Abstract
We have previously demonstrated that mutated DNA derived from the circulation can be detected in urine and predominantly exists as DNA fragments < 1 kb. To preferentially isolate the trans-renal DNA from urine, we developed a method using carboxylated beads to separate high-MW (1 kb or larger) from low-MW DNA in urine. A primer set for 18s rRNA (generating a PCR product of 872 bp) was designed and optimized for real-time PCR quantification of high-MW DNA templates. To evaluate the method, urine samples from 5 volunteers with no known diseases and 36 patients with various colorectal diseases were collected and tested. It was found that the average removal efficiency of high-MW DNA from total urine DNA using carboxylated beads is 92.72% ± 1.42%. Furthermore, compared with using total urine DNA, our method provides a greater ability to detect mutated K-ras in the urine of colorectal cancer patients. The concurrence of K-ras mutations detected in disease tissue and the corresponding urine specimen is significantly higher (P = 0.0015) when the samples were enriched in low-MW DNA.
Keywords: circulating DNA, disease biomarker, K-ras mutation, colon cancer
Introduction
We1 and others2,3 have shown that short, tumor-derived DNA can pass through the kidney barrier and be excreted into urine. We have also demonstrated previously that urine contains DNA in two size categories: small (150 to 250 bp) cell-free, nucleosome-sized DNA fragments [designated as low-molecular-weight (MW) urine DNA] mostly from the circulation, and large (1,000+ bp), cell-associated DNA from the urinary tract. Furthermore, we have shown that the preferential isolation of low-MW urine DNA by fractionation of total urine DNA through agarose gel electrophoresis was useful in enhancing the detection of circulating mutated K-ras DNA.1 However, this agarose gel fractionation method is very tedious and time-consuming, so other possibilities for low-MW DNA isolation from human urine were explored.
Solid-phase carboxylated magnetic beads (CMBs) have been used to purify nucleic acids from other contaminants, and the size of the nucleic acids that bind to CMBs has been shown to vary with the binding conditions.4,5 In this study a method to remove high-MW urine DNA by CMB was developed and evaluated for its efficacy, and its applications were further explored.
Materials and Methods
Study Subjects and Specimens
Participants were recruited from the Great Lakes—New England Clinical Epidemiological Center, which includes the University of Michigan, Dana–Farber Harvard Cancer Center, M.D. Anderson Medical Center, and the University of Toronto. Cancer patients were enrolled from surgical or oncologic services (prior to initiation of chemo- or radiation therapy or surgery). Most “healthy—no known neoplasia” controls were subjects enrolled at endoscopy suites, but had undergone a negative colonoscopy. Patient samples were obtained under IRB approvals.
Urine Collection and DNA Isolation
To freshly collected urine, 0.5 M EDTA, pH 8.0, was added to a final concentration of 10 mM EDTA to inhibit possible nuclease activity in the urine sample, and stored at −70°C. To isolate total urine DNA, the frozen urine sample was thawed at room temperature, and then placed immediately on ice prior to DNA isolation. DNA was isolated from thawed urine within an hour.
Urine samples were mixed with 1.5 volumes of 6 M guanidine thiocyanate by inverting 8 times. 1 mL of resin (Wizard DNA Isolation Kit, Promega, Madison, WI, USA) was added into the urine lysate and incubated for 2 h at room temperature with gentle mixing. The resin–DNA complex was centrifuged, transferred to a minicolumn (provided in the kit), and washed with a buffer provided by the manufacturer, and the DNA was then eluted with TE buffer. DNA from paraffin-embedded tissue sections was isolated using the MasterPure DNA Kit (Epicentre, Madison, WI, USA) according to the manufacturer's instructions.
Fractionation of High-MW (> 1 kb) and Low-MW (< 1 kb) DNA by Carboxylated Magnetic Beads
To fractionate high-MW (> 1 kb) and low-MW (< 1 kb) DNA, total urine DNA (resuspended in TE buffer) was mixed with 5 M NaCl and 20% polyethylene glycol 8000 (PEG) (AMRESCO Inc., Solon, OH, USA) to final concentrations of 0.3 M and 8% respectively (as illustrated in Fig. 2A). The CMB suspension (AgentCourt Bioscience Corporation, Beverly, MA, USA) was washed and resuspended with TE buffer prior to use. Ten μL of pre-washed CMB suspension was added in the DNA mix and incubated for 1−2 hours at room temperature to allow the binding of high-MW DNA to the beads. The beads were then removed from the suspension by using a magnetic plate (AgentCourt). The low-MW DNA that remained in the suspension was collected by addition of 10 μL of pre-washed CMBs in a solution of 1.2 M NaCl and 10% PEG. The beads were then washed with 75% ethanol and the DNA was eluted in TE buffer.
