Abstract
Background and objectives: B-type natriuretic peptide (BNP) and amino-terminal pro-B-type natriuretic peptide (NT-proBNP) are biomarkers of cardiovascular disease that is common in patients with chronic kidney disease (CKD). Conflicting data on the influence of glomerular filtration rate (GFR) on BNP and NT-proBNP levels in CKD may stem from failure to account fully for the effects of coexistent cardiac disease, dysfunction, and volume overload.
Design, setting, participants, & measurements: Prospective head-to-head comparison of plasma BNP and NT-proBNP in ambulatory euvolemic CKD patients with normal LV ejection fraction and no manifest cardiac or vascular disease. GFR was estimated by the Modification of Diet in Renal Disease formula, BNP and NT-proBNP measured using Abbott AxSYM and Roche Elecsys assays, respectively, and cardiac morphology and function assessed by transthoracic echocardiography.
Results: In 142 patients (42% female) of mean age 60 ± 11 yr, mean left ventricular ejection fraction was 71% ± 6%, GFR 38 ± 14 ml/min per 1.73 m2, and median BNP and NT-proBNP level 59 and 311 pg/ml, respectively. Multivariate predictors of NT-proBNP level were GFR, β-blocker usage, LV mass index, and hemoglobin level. Plasma BNP was independently predicted by LV mass index and β-blocker usage but not GFR. In the 74 patients without diastolic dysfunction, there was a significant rise in NT-proBNP but not BNP as GFR declined.
Conclusions: Unlike NT-proBNP, plasma BNP level is relatively independent of GFR. BNP may therefore be the more appropriate biomarker to screen for cardiac dysfunction in CKD.
Patients with chronic kidney disease (CKD) are at increased risk of cardiovascular disease. Natriuretic peptides (NPs), biomarkers of myocardial dysfunction (1), offer the potential for early detection and risk stratification of cardiac disease, as evident in emergency department (2) and community (3,4) settings. This screening utility could be extended to CKD patients asymptomatic of cardiovascular disease.
However, the precise influence of CKD on circulating levels of B-type natriuretic peptide (BNP) and amino-terminal pro-B-type natriuretic peptide (NT-proBNP), the two commonly used NPs in clinical practice, continues to be debated. Dependence of plasma BNP on glomerular filtration rate (GFR) has been reported among patients with and without heart failure (HF) (5,6), but this relationship may not be independent of cardiac or volume-related factors (7,8). The data on NT-proBNP in renal dysfunction are more concordant but were derived from populations that included patients with myocardial infarction, reduced left ventricular (LV) ejection fraction (LVEF), or HF (9,10). Indeed, most studies examining the impact of renal dysfunction on NPs uniformly included such patients (5,6,8–10). Recent Doppler myocardial imaging studies have shown that even HF patients with normal LVEF have reduced LV contractility compared with controls (11,12).
To limit confounding by cardiac dysfunction or volume overload, we prospectively constituted a clinically euvolemic CKD cohort without symptoms or history of cardiac disease and normal LVEF and regional function. We measured circulating levels of both NPs, hypothesizing that, in these patients, BNP can be shown to be relatively independent of GFR compared with NT-proBNP if cardiac and loading factors can be comprehensively accounted for.
Materials and Methods
Study Subjects
Adult Asian outpatients aged 19 to 75 yr attending a CKD clinic at National University Hospital, Singapore, from November 2004 through October 2005 were prospectively recruited. All patients with stable stages III and IV CKD with estimated GFR of 15 to 60 ml/min per 1.73 m2 body surface area (BSA) were invited to have an echocardiogram followed immediately by a blood draw. Exclusion criteria were 1) renal replacement therapy (dialysis or transplantation), 2) history of coronary artery disease, i.e., angina pectoris, previous myocardial infarction, and coronary artery intervention, 3) LVEF ≤50% or regional wall motion deficit assessed by any modality, 4) congenital or organic valvular heart disease, 5) cardiac arrhythmias including atrial fibrillation, 6) history of cerebrovascular disease, 7) peripheral vascular disease, i.e., a revascularization procedure or limb amputation, 8) hemoglobin <9 g/dl, 9) liver dysfunction, and 10) acute intercurrent illness. The study was approved by the Domain-Specific Review Board of the National Healthcare Group.
Information on age, gender, body mass index (BMI, calculated as weight divided by square of height), clinic blood pressure, regular medications, and associated comorbidities was recorded. Hypertension and diabetes mellitus were defined as documentation of the diagnosis or use of medications. GFR was estimated using the four-variable Modification of Diet in Renal Disease (MDRD) formula (13) and expressed in ml/min per 1.73 m2 BSA.
