned defeat testing.
2.6. Carry-Over Control
The effect of flesinoxan on the acquisition of conditioned defeat could be due to its effect on expression (i.e., a carry-over effect) if the drug is biologically active 24 h later at behavioral testing. In a carry-over control experiment, we tested whether injection of flesinoxan into the DRN 4 h after social defeat would reduce the expression of conditioned defeat. We performed injections 4 h after social defeat because it was a time point that we supposed would be outside the consolidation time window for conditioned defeat. At training, animals experienced a 15-min social defeat. Four hours later, we infused flesinoxan (800 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN and the next day animals were tested for conditioned defeat.
2.7. Experiments 3 and 4: 5-HT1A Receptor Antagonist
We designed Experiment 3 to test whether injection of a selective 5-HT1A receptor antagonist into the DRN would enhance the acquisition of conditioned defeat. We infused WAY 100635 (400 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN 10 min prior to a 5-min social defeat and 24 h later tested animals for conditioned defeat. The dose of WAY 100635 used here was established in a pilot study. Similarly, we designed Experiment 4 to test whether injection of a selective 5-HT1A receptor antagonist into the DRN would enhance the expression of conditioned defeat. In this case, hamsters experienced a 5-min social defeat, and we infused WAY 100635 (400 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN on the next day 10 min prior to conditioned defeat testing. Also, we exposed no defeat controls to a resident aggressor’s empty cage for 5 min and the next day infused WAY 100635 (400 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN 10 min prior to testing. No defeat controls were not included with Experiment 3 to reduce the number of animals required.
2.8. Histology
Following each experiment, hamsters were given a lethal dose of sodium pentobarbital and infused with 200 nl of India ink to verify the placement of injections. Brains were removed, frozen on dry ice, and stored at −80°C. Later, brains were sliced at 30 µm on a cryostat, and sections were stained with neutral red and coverslipped with DPX mountant. Brain sections were examined under a light microscope for evidence of ink in the DRN. Only hamsters with ink injections within 200 µm from the DRN were included in the data analysis (see Figure 1). Hamsters with ink injections further than 200 µm from the DRN were used as anatomical controls.
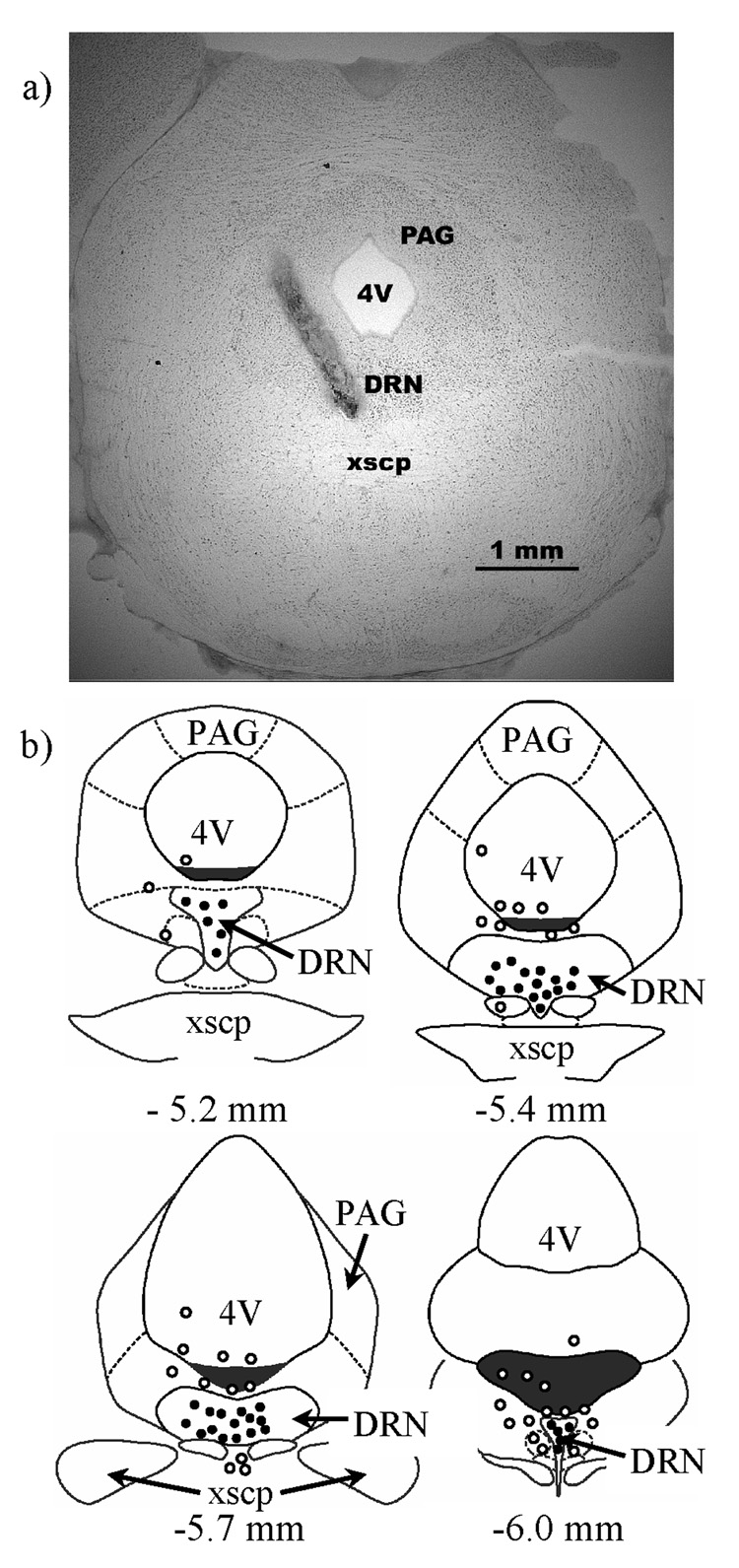
Figure 1.
The location of DRN injection sites are shown. a) A representative photomicrograph is shown of a hamster coronal brain section injected with India ink and stained with neutral red. The needle tract and ink injection are clearly visible and indicate an injection site approximately 5.4 mm behind bregma. b) Schematic representations are shown of hamster coronal brain sections adapted from the atlas of Morin and Wood (2001), and coordinates are reported from bregma. The illustrations represent injections from all four experiments and circles indicate the location of multiple injections. Black circles indicate the approximate site of accurately placed injections within the DRN, and open circles indicate injections placed outside the DRN. DRN – dorsal raphe nucleus, 4V – fourth ventricle, PAG – periaqueductal grey, xscp – decussation of the superior cerebellar peduncle.