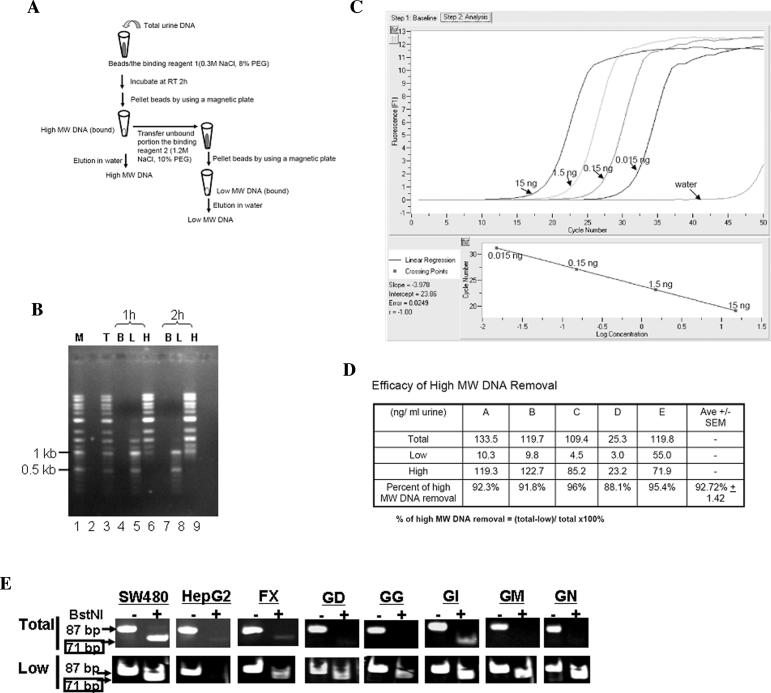
Figure 2.
Preferential isolation of low-MW urine DNA from total urine DNA using carboxylated magnetic beads (CMBs). (A) Schematic outline of the isolation procedure. (B) Estimation of the efficiency of CMB binding to high- and low-MW DNA. DNA markers (a mix of 1-kb and 100-bp DNA markers, as mentioned in the text) were subjected to the procedure outlined in panel A for the separation of high-and low-MW DNA fractions using a 1-hour (1 h) or 2-hour (2 h) incubation time with binding reagent 1. After separation, the total input DNA (T), the beads (B), the low-MW DNA fraction (L), and the high-MW DNA fraction (H) were resolved in a 1% agarose gel, stained with ethidium bromide, and photographed under the UV transilluminator. (C) Real-time PCR amplification of the 18s primer set # 872. Serial dilutions of human genomic DNA (as indicated) were subjected to real-time PCR amplification with the 18s primer #872, as described in text, using LightCycler FastStart DNA Master SYBR Green kit (Roche Diagnostics) according to the manufacturer's specifications (except for the concentration of MgCl2, which was 3 mM). The time and temperature in each step of the real-time PCR were 94°C (10 sec), 55°C (5 sec), and 72°C (20 sec) for 45 cycles. The linear regression fit was plotted. (D) Efficacy of high-MW DNA removal. Urine from five volunteers was collected and subjected to total urine DNA isolation as described previously.1 Half of total urine DNA was fractionated into high-and low-MW DNA fractions as outlined in panel A. The amount of high-MW DNA in each fraction was quantified by real-time PCR using 18s primers #872 as described in panel C. The percent of high-MW DNA removal was calculated as noted. (E) Detection of mutated K-ras DNA in low-MW urine DNA. Total and low-MW urine DNA were prepared as described in text and subjected to the RE-PCR assay for mutated K-ras DNA. The photos shown represent the difference in the outcome of RE-PCR between total and low-MW urine DNA from the same individuals listed in Table 1.