Biochemical Analysis
Biochemical assays were performed at the College of American Pathologists-accredited Department of Laboratory Medicine at National University Hospital, Singapore. Anticoagulant free venous blood samples were assayed for NT-proBNP by electrochemiluminescence immunoassay on the Elecsys 2010 Immunoanalyzer (Roche Diagnostics, Indianapolis, IN). BNP levels were measured in plasma samples stored in ethylene diamine tetra acetic acid-coated tubes, using Microparticle Enzyme Immunoassay on the Abbott AxSYM analyzer (Abbott Laboratories Diagnostic Division, Abbott Park, IL). Exact values of these NPs were used in data analysis.
Echocardiography
All patients had a comprehensive M-mode, two-dimensional, and Doppler echocardiogram performed by a single experienced sonographer (H.Y.) using Vivid 7 Dimension ultrasound equipment (General Electric, Milwaukee, WI) and reported by an echocardiographer (L.H.L.) blinded to clinical and biochemical data. Studies were performed and quantitated in accordance with guidelines of the American Society of Echocardiography (14,15).
LV end-diastolic volume (LVEDV) and end-systolic volume (LVESV) were calculated using the Teichholz correction of the cube formula (16). LVEF was calculated as 100 × (LVEDV − LVESV)/LVEDV. Echocardiographic LVM was derived using the modified cube formula 1.04 × [(LVIDd + IVSd + LVPWd)3 − LVIDd3 × 0.8] + 0.6 (17). LA volume was calculated as π/6 (A1 × A2 × A3), where A1 is the M-mode LA dimension and A2 and A3 are measurements of short- and long-axis LA dimensions, respectively, in the apical four-chamber view (18). LV mass (LVM), LVEDV, and LA volume were indexed for BSA and expressed as LVM/BSA, LVEDV/BSA, and LAV/BSA, respectively. Maximum inferior vena cava (IVC) diameter was measured at end-expiration about 2 cm from the right atrial-IVC junction, just proximal to the hepatic veins.
Standard diastolic filling indices were measured from pulsed Doppler mitral inflow, including early (E) and late (A) diastolic velocities and their ratio and pulmonary venous flow velocity curves. Early diastolic velocity of the mitral annulus (E′) was measured at the septal corner in the apical four-chamber view and the ratio of E to E′ velocity (E/E′) computed as a surrogate of LV filling pressure (19). By integrating these indices, global LV diastolic function was categorized as normal (grade 0), prolonged relaxation (grade 1), pseudonormal (grade 2), or restrictive (grades 3 and 4) (20).
Statistics
Data analysis was performed using SPSS 15.0 software (SPSS Inc, Chicago, IL). Continuous data were checked for normality using the Kolmogorov-Smirnov test and expressed as mean ± SD or median (range) as appropriate, and categorical data as percentages. Differences between groups were tested using one-way analysis of variance or a nonparametric alternative (Mann-Whitney or Kruskal-Wallis test) as appropriate. The Spearman rank sum test was used to correlate NP levels with a priori selected clinical and echocardiographic variables of LV remodeling, systolic function, relaxation, and preload. Linear regression vis-à-vis quartiles of NP levels was performed on variables that correlated at a threshold P ≤ 0.15 with either NP in univariate analysis. Preliminary analyses were conducted to ensure no important violation of the assumptions of linearity, multicollinearity, and homoscedasticity. Multicollinearity was assessed by first examining the pairwise relationships between variables (excluded if rho >0.70) and in the multivariate model, the tolerance of the variable (significant collinearity if >0.10). Multiple regression was done in two stages, first using a backward removal algorithm of candidate clinical (including GFR) and echocardiographic variables to identify the best predictors in either block, followed by hierarchical multiple regression, controlling for clinical covariates. The analysis was repeated after excluding statistically redundant variables. Models were separately constructed for BNP and NT-proBNP. Statistical significance was set at P < 0.05.
Based on reported correlations (in the range of −0.2 to −0.3) between BNP and GFR for patients with and without HF (5), a sample size of 140 to 150 patients was considered sufficient to achieve statistical significance with 85% power and allowable 2-sided error of 0.05.