2.9. Data Analysis
Total durations (sec) of submissive and defensive, social, nonsocial, and aggressive behavior were analyzed separately using either independent sample T-tests or between-subjects ANOVAs. Likewise, frequencies of attack, flight, and stretch-attend were analyzed separately using similar statistical tests. For no defeat controls in Experiments 1 and 2, data were analyzed using two-way ANOVAs to test for an interaction between social defeat and drug treatment. Tukey tests were used for pairwise comparisons when necessary. All comparisons were two-tailed and the alpha level was p < 0.05. Data are presented as mean ± SE.
3. Results
3.1. Experiment 1: Flesinoxan and Acquisition of Conditioned Defeat
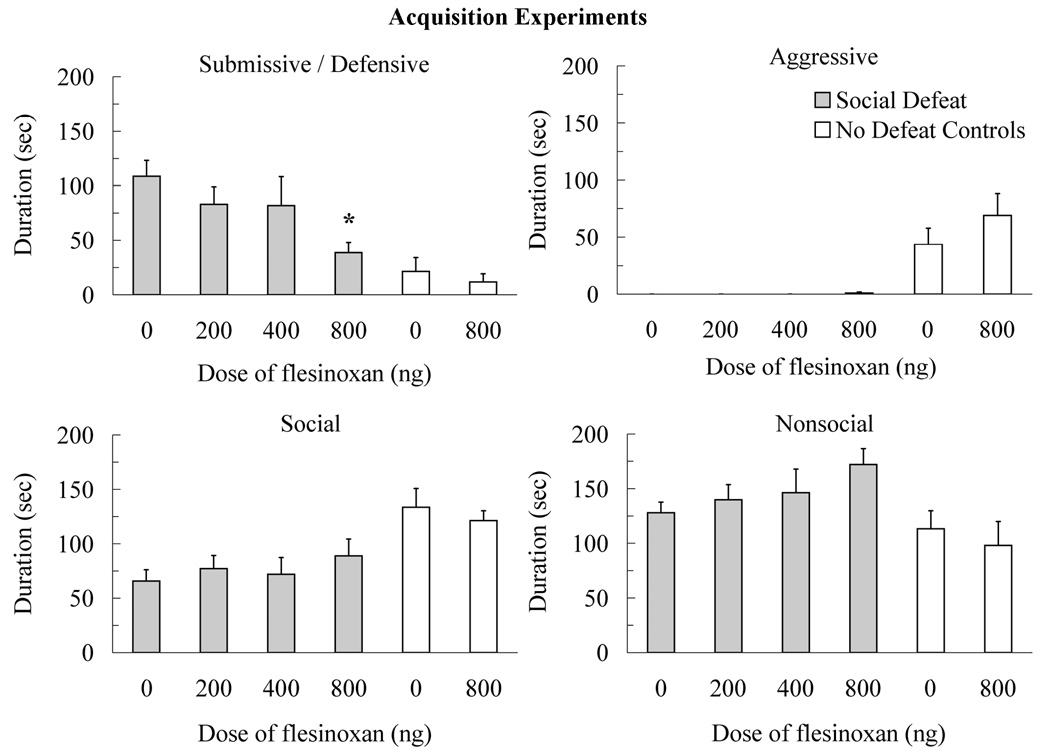
The injection of flesinoxan into the DRN prior to social defeat reduced the acquisition of conditioned defeat (Figure 2). Animals injected with 800 ng of flesinoxan into the DRN prior to social defeat showed a reduced duration of submissive and defensive behavior at testing compared to vehicle controls (F(3,38) = 2.93, p < 0.05, Tukey, p < 0.05). Similarly, the 800 ng dose of flesinoxan reduced the frequency of flight (F(3,38) = 3.13, p < 0.05) and stretch attend postures (F(3,38) = 5.02, p < 0.01) compared to vehicle controls (Table 1). Also, individuals injected with flesinoxan prior to social defeat did not show changes in the duration of other classes of behavior such as social (F(3,38) = 0.53, p > 0.05), nonsocial (F(3,38) = 1.48, p > 0.05), and aggressive (F(3,38) = 0.94, p > 0.05).
Figure 2.
Durations (mean ± SE) of submissive and defensive, aggressive, social, and nonsocial behavior are shown for a 5-min test with a novel, non-aggressive opponent. Defeated animals received an injection of flesinoxan (200 ng, N = 11; 400 ng, N = 10; 800 ng, N =11) or vehicle (N = 10) into the dorsal raphe nucleus (DRN) 10 min before defeat training. Likewise, no defeat controls received an injection of flesinoxan (800 ng, N = 10) or vehicle (N = 9) into the DRN 10 min before exposure to a resident aggressor’s empty cage. These data demonstrate reduced submissive and defensive behavior with increasing doses of flesinoxan given prior to training (asterisk indicates p < 0.05 compared to vehicle controls). Flesinoxan given prior to defeat training did not alter the behavior of no defeat controls. See the text for significant differences between defeated animals and no defeat controls.
Table 1.
Frequency of Flight and Stretch Attend Postures (Mean ± SE)
| Vehicle | 200 ng | 400ng | 800ng | p | |
|---|---|---|---|---|---|
| Experiment 1: Flesinoxan and Acquisition | |||||
| Flight | 9.0 ± 2.0 | 7.2 ± 1.6 | 7.9 ± 2.6 | 1.9 ± .79* | .037 |
| Stretch Attend | 3.3 ± .67 | 3.2 ± .63 | 2.2 ± .49 | 0.7 ± .30* | .005 |
| Experiment 2: Flesinoxan and Expression | |||||
| Flight | 7.2 ± 1.3 | 5.1 ± 1.3 | 4.4 ± 1.2 | 2.2 ± 1.0* | .049 |
| Stretch Attend | 5.3 ± .94 | 2.9 ± .55 | 2.8 ± .76 | 1.2 ± .57* | .004 |
| Experiment 3: WAY100635 and Acquisition | |||||
| Flight | 3.1 ± 1.3 | 8.7 ± 2.3 | .05 | ||
| Stretch Attend | 2.1 ± .57 | 3.4 ± .71 | ns | ||
| Experiment 4: WAY100635 and Expression | |||||
| Flight | 2.1 ± .82 | 8.6 ± 1.5 | .002 | ||
| Stretch Attend | 1.8 ± .74 | 2.8 ± .49 | ns | ||
Only one dose of WAY100635 (400 ng) was tested in Experiments 3 and 4.
indicates significantly different from vehicle controls (Tukey, p ≤ .05).