DNA Quantification by Real-Time Polymerase Chain Reaction (PCR)
Total or high-MW DNA was quantified by real-time PCR assays, performed using the LightCycler PCR instrument (Roche Applied Science, Indianapolis, IN, USA) and the LightCycler–Faststart DNA master SYBR Green kit (Roche) according to the manufacturer's specifications. The primer set used to quantify total DNA is specific to the albumin gene (forward, 5′-CCG TGG TCC TGA ACC AGT TA-3′; reverse, 5′-GTC GCC TGT TCA CCA AGG AT-3′) at an annealing temperature of 55°C. The primer set used to quantify the high-MW DNA is specific to the 18s rRNA gene (#872) (forward primer: 5′-TCCAGCTCCAATAGCG-3′, reverse primer: 5′-GGCATCACAGACCTGTT-3′) with an annealing temperature of 55°C. Serially diluted genomic DNA was included for calibration in each assay.
Restriction-Enriched Polymerase Chain Reaction (RE–PCR) Detection of the Codon 12 Mutation of the K-ras Gene
The RE–PCR assay, described previously,1 was used to distinguish wild-type and mutant K-ras sequences in DNA isolated from urine or tissue. Twenty cycles of hot-start PCR were performed as follows. DNA, equivalent to 0.2 mL of urine, with 1.5 mM MgCl2, 1 × PCR buffer (Qiagen, Valencia, CA, USA), 0.5 U Hot-Start Taq (Qiagen), 200 μM dNTP, 0.1 μM primers: L-Ext (5′ GCT CTT CGT GGT GTG GTG TCC ATA TAA ACT TGT GGT AGT TGG ACC T 3′) and R-Ext (5′ GCT CTT CGT GGT GTG GTG TCC CGT CCA CAA AAT GAT TCT GA 3′) were mixed and denatured at 95°C for 15 min, followed by 20 cycles of 94°C 30 sec, 52°C 30 sec, and 1 cycle of 72°C for 5 min. The first 20 cycles of PCR were used to amplify both wild-type and mutated DNA, but only to introduce an artificial BstNI site to the 5′ end of the amplified product derived from wild-type DNA. After the first round of PCR, the amplified products were digested with BstNI (New England Biolab, Beverly, MA, USA) to eliminate amplified products derived from wild-type DNA. 1/20 of the digested product was then subjected to a second hot-start PCR with 1 μM of the primers L (5′ ACT GAA TAT AAA CTT GTG GTA GTT GGA CCT 3′) and R-Bst (5′ GTC CAC AAA ATG ATC CTG GAT TAG C 3′) under the following conditions: 95°C 15min,40 cycles of 94°C 30sec, and 56°C 30 sec, followed by 1 cycle of 72°C 5 min. This second set of primers introduced a BstNI site to the 3′ end of amplified product derived from both wild-type and mutated templates serving as internal control for the BstNI diagnostic digestion after the second PCR. The amplified products (87 bp) of the second PCR were digested to completion with BstNI (as shown by disappearance of the 87-bp DNA fragment), and resolved through polyacrylamide gels. The appearance of the 71-bp DNA fragment after BstNI digestion is evidence of the K-ras mutation. Each sample was assayed 2−3 times. The samples were scored as “positive” for mutated K-ras when the 71-bp DNA fragments appeared after the second BstNI digestion of the PCR products in at least two of three repeated assays. As validation controls, the standard reconstitution samples were included in each assay.