Results
A total of 149 patients consented to participate. Of these, 6 were excluded on the basis of echocardiographic abnormalities, i.e., LVEF <50% (3 patients), regional wall motion abnormality (3 patients), and moderate mitral regurgitation (one patient). One patient was later excluded after magnetic resonance imaging showed evidence of remote ischemic stroke. The study cohort therefore comprised 142 patients of Chinese (90 subjects), Malay (41), and Indian (11) ethnicity whose clinical and echocardiographic characteristics are detailed in Table 1. Excluding one patient with uninterpretable diastolic function, diastolic dysfunction grade was 0, 1, and 2 in 74 (52%), 45 (32%), and 22 (15%) patients, respectively. No patient had frank restrictive LV filling.
Table 1.
Clinical, laboratory, and echocardiographic data of 142 patients with chronic kidney disease
| N (%), Mean ± SD, or Median (interquartile range) | |
|---|---|
| Age (yr) | 59.4 ± 10.6 |
| Female gender | 59 (42%) |
| Diabetes mellitus | 79 (55%) |
| Smoking history | 12 (8%) |
| Hypertension | 113 (79%) |
| Body mass index (kg/m2) | 25.4 (22.6–29.7) |
| Systolic BP (mmHg) | 144 (128–157) |
| Diastolic BP (mmHg) | 77 (72–85) |
| eGFR (ml/min per 1.73 m2 BSA) | 37 (27–52) |
| Hemoglobin (g/dl) | 12.5 ± 2.1 |
| BNP (pg/ml) | 59 (19–137) |
| NT-proBNP (pg/ml) | 129 (54–307) |
| LV systolic dimension (mm) | 28 (26–30) |
| LV diastolic dimension (mm) | 47 (44–49) |
| LV ejection fraction (%) | 71 (53–83) |
| LV mass/BSA (g/m2) | 99 ± 24 |
| LA dimension (mm | 40 ± 5 |
| LA volume/BSA (ml/m2) | 29 (24–35) |
| Maximum IVC diameter (mm) | 14 (8–24) |
| Mitral E/A ratio | 0.93 (0.79–1.21) |
| IVRT (ms) | 94 (83–104) |
| Septal E′ velocity (cm/s) | 7.0 (6.0–8.0) |
| Septal E/E′ ratio | 10.4 (8.4–12.9) |
BNP, B-type natriuretic peptide; BP, blood pressure; BSA, body surface area; eGFR, estimated glomerular filtration rate; IVC, inferior vena cava; LA, left atrial; LV, left ventricular; NT-proBNP, amino-terminal pro-B-type natriuretic peptide; E/A, ratio of early and late diastolic velocities; IVRT, isovolumic relaxation time.
CKD was attributed to diabetic nephropathy in 57 patients (39%), hypertensive nephrosclerosis in 14 (10%), small echogenic kidneys in 43 (30%), IgA nephropathy in 10 (7%) and miscellaneous causes in 19 patients (14%), including focal segmental glomerular sclerosis, autosomal dominant polycystic kidney disease, membranoproliferative and membranous glomerulonephritis, renal calculus disease, systemic lupus erythematosus, and chronic sclerosing glomerulonephritis. Medications used included angiotensin-converting enzyme inhibitors (89 patients, 62%), angiotensin receptor blockers (79, 55%), β-blockers (79, 55%) diuretics (76, 53%), and statins (106, 74%).
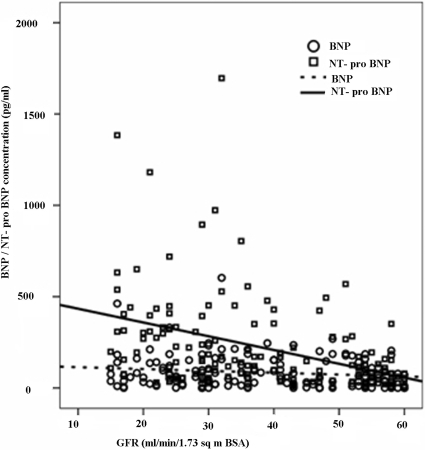
Figure 1 shows scatter plots of GFR against both NPs. With increasing severity of renal dysfunction, there was an increasing trend and scatter of circulating NT-proBNP levels that was apparent with BNP. Table 2 shows BNP, NT-proBNP, and LVEF values stratified by GFR groups; differences across groups were significant only for NT-proBNP.
Figure 1.
Scatter plot of plasma B-type natriuretic peptide (BNP) and amino-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations in relation to MDRD-estimated glomerular filtration rate (GFR). Lines of best linear fit are shown.
Table 2.