No defeat controls did not show elevated submissive and defensive behavior at testing, and injection of flesinoxan prior to training did not alter their behavior (Figure 2). Specifically, an interaction existed between social defeat and drug treatment for submissive and defensive behavior (F(3,36) = 7.18, p < 0.05), which indicated that flesinoxan reduced submissive and defensive behavior but the effect was restricted to socially defeated animals only. Likewise, no defeat controls showed more aggressive behavior (F(3,36) = 18.69, p < 0.01), more social behavior (F(3,36) = 13.95, p < 0.01), and less nonsocial behavior (F(3,36) = 7.43, p < 0.01) at testing than did defeated animals. Also, no defeat controls injected with flesinoxan (4.9 attacks ± 1.7) or vehicle (3.0 attacks ± 1.7) attacked the non-aggressive opponent at testing whereas defeated animals did not. Statistical analysis indicated that no defeat controls initiated more attacks at testing than did defeated animals (F(3,36) = 11.97, p < 0.01) and there was no interaction with drug treatment (F(3,36) = 0.69, p > .05).
Injection of flesinoxan into the DRN prior to social defeat did not alter the level of aggression shown by resident aggressors or the level of submission shown by subjects during social defeat training. Vehicle controls were the target of 297.9 sec (± 33.3) of aggression during social defeat and individuals injected with 200 ng, 400 ng, and 800 ng of flesinoxan received 344.1 (± 28.9), 296.0 (± 28.4), 327.4 (± 35.0) sec, respectively (F(3,38) = 0.55, p > 0.05). Also, vehicle controls received 13.3 (± 1.4) attacks during social defeat and individuals injected with 200 ng, 400 ng, and 800 ng of flesinoxan received 15.8 (± 1.2), 13.7 (± 0.9), 16.2 (± 1.3) attacks, respectively (F(3,38) = 1.41, p > 0.05). Further, vehicle controls responded to attacks with 506.1 (± 29.3) sec of submissive and defensive behavior, while individuals injected with 200 ng, 400 ng, and 800 ng of flesinoxan displayed 524.1 (± 25.7), 542.9 (± 31.2), 523.0 (± 31.9) sec, respectively (F(3,38) = 0.25, p > 0.05).
Nineteen animals had injections over 200 µm from the DRN and were used as anatomical controls. Most often anatomical controls had injection placements inside the fourth ventricle, but some animals had injections within the trochlear nucleus, lateral and ventrolateral periaqueductal grey, dorsal tegmental nucleus, and rhabdoid nucleus. Anatomical controls failed to show a reduction in the acquisition of conditioned defeat. Specficially, the duration of submissive and defensive behavior at testing was not significantly different between vehicle controls (99.8 ± 30.0, N = 7) and individuals injected with 200 ng (69.3 ± 45.8, N = 3), 400 ng (88.6 ± 33.5, N = 5), and 800 ng (131.2 ± 29.7, N = 4) of flesinoxan outside the DRN (F(3,15) = 0.44, p > 0.05).
3.2. Experiment 2: Flesinoxan and Expression of Conditioned Defeat
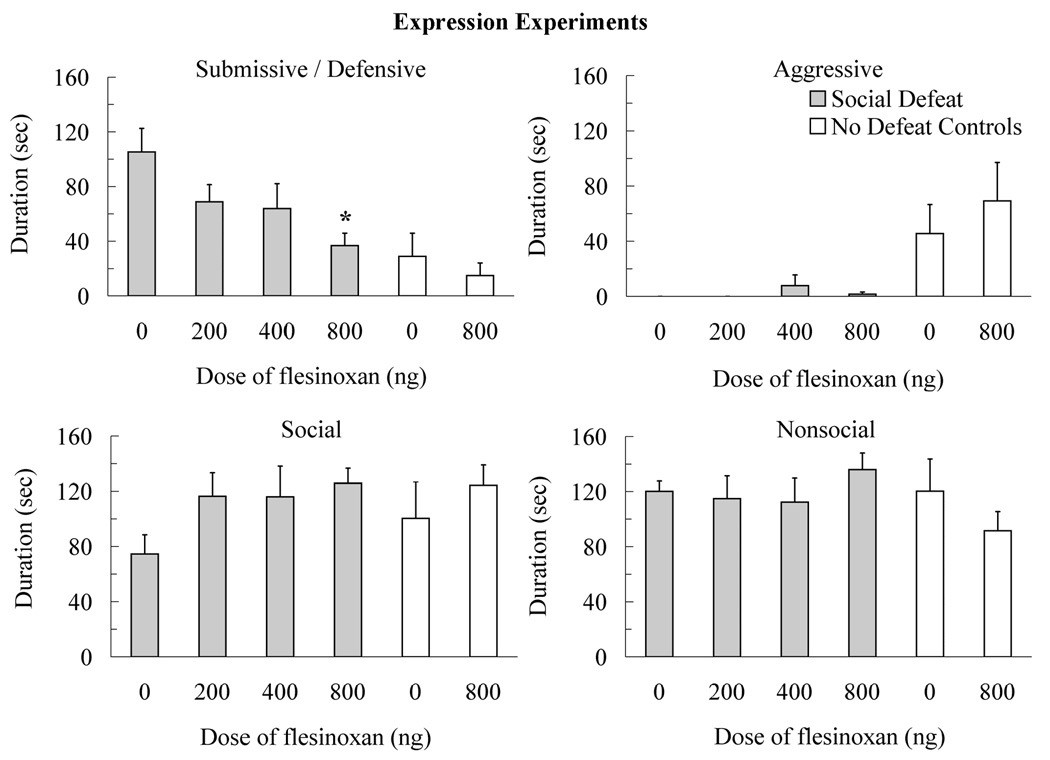
The injection of flesinoxan into the DRN prior to testing also reduced the expression of conditioned defeat (Figure 3). Animals injected with 800 ng of flesinoxan into the DRN prior to testing showed a reduced duration of submissive and defensive behavior compared to vehicle controls (F(3,31) = 3.67, p < 0.05, Tukey, p < 0.05). Similarly, 800 ng of flesinoxan reduced the frequency of flight (F(3,31) = 2.92, p < 0.05) and stretch attend postures (F(3,31) = 5.51, p < 0.01) compared to vehicle controls (Table 1). Also, animals injected with flesinoxan into the DRN prior to testing did not show differences in the duration of other classes of behavior such as social (F(3,31) = 1.93, p > 0.05), nonsocial (F(3,31) = 0.59, p > 0.05), and aggressive (F(3,31) = 0.83, p > 0.05).
Figure 3.
Durations (mean ± SE) of submissive and defensive, aggressive, social, and nonsocial behavior are shown for a 5-min test with a novel, non-aggressive opponent. Defeated animals received an injection of flesinoxan (200 ng, N = 8; 400 ng, N = 9; 800 ng, N = 9) or vehicle (N = 9) into the dorsal raphe nucleus (DRN) 10 min before conditioned defeat testing. Likewise, no defeat controls received an injection of flesinoxan (800 ng, N = 8) or vehicle (N = 8) into the DRN 10 min before testing. These data demonstrate reduced submissive and defensive behavior with increasing doses of flesinoxan given prior to testing (asterisk indicates p < 0.05 compared to vehicle controls). Flesinoxan given prior to testing did not alter the behavior of no defeat controls. See the text for significant differences between defeated animals and no defeat controls.