Results and Discussion
Heterogeneity of Urine DNA among Individuals
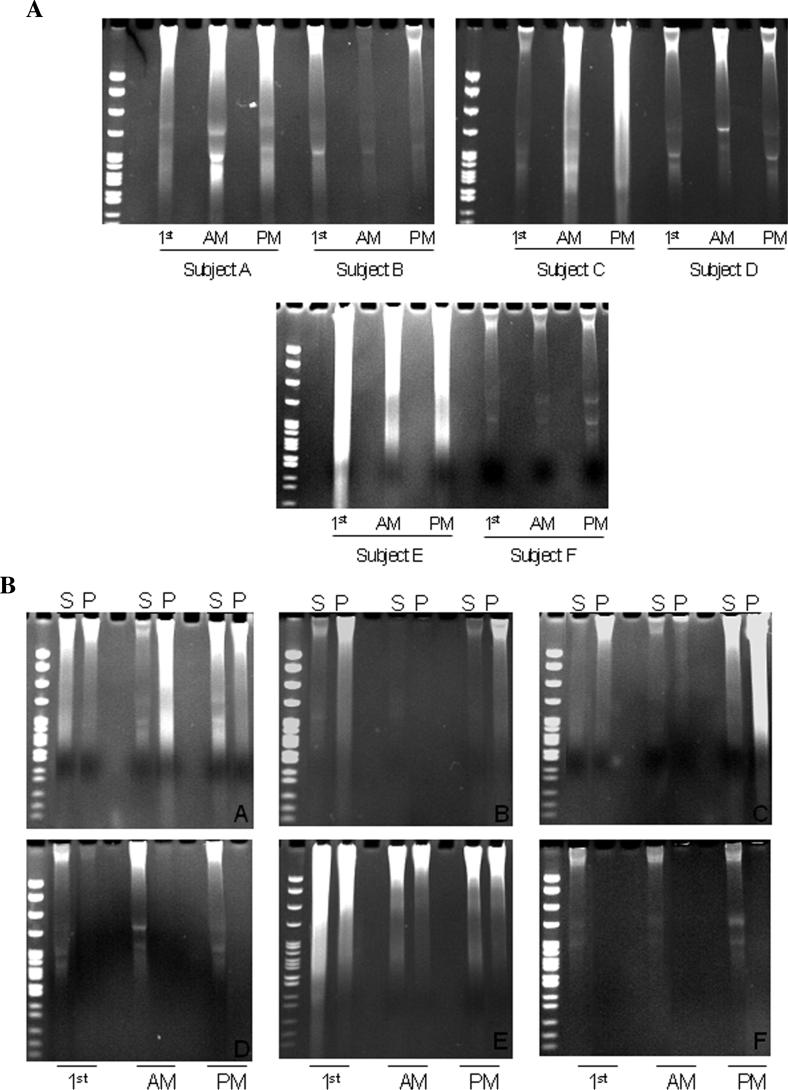
Before a method for the preferential isolation of circulatory DNA in urine could be designed, knowledge about urine DNA profiles in different individuals needed to be augmented. The urine of six different individuals (subjects A, B, C, D, E, and F) was collected at three different time points during a 24-hour period [after waking up (first caught), at 10 AM, and at 2 PM]. Half of the urine was subjected to total DNA isolation and the DNA profiles of these samples were analyzed, as shown in Figure 1A. Clearly, there was considerable heterogeneity in the total amount of DNA present in each sample, as well as in the ratio of high- to low-MW DNA derived from the urine of the same individual at different time points and from the urine of different individuals at similar time points. The other half of the collected urine was centrifuged at 2000 rpm for 10 min in order to fractionate the supernatant and the cell debris (pellet), and was then subjected to DNA isolation. DNA derived from both fractions was then analyzed in a polyacrylamide gel. As shown in Figure 1B, both the supernatant and the cell debris pellet from the urine of every individual contained both high- and low-MW DNA as revealed by the ethidium bromide staining after electrophoresis. It is clear that centrifugation alone is not able to efficiently separate high-MW DNA from low-MW DNA. This may have been caused by the stickiness of the DNA to the cell debris pellet.
Figure 1.
Heterogeneity of human urine. (A) Total urine DNA profile. Urine was collected from six human subjects (A-F) at three different time points of the day: early am (first-caught), around 10 am, and around 2 pm. Total urine DNA was isolated as previously described, resolved in 9% polyacrylamide gels, and visualized by ethidium bromide staining under UV transilluminator. (B) DNA profiles in the supernatant and cell debris–associated fractions. Urine was collected, frozen, and thawed before centrifugation at 2000 rpm for 10 min. DNA was isolated from the supernatant fraction (S) and cell pellet fraction (P), respectively, and then analyzed in polyacrylamide gels as described in (A).