Distribution of plasma natriuretic peptide levels, left ventricular (LV) ejection fraction, and frequency of diastolic dysfunction in relation to glomerular filtration rate (GFR), expressed in ml/min per 1.73 m2
| GFR Group A, 15–30 (n = 51) | GFR Group B, 31–45 (n = 40) | GFR Group C, 46–60 (n = 51) | P | |
|---|---|---|---|---|
| GFR | 24 (19–29) | 37 (34–41) | 54 (51–58) | — |
| BNP (pg/ml) | 64 (27–136) | 82 (23–153) | 40 (5–134) | 0.14 |
| NT-proBNP (pg/ml) | 270 (100–404) | 150 (76–327) | 77 (33–130) | <0.00001 |
| LV ejection fraction (%) | 72 (67–75) | 71 (67–76) | 71 (66–74) | 0.23 |
GFR, glomerular filtration rate; BNP, B-type natriuretic peptide; NT-proBNP, amino-terminal pro-B-type natriuretic peptide. Data are median (range).
Univariate correlations of clinical and echocardiographic variables with NP levels are shown in Table 3. GFR showed a moderate, highly significant correlation with NT-proBNP and a weak, borderline significant correlation with BNP. There were also moderate correlations between hemoglobin level and NT-proBNP and between LV mass/BSA and LA volume/BSA and both NPs.
Table 3.
Association of selected clinical and echocardiographic variables with B-type natriuretic peptide (BNP) and amino-terminal pro-B-type natriuretic peptide (NT-proBNP)
| BNP
|
NT-proBNP
|
|||
|---|---|---|---|---|
| rho | P | rho | P | |
| Age | 0.23 | 0.0058 | 0.28 | 0.00077 |
| Female gender | −0.02 | 0.81 | 0.14 | 0.10 |
| Hypertension | 0.24 | 0.0040 | 0.27 | 0.0013 |
| ACEI or ARB usage | −0.02 | 0.82 | −0.23 | 0.0061 |
| β-blocker usage | 0.26 | 0.0022 | 0.29 | 0.00064 |
| Diuretic usage | 0.09 | 0.30 | 0.24 | 0.0043 |
| Diabetes mellitus | 0.04 | 0.68 | 0.14 | 0.10 |
| Hemoglobin | −0.13 | 0.12 | −0.30 | 0.00033 |
| GFR | −0.17 | 0.047 | −0.48 | <0.0001 |
| LV end-diastolic volume | 0.18 | 0.030 | 0.15 | 0.068 |
| LV end-systolic volume | 0.11 | 0.18 | 0.05 | 0.57 |
| LV ejection fraction | 0.06 | 0.49 | 0.11 | 0.19 |
| LV mass/BSA | 0.32 | 0.00012 | 0.30 | 0.00032 |
| LA volume/BSAa | 0.34 | <0.0001 | 0.32 | <0.0001 |
| Maximum IVC diameter | 0.18 | 0.030 | 0.07 | 0.44 |
| Mitral E/A ratio | 0.05 | 0.57 | −0.10 | 0.22 |
| Mitral deceleration time | 0.06 | 0.48 | 0.17 | 0.50 |
| IVRT | −0.01 | 0.93 | 0.03 | 0.73 |
| Septal E′ velocity | −0.16 | 0.055 | −0.17 | 0.041 |
| Septal E/E′ ratio | 0.19 | 0.026 | 0.20 | 0.016 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; GFR, glomerular filtration rate; LV, left ventricular; BSA, body surface area; E/A, ratio of early and late diastolic velocities; IVRT, isovolumic relaxation time.
Not considered in multiple regression of echocardiographic variables.
Table 4 shows the final multiple regression models for each NP. Independent predictors of BNP level were LVM/BSA (P = 0.0016) and β-blocker usage (P = 0.0071), each variable accounting for approximately 7% of total variance. For NT-proBNP, multivariate predictors were GFR (P < 0.00001), β-blocker usage (P = 0.0010), LVM/BSA (P = 0.011), and hemoglobin level (P = 0.023). The clinical model alone explained 33% of the total variance with GFR accounting for just over half of this. After incorporation of LVM/BSA, the model explained 36% of NT-proBNP variance (R2 change, 3%). There was no significant multicollinearity in any of the analyses.
Table 4.