No defeat controls showed territorial aggression rather than conditioned defeat, and injection of flesinoxan prior to testing did not alter their behavior (Figure 3). A significant interaction between social defeat and drug treatment indicated that flesinoxan reduced submissive and defensive behavior in defeated animals only (F(3,30) = 5.19, p < 0.05). Also, no defeat controls showed and more aggressive behavior (F(3,30) = 11.83, p < 0.01) than did defeated animals, but they did not significantly differ from defeated animals in the duration of social (F(3,30) = 0.51, p > 0.05) or nonsocial behavior (F(3,30) = 2.21, p > 0.05). Similarly, no defeat controls attacked non-aggressive opponents at testing more often than did defeated animals (6.6 ± 1.9 and 0.0 ± 0.0, respectively; F(3,30) = 12.12, p < 0.01) and there was no interaction with drug treatment (F(3,30) = 0.09, p > 0.05).
Twenty animals had injections over 200 µm from the DRN. These animals were used as anatomical controls and most often had injection placements inside the fourth ventricle, but some animals had injections within the lateral and ventrolateral periaqueductal grey, dorsal tegmental nucleus, rhabdoid nucleus, and medial longitudinal fasciculus. Anatomical controls did not show a reduction in the expression of conditioned defeat since vehicle controls (104.1 ± 18.5, N = 7) and individuals injected with 200 ng (73.7 ± 18.2, N = 4), 400 ng (66.0 ± 25.2, N = 4), and 800 ng (105.7 ± 23.3, N = 5) of flesinoxan did not significantly differ in the duration of submissive and defensive behavior at testing (F(3,16) = 0.89, p > 0.05). In the carry-over control experiment, we investigated whether flesinoxan could affect the expression of conditioned defeated defeat when administered a day prior to testing. We found that animals injected with 800 ng of flesinoxan into the DRN 4 h after social defeat did not show a decrease in submissive and defensive behavior compared to vehicle controls (91.6 ± 15.7, N = 10; 114.9 ± 12.1, N = 9, respectively; t(17) = 1.16, p > 0.05).
3.3. Experiment 3: WAY 100635 and Acquisition of Conditioned Defeat
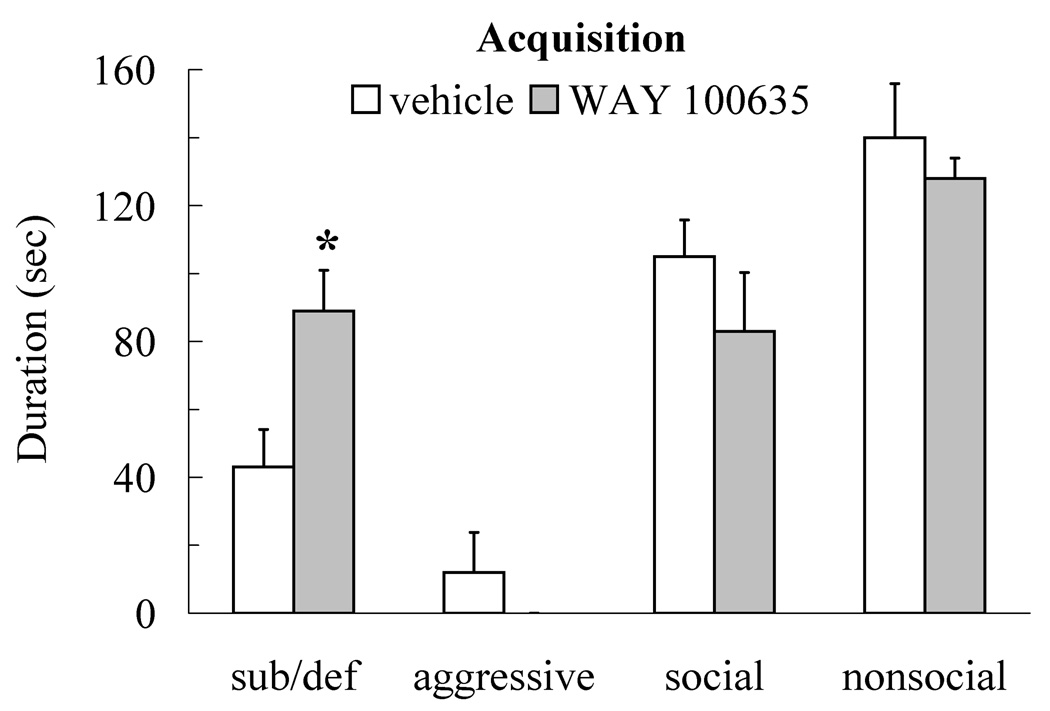
Injection of WAY 100635 into the DRN prior to social defeat enhanced the acquisition of conditioned defeat following a sub-optimal 5-min defeat experience (Figure 4). Individuals injected with 400 ng prior to social defeat showed increased submissive and defensive behavior at testing compared to vehicle controls (t(19) = 2.73, p < 0.05), but they did not differ from controls in social (t(19) = 1.07, p > 0.05), nonsocial (t(19) = 0.64, p > 0.05), or aggressive behavior (t(19) = 1.05, p > 0.05). Individuals injected with 400 ng fled from non-aggressive opponents at testing more often than did vehicle controls (t(19) = 2.13, p < 0.05; Table 1), although they did not differ from vehicle controls in the frequency of stretch attend posture (t(19) = 1.48, p > 0.05; Table 1). The accuracy of our DRN cannulations improved in Experiment 3 and anatomical controls were unavailable.
Figure 4.
Durations (mean ± SE) of submissive and defensive (sub/def), aggressive, social, and nonsocial behavior are shown for a 5-min test with a novel, non-aggressive opponent. Animals received an injection of WAY 100635 (400 ng, N = 11) or vehicle (N = 10) into the dorsal raphe nucleus 10 min before sub-optimal social defeat training. Administration of WAY 100635 prior to social defeat significantly increased submissive and defensive behavior compared to vehicle controls (asterisk indicates p < 0.05).
Injection of WAY 100635 into the DRN prior to social defeat did not alter the aggressive behavior of resident aggressors or the submissive behavior of subjects during social defeat training. Specifically, vehicle and WAY 100635 animals did not significantly differ in the duration of aggression received from the resident aggressor at training (113.4 ± 13.7 and 110.4 ± 10.4, respectively; t(19) = 0.17, p > 0.05) or the number of attacks received (9.9 ± 1.8 and 8.3 ± 1.6, respectively; t(19) = 0.68, p > 0.05). Further, vehicle and WAY 100635 animals did not significantly differ in the duration of submissive and defensive behavior displayed during attack by the resident aggressor (201.3 ± 15.1 and 204.2 ± 8.8, respectively; t(19) = 0.19, p > 0.05).