Removal of High-MW DNA by Carboxylated Magnetic Beads
Solid-phase carboxylated magnetic beads (CMBs) have been used to purify nucleic acids from other contaminants, and the size of the nucleic acids that bind to CMB has been shown to vary with the binding conditions.4,5 To establish conditions in which CMBs (AgentCourt) selectively bind to DNA ≥ 1 kb in size(reagent 1), and to develop a binding condition to bind the DNA remaining in the supernatant (reagent 2) following high-MW DNA removal, solutions were prepared that ranged from 0.1 M−1.2 M NaCl and 5%−12% polyethylene glycol (PEG). The test DNA was a mixture of two DNA molecular-weight markers, the 100-bp DNA ladder (Invitrogen, Carlsbad, CA, USA) and the 1-kb DNA ladder (Promega).
A procedure to remove high-MW DNA and preferentially isolate low-MW DNA was established as outlined in Figure 2A and described in the Materials and Methods section. In brief: the tested DNA was mixed with beads in reagent 1 (0.3 M NaCl, 8% PEG) for 1 or 2 h. The DNA–bead complex was then sedimented using a magnetic plate (AgentCourt), and the supernatant was transferred to reagent 2 to collect the remaining DNA. To estimate the binding efficiency, the fractions were resolved in a 1.0% agarose gel and stained with ethidium bromide. As shown in Figure 2B, after 2 hours’ incubation at room temperature, almost no detectable high-MW DNA was left unbound in the solution (lane 8). There was very limited, if any, small DNA (less than 1 kb) detected in the high-MW DNA fraction (lane 9). However, in the 1-hour incubation group, some high-MW DNA remained in the supernatant (lane 5). Thus, a 2-hour incubation period was used for the subsequent experiments. Almost all the bound DNA was eluted in water, as no DNA was detected in the beads fraction (lanes 4 and 7), suggesting that the elution of DNA from the beads was efficient.
To quantitatively measure the efficacy of high-MW DNA removal using this method, a real-time polymerase chain reaction (PCR) assay was devised. A primer set 18s#872 (forward primer: 5′-TCCAGCTCCAATAGCG-3′; reverse primer: 5′-GGCATCACAGACCTGTT-3′) generating a PCR product of 872 bp from the 18s ribosome gene was designed and optimized for real-time PCR quantification using the LightCycler (Roche Diagnostics, Indianapolis, IN, USA). The linearity of the amplification of serial dilutions of the human control DNA (Roche) by the 18s#872 primers is shown in Figure 2C.
To evaluate the efficacy of high-MW DNA removal from total urine DNA by CMBs, urine from 5 volunteers with no known disease was collected and subjected to total DNA isolation. Half of the total urine DNA from each individual was then subjected to high- and low-MW DNA fractionation using the developed method. The amount of high-MW DNA of each fraction (total, high and low) was then quantified by real-time PCR using the 18s#872 primer set. Since this primer set only amplifies high-MW DNA, it should effectively determine the efficacy of high-MW DNA removal. In the five sets of fractionated urine DNA samples, the average efficacy of high-MW DNA removal ranged from 88.1% to 96%, with an average of 92.72% ± 1.42%, as shown in Figure 2D. Thus, CMB was shown to be able to effectively remove high-MW DNA from total urine DNA.
Removal of High-MW DNA by Carboxylated Magnetic Beads Enhanced the Detection of Mutated K-ras DNA in Urine of Patients with Colorectal Diseases
After the validity of the method was established, this method was applied to the detection of a colorectal cancer (CRC)–related biomarker, the K-ras codon 12 mutations. Thirty-six blinded urine specimens, collected from subjects with various colorectal diseases, were subjected to total urine DNA isolation, and then to the CMB procedure to obtain the low-MW DNA fraction. The matched paraffin-embedded disease tissue sections (if available) were subjected to DNA isolation using a MasterPure DNA kit (Epicentre). To detect K-ras codon 12 mutations, restriction endonuclease-enriched polymerase chain reaction (RE-PCR) was performed as previously described1 on total and low-MW urine DNA and tissue DNA (if available). Patient information such as diagnosis, age, and gender were revealed after the assays were performed.