Multivariate predictors of plasma B-type natriuretic peptide (BNP) and amino-terminal pro-B-type natriuretic peptide (NT-proBNP)
| B | SE | 95% CI | β | P | |
|---|---|---|---|---|---|
| BNP | |||||
| LV mass/BSA | 0.012 | 0.004 | 0.005 to 0.019 | 0.258 | 0.0016 |
| β-blocker usage | 0.489 | 0.179 | 0.135 to 0.843 | 0.219 | 0.0071 |
| NT-proBNP | |||||
| GFR | −0.035 | 0.006 | −0.046 to −0.023 | −0.426 | <0.00001 |
| β-blocker usage | 0.527 | 0.157 | 0.217 to 0.837 | 0.235 | 0.0010 |
| LV mass/BSA | 0.008 | 0.003 | 0.002 to 0.015 | 0.179 | 0.011 |
| hemoglobin | −0.086 | 0.038 | −0.161 to −0.012 | −0.165 | 0.023 |
B, unstandardized regression coefficient; β, standardized regression coefficient; CI, confidence intervals; SE, standard error of the estimate; LV, left ventricular; BSA, body surface area; GFR, glomerular filtration rate.
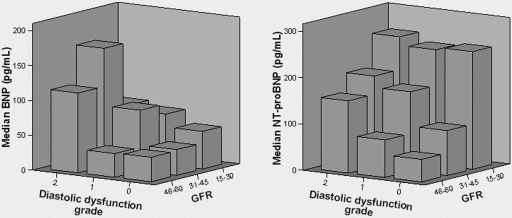
Figure 2 shows three-dimensional plots trending median NP levels by GFR and diastolic dysfunction grade. These illustrate the general trend, especially among patients with GFR >30 ml/min per 1.73 m2 that NP levels increase with progressive diastolic dysfunction. In the 74 patients with grade 0 diastolic dysfunction, median NT-proBNP level increased significantly with each lower GFR strata (test for trend, P = 0.001). This was not observed with plasma BNP (P = 0.58).
Figure 2.
Three-dimensional bar graphs of median levels of B-type natriuretic peptide (BNP, left panel) and N-terminal pro-B-type natriuretic peptide (NT-proBNP, right panel) versus glomerular filtration rate (GFR, expressed in ml/min per 1.73 m2) and diastolic dysfunction grade.
Discussion
NPs are increasingly used in clinical practice to diagnose myocardial dysfunction. Their growing importance as reflected in a recent consensus statement on HF (21) mandates a clear characterization of the dependence of NP levels on renal dysfunction, a common comorbidity among HF patients. The ideal NP to diagnose or monitor cardiac dysfunction in the setting of CKD remains debated. Clearance of plasma BNP, the biologically active fragment of proBNP, occurs via endocytosis and lysosomal degradation after binding to NP clearance receptor type C and secondarily via proteolysis by neutral endopeptidase 24.11 (22). Peripheral receptors for NT-proBNP are not known and its clearance less well established, but renal excretion may play a role (23). Nevertheless, few data convincingly support the presumption that NT-proBNP is more prone than BNP to be influenced by renal dysfunction (10,24). In this head-to-head study, we demonstrate an independent inverse correlation between GFR and NT-proBNP but not BNP in asymptomatic stage III and IV CKD outpatients in whom cardiac risk stratification is of particular importance.
The large Breathing Not Properly Multinational Study (5) established a weak but statistically significant relationship between GFR and BNP in patients presenting emergently with acute dyspnea, 35% of whom had a history of HF and 25% myocardial infarction. In this setting, the impact of renal dysfunction on NP levels is difficult to ascertain given additional and variable contributions of LV and right ventricular systolic and diastolic dysfunction, myocardial ischemia, volume overload, and mitral regurgitation (25). Thus, in the study of Tsutamoto et al. (6), HF patients with estimated GFR <40 ml/min and the highest BNP levels did indeed have higher median LV end-diastolic pressure compared with those with better preserved GFR. Such patients with presumed “cardiorenal syndrome” (26) may also have higher NP levels arising from advanced heart disease, greater preload, and multiple other comorbidities (27,28). Falsely low estimated GFR caused by poor renal perfusion or diuretic use in acute HF further complicate the relationship with NPs (5). To minimize some of these confounders, Takami et al. (8) excluded patients with LVEF <40%, regional wall motion abnormality, and valvular disease in their study of 103 Japanese predialysis inpatients with more severe volume or pressure overload than our cohort, as evidenced by larger IVC diameter and LV mass index. Consistent with our study, they found that BNP level in CKD patients was independent of GFR but dependent on echocardiographic surrogates of LV volume overload and LV end-diastolic pressure.