3.4. Experiment 4: WAY 100635 and Expression of Conditioned Defeat
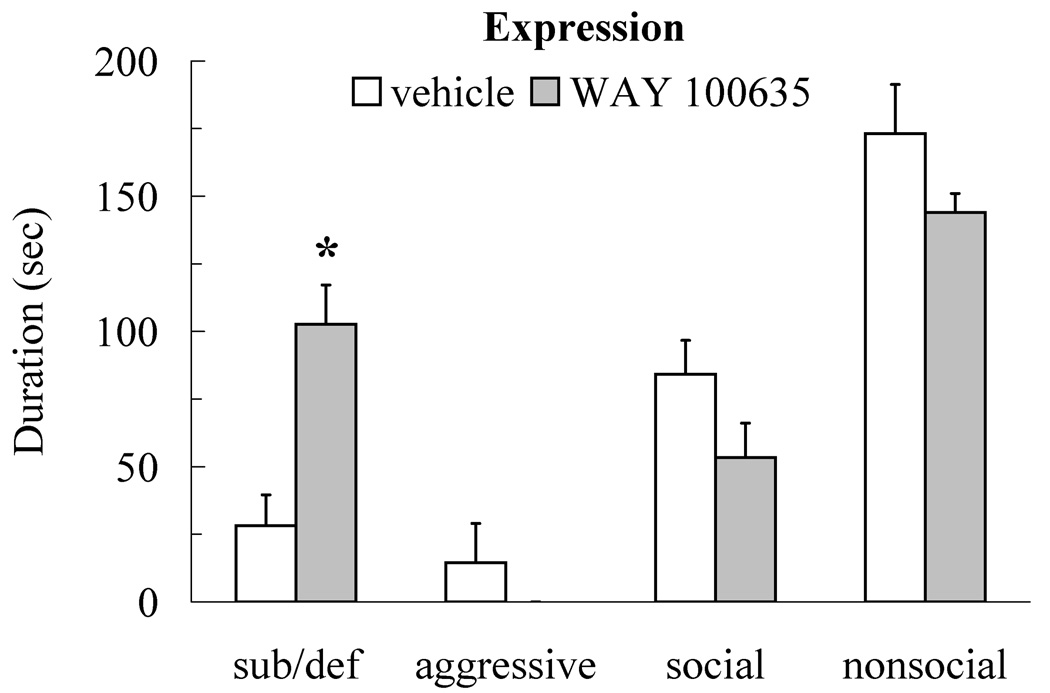
Injection of WAY 100635 into the DRN prior to testing enhanced the expression of conditioned defeat (Figure 5). Individuals that received 400 ng of WAY 100635 showed an increased duration of submissive and defensive behavior at testing compared to vehicle controls (t(17) = 3.96, p < 0.01) but they did not differ from controls in social (t(17) = 1.72, p > 0.05), nonsocial (t(17) = 1.58, p > 0.05), or aggressive behavior (t(17) = 1.06, p > 0.05). Individuals injected with 400 ng of WAY 100635 also fled from non-aggressive opponents at testing more frequently than did vehicle controls (t(17) = 3.75, p < 0.01; Table 1), although they did not significantly differ from vehicle controls in stretch attend posture (t(17) = 1.17, p > 0.05; Table 1).
Figure 5.
Durations (mean ± SE) of submissive and defensive (sub/def), aggressive, social, and nonsocial behavior are shown for sub-optimally defeated animals during a 5-min test with a novel, non-aggressive opponent. Animals received an injection of WAY 100635 (400 ng, N = 10) or vehicle (N = 9) into the dorsal raphe nucleus 10 min before conditioned defeat testing. Injection of WAY 100635 prior to testing significantly increased submissive and defensive behavior compared to vehicle controls (asterisk indicates p < 0.05).
Sixteen animals had injections over 200 µm from the DRN and served as anatomical controls. Injection sites for this set of anatomical controls were similar to those in Experiments 1 and 2. Injection of WAY 100635 (400ng) outside the DRN did not significantly increase the duration of submissive and defensive behavior shown at conditioned defeat testing compared to vehicle controls (32.0 ± 11.3, N = 9 and 40.7 ± 17.4, N = 7 respectively; t(14) = 0.44, p > 0.05).
We also included no defeat controls in Experiment 4 and found that injection of WAY 100635 prior to testing did not alter agonistic behavior (Table 2). Specifically, no defeat controls that received injection of WAY 100635 (400ng) into the DRN did not significantly differ from vehicle controls in the duration of submissive and defensive (t(15) = 0.86, p > 0.05), aggression (t(15) = 0.46, p > 0.05), social (t(15) = 1.69, p > 0.05), and nonsocial behavior (t(15) = 1.34, p > 0.05) shown during testing. Also, they did not significantly differ from vehicle controls in the frequency of attacks directed toward novel opponents during testing (t(15) = 0.75, p > 0.05).
Table 2.
Experiment 4: no defeat controls
| Vehicle | 400ng | p | |
|---|---|---|---|
| Sub/Def | 13.7 ± 8.5 | 6.1 ± 3.4 | ns |
| Aggressive | 32.4 ± 13.4 | 43.6 ± 20.0 | ns |
| Social | 154.9 ± 16.6 | 115.7 ± 16.4 | ns |
| Nonsocial | 99.1 ± 15.8 | 134.5 ± 20.6 | ns |
| Attack | 2.3 ± 0.9 | 3.8 ± 1.8 | ns |
No defeat controls received WAY 100635 (400ng, N = 9) or vehicle (N = 8) into the dorsal raphe nucleus 10 min prior to testing (Mean ± SE). Sub/def – submissive/defensive.
4. Discussion
The results of the present experiments strongly support the hypothesis that the activity of 5-HT cells in the DRN modulates the formation and display of stress-induced changes in social behavior. We found that injection of flesinoxan, a selective 5-HT1A agonist, into the DRN prior to defeat training reduces the acquisition of conditioned defeat and that a similar injection given prior to testing reduces the expression of conditioned defeat. These behavioral effects are very likely to have been produced by activation of somatodendritic 5-HT1A autoreceptors in the DRN, and autoreceptor activation has been shown to inhibit the firing of DRN neurons and reduce 5-HT release in DRN projection regions (Sprouse and Aghajanian, 1987; Sharp et al., 1989). Our results are strengthened by the finding that injection of WAY 100635 into the DRN, and likely blockade of 5-HT1A autoreceptors, enhances the acquisition and expression of conditioned defeat. The results of our carry-over control experiment indicate that the effect of flesinoxan on the acquisition of conditioned defeat is not due to residual drug still biologically active at testing. Also, our data for no defeat controls indicate that both flesinoxan and WAY 10635 were unable to alter agonistic behavior in the absence of prior social defeat and thus primarily affect the submissive and defensive behavior of defeated animals.