In brief: DNA derived from either 200 μL urine or 1/10 of a tissue section was used in each assay. The PCR product from the REPCR assay is 87 bp, and the appearance of a 71-bp fragment after the second BstNI digestion is evidence of mutated K-ras DNA in the DNA sample. The detection limit of the assay is 15 copies of the mutant K-ras per 100 ng of wild-type DNA per reaction (data not shown). As validation controls, DNA prepared from sources known to have either mutant (human adeno-carcinoma SW480 cells) or wild-type (human hepatoblastoma HepG2 cells) K-ras sequences was subjected to PCR. As expected, and shown in Figure 2E, DNA prepared from HepG2 cells contained no detectable levels of mutant K-ras (no appearance of the 71-bp fragment after the second BstNI digestion), whereas DNA prepared from SW480 cells contained mutant K-ras sequences (the 71-bp fragment was detected after the second BstNI digestion). For each urine sample that contained detectable mutant K-ras DNA, the presence of the mutant K-ras sequence was more evident when low-MW DNA was used in the assay as compared to the total urine DNA, as demonstrated by the six examples in Figure 2E. The data are summarized in Table 1.
TABLE 1.
Detection of Mutated K-ras DNA in Tissue DNA and its Corresponding Total and Low-MW Urine DNA
| CRCa (n = 16, mean age = 64, range 33−87, 6 females) |
Adn polypsa (n = 12, mean age = 60, range 41−80, 5 females) |
Hypl polypsa (n = 2, mean age = 51.5, range 51−52, 0 female) |
Nkna,b (n = 7, mean age = 52.7, range 50−61, 5 females) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Codec | Tissued | Td | Ld | Codec | Tissued | Td | Ld | Codec | Tissued | Td | Ld | Codec | Td | Ld |
| GA | + | − | + | FX | NA | + | + | GJ | − | − | − | FY | − | − |
| GC | NA | + | + | FZ | NA | − | − | GN | + | − | + | GD | − | + |
| GE | NA | − | + | GF | + | − | + | GL | − | − | ||||
| GG | + | − | + | GM | − | − | + | HI | − | − | ||||
| GH | + | + | + | HN | NA | − | + | GQ | − | − | ||||
| GI | − | + | + | GO | + | − | − | GW | − | − | ||||
| GK | + | + | + | GP | + | − | + | HF | − | − | ||||
| HK | NA | − | + | GR | + | + | + | |||||||
| GS | + | − | + | GV | − | − | − | |||||||
| GT | NA | − | − | HA | + | − | + | |||||||
| GU | NA | − | − | HB | + | − | + | |||||||
| GX | NA | + | + | HC | + | − | + | |||||||
| GY | + | − | + | |||||||||||
| GZ | + | − | + | |||||||||||
| HD | + | + | + | |||||||||||
| HE | + | + | + | |||||||||||
| # tested | # of “+”e | # of “+”e | # of “+”e | # tested | # tested | # of “+”e | # of “+”e | # tested | # tested | # of “+”e | # of “+”e | # tested | # of “+”e | # of “+”e |
| 16 | 10 | 7 | 14 | 12 | 9 | 2 | 9 | 2 | 2 | 0 | 1 | 7 | 0 | 1 |
| % posf | 43.8% | 87.5% | 16.7% | 75% | 0% | 50% | 0% | 14.2% | ||||||
| P valueg | P = 0.0233 | P = 0.0123 | NA | |||||||||||
Urine samples were collected from patients grouped in four groups based on the diagnosis as colorectal cancer (CRC), adenomatous (Adn) polyps, hyperplastic (Hypl) polyps, or no know neoplasia (Nkn). Patients’ samples were collected under IRB approval.
No tissue sample was available in the Nkn group.
Each patient's urine samples were given a unique code, blinded to diagnosis. DNA was isolated and subjected to the RE-PCR assay for mutated K-ras DNA. Diagnosis of each subject was unblinded thereafter.
Total DNA was isolated from each urine sample and its corresponding tissue samples (if available, NA: not available). Half of total urine DNA was subjected to carboxylated magnetic bead isolation method to obtain low-MW urine DNA (L) fraction. Tissue DNA (Tissue) and both total (T) and low (L) MW urine DNA were subjected to the RE-PCR assay for mutated K-ras DNA. The level of K-ras mutated DNA by RE-PCR is 2 copies per reaction. The presence (+) or absence (−) of detectable mutated K-ras DNA in each DNA preparation is listed.
Number of samples shown to contain detectable mutated K-ras DNA by the RE-PCR assay.
Percent of samples in each group that contain detectable mutated K-ras DNA as determined by RE-PCR assay.
Statistical analysis was performed using the Fisher's exact test.