To date, few studies have compared NPs head-to-head in patients with CKD (10,11). Vickery et al. (9) showed increases in NP concentrations with declining GFR, which was greater for NT-proBNP. Although median LVEF was normal for all CKD strata, 6% of patients had prevalent HF and 35% had known cardiovascular disease. Because detailed evaluation of cardiac function was not performed, the observed rise in BNP may not relate solely to GFR, but there is consensus with the present study on the greater GFR dependence of NT-proBNP. In contrast, Luchner et al. (10) showed, among survivors of myocardial infarction, virtually equal (2-fold) increases in median values of both NPs in those with renal dysfunction. Because the assigned creatinine clearance partition value for renal dysfunction was 85 ml/m2 with mean clearance being 71 ml/min in the renally impaired patients and because both groups with and without renal dysfunction had identically reduced LVEF (mean, 50%) and self-reported HF symptoms (1 of 5 cases), their findings could reflect preponderant cardiac dysfunction in the face of relatively mild renal impairment.
Our study attempts to account for the confounding influence of systolic and, in a more limited fashion, diastolic dysfunction on NP levels. The degree of diastolic dysfunction was not severe in our cohort, consistent with the asymptomatic population. In patients without diastolic dysfunction or raised LV filling pressure, there was again a clear inverse relationship of NT-proBNP level with GFR that was not observed with BNP.
Apart from GFR, the observed associations of NP levels with β-blocker treatment (29–32), LVM/BSA (33,34), and hemoglobin concentration (35,36) have previously been highlighted in isolation. Similar to previous studies (34), LVM/BSA better predicted NP levels than any echocardiographic variable assessed and, in particular, was superior to both blood and tissue Doppler indices of diastolic dysfunction. Such may not be the case for less homogeneous populations. Additionally, the predictor model for BNP explained only 13% of total variance, suggesting that multiple, more complex factors modulate plasma BNP. These may include LV end-diastolic wall stress (37), altered regional myocardial deformation (38), and right ventricular dysfunction (25). NT-proBNP level was more readily predicted by candidate variables, most notably GFR, which explained more than one third of the variance.
A correlation of NP levels with age, especially NT-proBNP, has been reported in both Western (39,40) and Asian populations (41). In our patients, age correlated with plasma NP levels in univariate analysis but lacked independent predictive value. This is not unexpected in a CKD cohort where LV hypertrophy, a variable highly correlated with age, is frequently encountered.
Limitations
Despite careful patient selection, occult myocardial ischemia, which may raise circulating NP levels (42), cannot be ruled out in some of our CKD patients. Intensive screening for coronary artery disease was not systematically performed as all patients were free of symptoms or signs of cardiovascular disease.
LVEF was assessed using volumes derived from the M-mode rather than Simpson's method. The former technique provides excellent temporal resolution of endocardial motion, is highly reproducible and appropriate for use in our patients with symmetric LV morphology, and validated by a wealth of clinical data (43). Despite known limitations, LVEF was used as the index of systolic function in our study to permit comparison across studies.
Use of the MDRD formula to estimate GFR is controversial, especially in Asians (44,45). Compared with other formulas, however, MDRD provides the most precise estimate of true renal function, especially in the lower GFR ranges, and is hence the most reliable in clinical practice (46). Indeed, a good correlation in patients with GFR <60 ml/min has been recently reported in a large, diverse population (47).
Conclusion
CKD and consequent end-stage renal failure are threatening to reach epidemic proportions worldwide over the next decade (48). Thus, an increasing number of CKD patients will require early institution of retardation strategies and screening for comorbidity, in particular, cardiac BNP appears not to be significantly influenced by renal dysfunction and may therefore be the biomarker of choice for detection and surveillance of myocardial dysfunction in CKD patients. This recommendation is predicated on the demonstrated equivalence of BNP and NT-proBNP in detection of LV systolic and diastolic dysfunction among predominantly non-CKD patients (40,49). However, further studies should be done to verify the performance characteristics of both NPs in prospective detection of cardiac dysfunction in the CKD population.
Disclosures
This study was funded by a research grant (0862/2004) from the National Medical Research Council, Singapore. BNP and NT-proBNP test kits were kindly gifted to the investigators by Abbott Laboratories and Roche Diagnostics, Singapore, respectively.