Our findings compliment those showing a critical role of 5-HT in the control of learned helplessness. Maier and colleagues have proposed that increased 5-HT release in DRN projection regions at the time of testing mediates the enhanced fear conditioning and impaired escape behavior that occur following inescapable shock (Maier et al., 1995; Maier and Watkins, 2005). Further, Maier and colleagues have suggested that exaggerated 5-HT release by the DRN during inescapable shock training sensitizes the DRN and leads to an exaggerated serotonergic response during subsequent escape testing and fear conditioning (Maier et al., 1995; Greenwood et al., 2003). Several types of evidence support Maier’s model. Injection of the 5-HT1A agonist 8-hydroxy-2-(di-η-propylamino)tetralin (8-OH-DPAT) into the DRN prior to training or prior to testing has been shown to block the behavioral effects of inescapable shock (Maier et al., 1995). Inescapable shock has also been shown to increase c-Fos immunoreactivity in select DRN subregions (Grahn et al., 1999), as well as 5-HT efflux in the DRN (Maswood et al., 1998), ventral hippocampus (Amat et al., 1998b), basolateral amygdala (Amat et al., 1998a), and medial prefrontal cortex (Bland et al., 2003). Further, freewheel running up-regulates 5-HT1A receptor mRNA in the DRN and reduces vulnerability to learned helplessness (Greenwood et al., 2003; Greenwood et al., 2005). It may be that social defeat similarly sensitizes the DRN and leads to an exaggerated serotonergic response to the presence of the non-threatening opponent at testing, and the effect of flesinoxan and WAY 100635 on the firing of DRN neurons would support this hypothesis. However, 5-HT1a autoreceptors are prone to rapid desensitization (Riad et al., 2004), and if flesinoxan desensitized 5-HT1A autoreceptors in our experiments it could have reduced conditioned defeat by augmenting 5-HT release, especially in animals tested 24 h later. Although we can not rule out the possibility of acute flesinoxan treatment down-regulating 5-HT1A autoreceptors in our experiments, a recent study found that chronic flesinoxan treatment administered peripherally did not desensitize 5-HT1A autoreceptors, whereas a higher affinity 5-HT1A receptor agonist did (Assie et al., 2006).
Although Maier’s model states that increased 5-HT release in DRN projection regions underlies the behavioral consequences of uncontrollable stress, the efficacy of SSRI’s suggests that underactivation of serotonergic function is associated with depression and anxiety disorders (Ressler and Nemeroff, 2000). Reconciling the evidence implicating 5-HT in depression, anxiety and stress-related behavior will likely require an improved understanding of the net effects of 5-HT release in DRN projection regions. For instance, activation of 5-HT1A receptors in forebrain regions has been shown to reduce shock-induced ultrasonic vocalizations (Schreiber and De Vry, 1993), fear conditioning (Li et al., 2006), fear potentiated startle (Groenink et al., 2000), and learned helplessness (Martin et al., 1990; Martin et al., 1991). In contrast, activation of 5-HT2 receptors has been shown to increase anxiety-like behavior on the social interaction test (Bagdy et al., 2001), the open field test (Campbell and Merchant, 2003), and following ethanol withdrawal (Overstreet et al., 2006). The role of 5-HT in anxiety-like and stress-related behavior likely depends on the selective release of 5-HT in specific brain regions and the distribution of 5-HT receptor subtypes localized in those areas.
Graeff and colleagues have used a T-maze test to investigate the differential contributions of the serotonergic system to conditioned defensive responses, as indicated by inhibitory avoidance on the T-maze, and to unconditioned defensive responses, as indicated by one-way escape (Graeff et al., 1997). They have proposed a model in which two separate 5-HT systems regulate defensive behavior. The model contains an ascending DRN serotonergic pathway that innervates the amygdala and facilitates defensive responses to cued, distal threats, and a DRN serotonergic pathway that innervates the dorsal periaqueductal grey (dPAG) and inhibits innate flight reactions in response to proximal danger. In support of Graeff’s model, Sena et al. (2003) found that treatments that decrease 5-HT activity in the DRN, such as intra-DRN injection of 8-OH-DPAT, impair inhibitory avoidance while they facilitate one-way escape on the T-maze. Graeff’s model is consistent with our hypothesis that 5-HT modulates the acquisition and expression of conditioned defeat by activating basolateral amygdala circuits known to underlie conditioned fear (Jasnow et al., 2004; Jasnow et al., 2005). Nevertheless, it is possible that our pharmacological treatments might produce an effect via the DRN-dPAG serotonergic pathway. For several reasons, however, it seems unlikely that our treatments altered serotonin release in the dPAG or that the drugs diffused into the dPAG, itself. Our anatomical controls included individuals with PAG and fourth ventricle injection sites, and they were ineffective. Also, the unconditioned defensive responses associated with the DRN-dPAG pathway are mediated by 5-HT2 receptors in the dPAG (Oliveira et al., 2007). Further, if our results were mediated by the DRN-dPAG serotonergic pathway, Graeff’s model would have predicted the opposite changes in submissive and defensive behavior.
The elevated T-maze measures conditioned fear as defensive responses which require behavioral inhibition, whereas our conditioned defeat model is characterized by an increase in active responses such as flight and stretch-attend posture. Flight is a well-characterized defensive avoidance response, whereas stretch-attend posture is a measure of risk assessment that appears analogous to defensive approach (Blanchard and Blanchard, 1989). McNaughton and Corr (2004) have proposed that defensive avoidance and defensive approach are controlled by separate neural circuits and suggest that they are related to fear and anxiety, respectively. We found that intra-DRN injection of flesinoxan reduced both the frequency of flight and stretch-attend posture at testing. Our results suggest that the activation of 5-HT1A autoreceptors reduces both defensive avoidance and defensive approach and are consistent with McNaughton and Corr’s (2004) model which claims that 5-HT innervates the entire defense system. We also found that blockade of 5-HT1A autoreceptors is sufficient to modulate fleeing from a non-aggressive opponent, although the lack of effect on stretch-attend posture should be taken with caution since we used only a single dose of WAY 100635. Interestingly, we have previously shown that blockade of corticotropin-releasing factor (CRF) receptors in the DRN affects flight but not stretch-attend posture suggesting that CRF neurotransmission in the DRN may activate a subset of 5-HT neurons that selectively modulate defensive avoidance (Cooper and Huhman, 2007).