The participants in this blind study either had colorectal cancer (n = 16), adenomatous polyps (n = 12), hyperplastic polyps (n = 2), or no known neoplasia (n = 7). For the colorectal cancer group, 43.8% of total urine DNA samples were positive for the K-ras mutations, whereas 87.5% of samples were positive when the low-MW DNA fraction was used as the substrate for the assay. For the group with adenomatous polyps, 16.7% of urine samples were positive for the K-ras mutations when total urine DNA was used, whereas 75% of urine samples were positive for the K-ras mutations if the low-MW DNA fraction was used. When the detection of K-ras mutations using total urine DNA and low-MW DNA were compared using the Fisher's exact test, the P-value was 0.0233 for the colorectal cancer group and 0.0123 for the adenomatous polyps group, suggesting there is a significant difference between the number of positive total urine DNA samples and the number of positive low-MW DNA samples. Statistical analysis was not performed for the hyperplastic polyps and no known neoplasia groups because of small sample sizes, and there was no tissue DNA available for the latter group. Furthermore, the concurrence of K-ras mutations detected in disease tissue (colorectal cancer, adenomatous polyps, and hyperplastic polyps) and the corresponding urine specimen is also higher when the low-MW urine DNA fraction was used for the assays (86%, 18/21) as compared to total urine DNA (38%, 8/21), with a P-value of 0.0015 as analyzed by the chi-square test. Of interest, this 86% concurrence is similar to our previous findings using a smaller sample size in which the detection of mutated K-ras DNA was found to be positive using total urine DNA or DNA isolated from cell-free urine. The latter option of using DNA isolated from cell-free urine was only utilized if the mutated K-ras DNA was not detected using the total urine DNA. This strongly indicates that the low-MW urine DNA fraction is better than total urine DNA for detection of K-ras mutations in urine of colorectal disease patients.
One possible contributor to this discrepancy in assay results between total urine DNA and low-MW urine DNA from the same individual could be due to a better ratio of the target DNA to the nonspecific DNA. The removal of high-MW DNA from the total urine DNA would result in a better ratio of K-ras-mutated DNA (presumably from the circulation) to wild-type DNA (from the circulation and sloughed off urinary tract cells). This is particularly important in light of the nature of the RE-PCR K-ras mutation assay. The detection sensitivity of the target mutant DNA sequences could be reduced as a result of interference from an excessive amount of wild-type DNA in the PCR. This would reduce the amplification efficiency of the DNA sequence of interest.
In summary, this study clearly demonstrates that (1) there is significant heterogeneity among the urine DNA profiles of different individuals and among the urine DNA profiles at different times of urine collection; (2) CMBs can be used to preferentially isolate low-MW urine DNA from total urine DNA; and (3) the low-MW urine DNA fraction rather than total urine DNA should be used to increase assay sensitivity for detection of circulation-derived, cancer-related DNA biomarkers in urine for cancer detection, monitoring, and/or prognosis.
Acknowledgments
We thank Dr. Pamela A. Norton (Drexel University, Philadelphia, PA) for the critical reading of the manuscript and Dr. Gang Chen (Drexel University, Philadelphia, PA) for the assistance in the statistical analysis. This work was supported, in part, by the Early Detection Research Network (EDRN) of the National Cancer Institute, the Hepatitis B Foundation of America, and an appropriation from the Commonwealth of Pennsylvania.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Su YH, et al. Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer. J. Mol. Diagn. 2004;6:101–107. doi: 10.1016/S1525-1578(10)60497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botezatu I, et al. Genetic analysis of DNA excreted in urine: a new approach for detecting specific genomic DNA sequences from cells dying in an organism. Clin. Chem. 2000;46:1078–1084. [PubMed] [Google Scholar]
- 3.Serdyuk OI, et al. Detection of mutant k-ras sequences in the urine of cancer patients. Bull. Exp. Biol. Med. 2001;131:283–284. doi: 10.1023/a:1017624120807. [DOI] [PubMed] [Google Scholar]
- 4.DeAngelis M, et al. Solid-phase reversible immobilization for the isolation of PCR products. Nucl. Acids Res. 1995;23:4742–4743. doi: 10.1093/nar/23.22.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins T, et al. DNA purification and isolation using a solid-phase. Nucl. Acids Res. 1994;22:4543–4544. doi: 10.1093/nar/22.21.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]