Acknowledgments
Part of the data were presented as posters at the 43rd Annual Congress of the European Renal Association-European Dialysis and Transplant Association held in Glasgow, United Kingdom in July 2006 and at the American Society of Nephrology Renal Week 2007 held in San Francisco, California, November 2007.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Daniels LB, Maisel AS: Natriuretic peptides. J Am Coll Cardiol 50: 2357–2368, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Korenstein D, Wisnivesky JP, Wyer P, Adler R, Ponieman D, McGinn T: The utility of B-type natriuretic peptide in the diagnosis of heart failure in the emergency department: a systematic review. BMC Emerg Med 7: 6, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuat A, Murphy JJ, Hungin AP, Curry J, Mehrzad AA, Hetherington A, Johnston JI, Smellie WS, Duffy V, Cawley P: The diagnostic accuracy and utility of a B-type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract 56: 327–333, 2006 [PMC free article] [PubMed] [Google Scholar]
- 4.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL: Systolic and diastolic heart failure in the community. JAMA 296: 2209–2216, 2006 [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA, Duc P, Omland T, McCord J, Nowak RM, Hollander JE, Herrmann HC, Steg PG, Westheim A, Knudsen CW, Storrow AB, Abraham WT, Lamba S, Wu AH, Perez A, Clopton P, Krishnaswamy P, Kazanegra R, Maisel AS: B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis 41: 571–579, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Tsutamoto T, Wada A, Sakai H, Ishikawa C, Tanaka T, Hayashi M, Fujii M, Yamamoto T, Dohke T, Ohnishi M, Takashima H, Kinoshita M, Horie M: Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. J Am Coll Cardiol 47: 582–586, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Zoccali C, Mallamaci F, Benedetto FA, Tripepi G, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Cottini E, Malatino LS: Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol 12: 1508–1515, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Takami Y, Horio T, Iwashima Y, Takiuchi S, Kamide K, Yoshihara F, Nakamura S, Nakahama H, Inenaga T, Kangawa K, Kawano Y: Diagnostic and prognostic value of plasma brain natriuretic peptide in non-dialysis-dependent CRF. Am J Kidney Dis 44: 420–428, 2004 [PubMed] [Google Scholar]
- 9.Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, Lamb EJ: B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis 46: 610–620, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Luchner A, Hengstenberg C, Lowel H, Riegger GA, Schunkert H, Holmer S: Effect of compensated renal dysfunction on approved heart failure markers: direct comparison of brain natriuretic peptide (BNP) and N-terminal pro-BNP. Hypertension 46: 118–123, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW: Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation 105: 1195–1201, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Vinereanu D, Nicolaides E, Tweddel AC, Fraser AG: “Pure” diastolic dysfunction is associated with long-axis systolic dysfunction: implications for the diagnosis and classification of heart failure. Eur J Heart Fail 7: 820–828, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al: Recommendations for quantitation of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 2: 358–367, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ: Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Teichholz LE, Kreulen T, Herman MV, Gorlin R: Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 37: 7–11, 1976 [DOI] [PubMed] [Google Scholar]
- 17.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N: Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57: 450–458, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM: Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol 41: 1036–1043, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ: Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102: 1788–1794, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ: Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289: 194–202, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL: How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28: 2539–2550, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Vanderheyden M, Bartunek J, Goethals M: Brain and other natriuretic peptides: molecular aspects. Eur J Heart Fail 6: 261–268, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Wahl HG, Graf S, Renz H, Fassbinder W: Elimination of the cardiac natriuretic peptides B-type natriuretic peptide (BNP) and N-terminal proBNP by hemodialysis. Clin Chem 50: 1071–1074, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Hall C: NT-ProBNP: the mechanism behind the marker. J Card Fail 11[Suppl]: S81–S83, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Troughton RW, Prior DL, Pereira JJ, Martin M, Fogarty A, Morehead A, Yandle TG, Richards AM, Starling RC, Young JB, Thomas JD, Klein AL: Plasma B-type natriuretic peptide levels in systolic heart failure: importance of left ventricular diastolic function and right ventricular systolic function. J Am Coll Cardiol 43: 416–422, 2004 [DOI] [PubMed] [Google Scholar]