Low 5-HT activity is most often thought to promote aggression. In Syrian hamsters, serotonin inhibits vasopressin-facilitated aggression by activating 5-HT1A receptors in the anterior hypothalamus, and DRN neurons account for at least part of the serotonergic innervation into the anterior hypothalamus (Ferris et al., 1999). One possible explanation for our results is that flesinoxan injection into the DRN altered aggression and only indirectly affected submissive and defensive behavior. This is improbable, however, because flesinoxan in the DRN did not reinstate aggression following social defeat. In contrast to the prevailing view of 5-HT and aggression, there are data indicating that injection of a 5-HT1A agonist into the DRN can reduce aggression in rat resident-intruder tests (Mos et al., 1993). Similarly, elevated c-Fos expression has been found in 5-HT-positive DRN neurons in highly aggressive rats following a fight, suggesting that aggression is associated with a brief activation of serotonergic neurotransmission (van der Vegt et al., 2003). If increased 5-HT activity in the DRN facilitates aggression in our model, then we might have expected WAY 100635 to reinstate aggression following social defeat. The data for WAY 100635, however, were in the opposite direction. Furthermore, both flesinoxan and WAY 100635 failed to alter agonistic behavior in no defeat controls suggesting that in our model neither activation nor blockade of 5-HT1A receptors in the DRN modulates agonistic behavior in the absence of social defeat. Thus, our data support the hypothesis that 5-HT neurons in the DRN are a critical component of the neural circuitry underlying conditioned defeat because they are part of the circuitry modulating fear and defensive behavior, and not because they modulate aggression.
In summary, our data indicate that the activity of 5-HT neurons in the DRN contributes to both the acquisition and expression of conditioned defeat. We found that activation of 5-HT1A autoreceptors in the DRN during both social defeat and subsequent behavioral testing had a pronounced effect on defensive avoidance behavior such as flight, which is thought to be regulated by conditioned fear mechanisms in the amygdala and other limbic regions. Moreover, our data compliment other studies suggesting that elevated 5-HT release in DRN projection regions contributes to the formation and display of learned helplessness and other stress-induced changes in behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agid O, Kohn Y, Lerer B. Environmental stress and psychiatric illness. Biomed. Pharmacother. 2000;54:135–141. doi: 10.1016/S0753-3322(00)89046-0. [DOI] [PubMed] [Google Scholar]
- Albers HE, Huhman KL, Meisel RL. Hormonal Basis of Social Conflict and Communication. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego, CA: Academic Press; 2002. pp. 393–433. [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998a;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res. 1998b;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Anderson IM, Mortimore C. 5-HT and human anxiety. Evidence from studies using acute tryptophan depletion. Adv. Exp. Med. Biol. 1999;467:43–55. doi: 10.1007/978-1-4615-4709-9_6. [DOI] [PubMed] [Google Scholar]
- Assie MB, Lomenech H, Ravailhe V, Faucillon V, Newman-Tancredi A. Rapid desensitization of somatodendritic 5-HT1A receptors by chronic administration of the high-efficacy 5-HT1A agonist, F13714: a microdialysis study in the rat. Br. J. Pharmacol. 2006;149:170–178. doi: 10.1038/sj.bjp.0706859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int. J. Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology. 2004;29:899–910. doi: 10.1016/j.psyneuen.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Bergqvist PB, Bouchard C, Blier P. Effect of long-term administration of antidepressant treatments on serotonin release in brain regions involved in obsessive-compulsive disorder. Biol. Psychiatry. 1999;45:164–174. doi: 10.1016/s0006-3223(98)00154-1. [DOI] [PubMed] [Google Scholar]
- Berton O, Aguerre S, Sarrieau A, Mormede P, Chaouloff F. Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience. 1998;85:147–159. doi: 10.1016/s0306-4522(97)00282-0. [DOI] [PubMed] [Google Scholar]
- Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience. 1999;92:327–341. doi: 10.1016/s0306-4522(98)00742-8. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J. Comp. Psychol. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–1596. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacology. 1999;21:91S–98S. doi: 10.1016/S0893-133X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor receptors in the dorsal raphe nucleus modulate social behavior in Syrian hamsters. Psychopharmacology. 2007;194:297–307. doi: 10.1007/s00213-007-0849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Delville Y. Serotonin regulation of aggressive behavior in male golden hamster (Mesocricetus auratus) Behav. Neurosci. 1999;113:804–815. doi: 10.1037//0735-7044.113.4.804. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE. Anxiolytic effects in the plus-maze of 5-HT1A-receptor ligands in dorsal raphe and ventral hippocampus. Pharmacol. Biochem. Behav. 1996;54:123–128. doi: 10.1016/0091-3057(95)02108-6. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J. Neurosci. 1996;16:4810–4815. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugge G, Kramer M, Rensing S, Fuchs E. 5HT1A-receptors and behaviour under chronic stress: selective counteraction by testosterone. Eur. J. Neurosci. 1998;10:2685–2693. [PubMed] [Google Scholar]
- Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1284–R1293. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Kramer M, Hermes B, Netter P, Hiemke C. Psychosocial stress in tree shrews: clomipramine counteracts behavioral and endocrine changes. Pharmacol. Biochem. Behav. 1996;54:219–228. doi: 10.1016/0091-3057(95)02166-3. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Viana MB, Mora PO. Opposed regulation by dorsal raphe nucleus 5-HT pathways of two types of fear in the elevated T-maze. Pharmacol. Biochem. Behav. 1996;53:171–177. doi: 10.1016/0091-3057(95)02012-8. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Viana MB, Mora PO. Dual role of 5-HT in defense and anxiety. Neurosci. Biobehav. Rev. 1997;21:791–799. doi: 10.1016/s0149-7634(96)00059-0. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005;1033:164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J. Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink L, Joordens RJ, Hijzen TH, Dirks A, Olivier B. Infusion of flesinoxan into the amygdala blocks the fear-potentiated startle. Neuroreport. 2000;11:2285–2288. doi: 10.1097/00001756-200007140-00043. [DOI] [PubMed] [Google Scholar]
- Hamon M, Fattaccini CM, Adrien J, Gallissot MC, Martin P, Gozlan H. Alterations of central serotonin and dopamine turnover in rats treated with ipsapirone and other 5-hydroxytryptamine1A agonists with potential anxiolytic properties. J. Pharmacol. Exp. Ther. 1988;246:745–752. [PubMed] [Google Scholar]