- 26.van Kimmenade RR, Pinto Y, Januzzi, JL Jr: When renal and cardiac insufficiencies intersect: is there a role for natriuretic peptide testing in the “cardio-renal syndrome?” Eur Heart J 28: 2960–2961, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Gardner RS, Chong KS, O'Meara E, Jardine A, Ford I, McDonagh TA: Renal dysfunction, as measured by the modification of diet in renal disease equations, and outcome in patients with advanced heart failure. Eur Heart J 28: 3027–3033, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM: Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Davis ME, Richards AM, Nicholls MG, Yandle TG, Frampton CM, Troughton RW: Introduction of metoprolol increases plasma B-type cardiac natriuretic peptides in mild, stable heart failure. Circulation 113: 977–985, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ohta Y, Watanabe K, Nakazawa M, Yamamoto T, Ma M, Fuse K, Ito M, Hirono S, Tanabe T, Hanawa H, Kato K, Kodama M, Aizawa Y: Carvedilol enhances atrial and brain natriuretic peptide mRNA expression and release in rat heart. J Cardiovasc Pharmacol 36[Suppl 2]: S19–S23, 2000 [DOI] [PubMed] [Google Scholar]
- 31.van den Meiracker AH, Lameris TW, van de Ven LL, Boomsma F: Increased plasma concentration of natriuretic peptides by selective beta1-blocker bisoprolol. J Cardiovasc Pharmacol 42: 462–468, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Luchner A, Burnett JC Jr, Jougasaki M, Hense HW, Riegger GA, Schunkert H: Augmentation of the cardiac natriuretic peptides by beta-receptor antagonism: evidence from a population-based study. J Am Coll Cardiol 32: 1839–1844, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Mallamaci F, Zoccali C, Tripepi G, Benedetto FA, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Stancanelli B, Malatino LS: Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int 59: 1559–1566, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Lukowicz TV, Fischer M, Hense HW, Doring A, Stritzke J, Riegger G, Schunkert H, Luchner A: BNP as a marker of diastolic dysfunction in the general population: importance of left ventricular hypertrophy. Eur J Heart Fail 7: 525–531, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Kanda H, Kita Y, Okamura T, Kadowaki T, Yoshida Y, Nakamura Y, Ueshima H: What factors are associated with high plasma B-type natriuretic peptide levels in a general Japanese population? J Hum Hypertens 19: 165–172, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Tsuji H, Nishino N, Kimura Y, Yamada K, Nukui M, Yamamoto S, Iwasaka T, Takahashi H: Haemoglobin level influences plasma brain natriuretic peptide concentration. Acta Cardiol 59: 527–531, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Alter P, Rupp H, Rominger MB, Vollrath A, Czerny F, Klose KJ, Maisch B: Relation of B-type natriuretic peptide to left ventricular wall stress as assessed by cardiac magnetic resonance imaging in patients with dilated cardiomyopathy. Can J Physiol Pharmacol 85: 790–799, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Ogawa T, Linz W, Stevenson M, Bruneau BG, Kuroski de Bold ML, Chen JH, Eid H, Scholkens BA, de Bold AJ: Evidence for load-dependent and load-independent determinants of cardiac natriuretic peptide production. Circulation 93: 2059–2067, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Barnes SC, Collinson PO, Galasko G, Lahiri A, Senior R: Evaluation of N-terminal pro-B type natriuretic peptide analysis on the Elecsys 1010 and 2010 analysers. Ann Clin Biochem 41: 459–463, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Costello-Boerrigter LC, Boerrigter G, Redfield MM, Rodeheffer RJ, Urban LH, Mahoney DW, Jacobsen SJ, Heublein DM, Burnett JC Jr: Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol 47: 345–353, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi X, Xu G, Xia T, Song Y, Lin Q: N-terminal-pro-B-type natriuretic peptide (NT-proBNP): reference range for Chinese apparently healthy people and clinical performance in Chinese elderly patients with heart failure. Clin Chim Acta 360: 122–127, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Sabatine MS, Morrow DA, de Lemos JA, Omland T, Desai MY, Tanasijevic M, Hall C, McCabe CH, Braunwald E: Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol 44: 1988–1995, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W: Recommendations for chamber quantification. Eur J Echocardiogr 7: 79–108, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Jafar TH, Schmid CH, Levey AS: Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol 16: 1413–1419, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Smilde TD, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL: Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation 114: 1572–1580, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, Rahman M, Deysher AE, Zhang YL, Schmid CH, Levey AS: Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 18: 2749–2757, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Meguid El Nahas A, Bello AK: Chronic kidney disease: the global challenge. Lancet 365: 331–340, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Grewal J, McKelvie R, Lonn E, Tait P, Carlsson J, Gianni M, Jarnert C, Persson H: BNP and NT-proBNP predict echocardiographic severity of diastolic dysfunction. Eur J Heart Fail 10: 252–259, 2008 [DOI] [PubMed] [Google Scholar]