- Harper DG, Tornatzky W, Miczek KA. Stress induced disorganization of circadian and ultradian rhythms: comparisons of effects of surgery and social stress. Physiol. Behav. 1996;59:409–419. doi: 10.1016/0031-9384(95)02012-8. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm. Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Cooper MA, Huhman KL. N-methyl-D-aspartate receptors in the amygdala are necessary for the acquisition and expression of conditioned defeat. Neuroscience. 2004;123:625–634. doi: 10.1016/j.neuroscience.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav. Neurosci. 2005;119:1125–1130. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J. Neuroendocrinol. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J. Neuroendocrinol. 1999;11:547–559. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol. Scand. Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Li X, Inoue T, Abekawa T, Weng S, Nakagawa S, Izumi T, Koyama T. 5-HT1A receptor agonist affects fear conditioning through stimulations of the postsynaptic 5-HT1A receptors in the hippocampus and amygdala. Eur. J. Pharmacol. 2006;532:74–80. doi: 10.1016/j.ejphar.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav. Neurosci. 1995;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Martin P, Beninger RJ, Hamon M, Puech AJ. Antidepressant-like action of 8-OH-DPAT, a 5-HT1A agonist, in the learned helplessness paradigm: evidence for a postsynaptic mechanism. Behav. Brain Res. 1990;38:135–144. doi: 10.1016/0166-4328(90)90011-3. [DOI] [PubMed] [Google Scholar]
- Martin P, Tissier MH, Adrien J, Puech AJ. Antidepressant-like effects of buspirone mediated by the 5-HT1A post-synaptic receptors in the learned helplessness paradigm. Life Sci. 1991;48:2505–2511. doi: 10.1016/0024-3205(91)90605-b. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Kataoka Y, Mataki S, Kato Y, Oi K. Conflict situation increases serotonin release in rat dorsal hippocampus: in vivo study with microdialysis and Vogel test. Neurosci. Lett. 1996;215:197–200. doi: 10.1016/0304-3940(96)12982-7. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol. Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Meerlo P, de Boer SF, Koolhaas JM, Daan S, Van Den Hoofdakker RH. Changes in daily rhythms of body temperature and activity after a single social defeat in rats. Physiol. Behav. 1996;59:735–739. doi: 10.1016/0031-9384(95)02182-5. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, Turek FW. The effects of social defeat and other stressors on the expression of circadian rhythms. Stress. 2002;5:15–22. doi: 10.1080/102538902900012323. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Doucet E, Riad M, Adrien J, Verge D, Hamon M. Effect of the selective lesion of serotoninergic neurons on the regional distribution of 5-HT1A receptor mRNA in the rat brain. Brain Res. Mol. Brain Res. 1992;14:357–362. doi: 10.1016/0169-328x(92)90104-j. [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. New York, NY: Academic Press; 2001. [Google Scholar]
- Mos J, Olivier B, Poth M, Van Oorschot R, Van Aken H. The effects of dorsal raphe administration of eltoprazine, TFMPP and 8-OH-DPAT on resident intruder aggression in the rat. Eur. J. Pharmacol. 1993;238:411–415. doi: 10.1016/0014-2999(93)90877-k. [DOI] [PubMed] [Google Scholar]
- Mos J, Van Hest A, Van Drimmelen M, Herremans AH, Olivier B. The putative 5-HT1A receptor antagonist DU125530 blocks the discriminative stimulus of the 5-HT1A receptor agonist flesinoxan in pigeons. Eur. J. Pharmacol. 1997;325:145–153. doi: 10.1016/s0014-2999(97)00131-3. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Edwards E, Plotsky PM. 5-HT(1B) mrna regulation in two animal models of altered stress reactivity. Biol. Psychiatry. 2002;51:902–908. doi: 10.1016/s0006-3223(01)01371-3. [DOI] [PubMed] [Google Scholar]
- Oliveira LC, Broiz AC, de Macedo CE, Landeira-Fernandez J, Brandao ML. 5-HT2 receptor mechanisms of the dorsal periaqueductal gray in the conditioned and unconditioned fear in rats. Psychopharmacology. 2007;191:253–262. doi: 10.1007/s00213-006-0653-3. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Angel RA, Navarro M, Breese GR. Reduction in repeated ethanol-withdrawal-induced anxiety-like behavior by site-selective injections of 5-HT1A and 5-HT2C ligands. Psychopharmacology. 2006;187:1–12. doi: 10.1007/s00213-006-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin. Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus) Behav. Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress. Anxiety. 2000;12 Suppl. 1:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. J. Neurosci. 2004;24:5420–5426. doi: 10.1523/JNEUROSCI.0950-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC. Anxiety enhancement in the murine elevated plus maze by immediate prior exposure to social stressors. Physiol. Behav. 1993;53:383–388. doi: 10.1016/0031-9384(93)90222-2. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res. 1996;739:57–69. doi: 10.1016/s0006-8993(96)00809-8. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav. Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Schoeffter P, Hoyer D. Centrally acting hypotensive agents with affinity for 5-HT1A binding sites inhibit forskolin-stimulated adenylate cyclase activity in calf hippocampus. Br. J. Pharmacol. 1988;95:975–985. doi: 10.1111/j.1476-5381.1988.tb11728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, De Vry J. Neuronal circuits involved in the anxiolytic effects of the 5-HT1A receptor agonists 8-OH-DPAT ipsapirone and buspirone in the rat. Eur. J. Pharmacol. 1993;249:341–351. doi: 10.1016/0014-2999(93)90531-l. [DOI] [PubMed] [Google Scholar]
- Sena LM, Bueno C, Pobbe RL, Andrade TG, Zangrossi H, Jr., Viana MB. The dorsal raphe nucleus exerts opposed control on generalized anxiety and panic-related defensive responses in rats. Behav. Brain Res. 2003;142:125–133. doi: 10.1016/s0166-4328(02)00399-6. [DOI] [PubMed] [Google Scholar]
- Sharp T, Bramwell SR, Grahame-Smith DG. 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br. J. Pharmacol. 1989;96:283–290. doi: 10.1111/j.1476-5381.1989.tb11815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R283–R290. doi: 10.1152/ajpregu.00330.2006. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- van der Vegt BJ, Lieuwes N, van de Wall EH, Kato K, Moya-Albiol L, Martinez-Sanchis S, de Boer SF, Koolhaas JM. Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav. Neurosci. 2003;117:667–674. doi: 10.1037/0735-7044.117.4.667. [DOI] [PubMed] [Google Scholar]
- Vieweg WV, Julius DA, Fernandez A, Beatty-Brooks M, Hettema JM, Pandurangi AK. Posttraumatic stress disorder: clinical features, pathophysiology, and treatment. Am. J. Med. 2006;119:383–390. doi: 10.1016/j.amjmed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Walletschek H, Raab A. Spontaneous activity of dorsal raphe neurons during defensive and offensive encounters in the tree-shrew. Physiol. Behav. 1982;28:697–705. doi: 10.1016/0031-9384(82)90054-3. [DOI] [PubMed] [Google Scholar]