Abstract
Background and objectives: Encapsulating peritoneal sclerosis (EPS) is a severe peritoneal fibrotic reaction in patients on long-term peritoneal dialysis (PD). The early clinical features may be nonspecific. The purpose of the study is to assess the reliability and diagnostic utility of abdominal CT scanning in the diagnosis of EPS.
Design, setting, participants, & measurements: Abdominopelvic CT scans of 27 patients diagnosed with EPS on clinical and radiologic grounds in our unit from 1997 to 2006 were retrospectively analyzed. In addition, 35 control CT scans were scored: 15 from hemodialysis patients (HD controls) and 20 from patients on PD (PD controls). Scans were anonymized and scored independently by three radiologists.
Results: Inter-rater agreement was moderate to very good (kappa = 0.40 to 0.75) for peritoneal calcification, bowel distribution, bowel wall thickening, and bowel dilation but poorer for loculation of ascites and peritoneal thickening. There was a strongly significant difference between the total CT scan scores at EPS diagnosis and controls (P < 0.00001). Each individual parameter also showed significant differences between EPS and controls (P < 0.006). Bowel tethering and peritoneal calcification were the most specific parameters, and. loculation was the least discriminatory parameter. Interestingly, prediagnostic scans a median of 1.5 yr before EPS diagnosis were normal or near-normal in 9 of 13 EPS patients.
Conclusions: CT scanning is a valid and reliable adjunct to the diagnosis of EPS but may not be useful as a screening tool, as the prediagnostic scans did not show abnormalities in many patients who subsequently developed EPS.
Peritoneal dialysis (PD) is an important form of renal replacement therapy enabling patients to have a home-based treatment. Encapsulating peritoneal sclerosis (EPS) is a rare but devastating complication in patients on PD, in which the peritoneum becomes progressively thickened and scarred, enclosing the bowel in fibrous tissue. Clinically, the patients present with recurrent small bowel obstruction, blood-stained dialysis effluent or ascites, and/or malnutrition (1,2). The incidence in case series of PD patients is approximately 1% to 3% and increases with length of time on PD, especially more than 5 yr (3–5). The early clinical features of EPS may be nonspecific and are often not recognized until the patient develops complications, such as bowel obstruction. Abdominopelvic CT scan abnormalities in EPS have previously been documented, including peritoneal thickening, peritoneal calcification, loculated fluid collections, tethering of the small bowel, and bowel wall thickening (6–9). Several of these are open to subjective interpretation, which could result in varying reports when scans are reviewed by different radiologists. Furthermore, the clinical utility, diagnostic importance, and longitudinal changes in the various CT abnormalities have not been systematically analyzed.
In this study, CT scans of EPS patients diagnosed in our center and CT scans of control PD and hemodialysis (HD) patients were anonymized and scored in random order by three independent radiologists. Two main questions have been addressed. First, which CT scan abnormalities are detectable in patients with EPS, and do these differ in type or degree from PD patients without clinical EPS or patients with end-stage kidney disease not on PD? Second, are radiologists able to identify these changes sufficiently reliably to make CT scanning diagnostically useful? Longitudinal CT data were also available predating and postdating the diagnosis of EPS in several patients, which gives an insight into temporal changes in CT scans in this disease.
Materials and Methods
Case Identification and Diagnosis
Case notes of patients diagnosed with EPS on clinical grounds, supported by imaging studies, at Hammersmith Hospitals NHS Trust between June 1, 1997 and December 1, 2006 (9.5 yr) were retrospectively reviewed (30 patients). CT scans were available for 27 of 30 EPS patients. Twenty-one of 27 EPS patients had additional CT scans taken before and/or after the diagnosis of EPS (total 75 scans), and these were also scored. As a control group, CT scans of 20 PD patients without symptoms of EPS and 15 HD patients were also anonymized and analyzed. The control scans had been taken for various indications, including weight loss, anemia, fever of unknown origin, and screening for renal cystic disease or abdominal aortic aneurysms. PD patients with infective peritonitis at the time of the CT scan were not included.
CT Scans
CT scans including the abdomen and pelvis were included in our study. The CT scans were viewed on GE PACS workstations and were considered to be of diagnostic quality by the radiologists involved. Fifty-eight percent of the CT scans were performed at Hammersmith Hospital using a GE Lightspeed 8 slice scanner (GE, Milwaukee, WI). Forty-two percent of the scans were done at Charing Cross Hospital, using a Siemens Somaton Sensation 16 slice scanner (Siemens, Erlangen, Germany). Eighty-seven percent of the scans were contrast enhanced using Ultravist 300 (Bracco, Milan, Italy). All of the CT scans in the study were assigned numbers randomly and digitally anonymized on individual CD ROMS. The blinded CT scans were then scored independently by 3 experienced radiologists working on different sites of our institution. Clinical information was not available to the radiologists at the time of the assessments.
Before the study, the radiologists and clinicians involved agreed a scoring system based on our own observations of CT abnormalities in EPS and previously published data relating to CT abnormalities found in EPS (6,10). Scoring parameters are given in Table 1. All of the parameters were scored by all three radiologists, except bowel dilation, which was scored by two of the three radiologists. Bowel tethering refers to in-drawing of the bowel to the center of the abdomen, giving an irregular, speculated, or fibrotic appearance.
Table 1.
CT scan scoring parameters
| Peritoneal Calcification | Peritoneal Thickening | Bowel Wall Thickening | |||
|---|---|---|---|---|---|
| 0 | not identified | 0 | not identified | 0 | not identified |
| 1 | localized area <20% | 1 | localized area <20% | 1 | localized bowel |
| 2 | 20% of peritoneum | 2 | localized <20% | 2 | 20% of bowel |
| 3 | 50% of peritoneum | 3 | 50% of peritoneum | 3 | 50% of bowel |
| 4 | extensive >80% | 4 | extensive >80% | 4 | extensive >80% |
| Bowel Tethering
|
Loculation
|
Bowel Dilatation
|
|||
| 0 | not present | 0 | not present | 0 | not identified |
| 1 | mild tethering | 1 | <3 locules | 1 | localized bowel |
| 2 | moderate tethering | 2 | 3–6 locules | 2 | 20% of bowel |
| 3 | marked tethering | 3 | multiloculated | 3 | 50% of bowel |
| 4 | extensive >80% | ||||
Statistical Analysis
Statistical analysis was performed by Joseph Eliahoo of the Statistics Department, Imperial College London. Nonparametric tests were used throughout. The kappa test was used to assess pairwise interobserver variability between the radiologists, and the results of each of the three comparisons are presented for each parameter. A kappa score of 1 indicates perfect agreement, and a score of 0 indicates random agreement. For purposes of the kappa testing, CT scans were either scored as normal (score 0) or abnormal (scores 1 to 4 considered equivalent). When testing for significance of differences between the EPS patients and controls, the median score between the radiologists for each parameter was taken. Fisher's exact test was used to compare CT scores of the EPS patients with HD and PD control groups for each of the parameters (peritoneal calcification, peritoneal thickening, bowel thickening, bowel tethering, bowel dilation, and loculation).
The “total CT score” for EPS is the sum of the median scores for each parameter (out of a total maximum of 22). The Kruskal-Wallis equality of ranks test with Bonferroni correction was used to compare the total scores between the three groups. The Wilcoxon rank-sum test was used to compare the total score between the EPS patients and the HD controls and PD controls individually and also between the HD and PD controls. The Wilcoxon rank-sum test was also used to compare total CT scan score between the poor and better outcome EPS patients.
Results
Demographic Information
Summary demographic information on subjects included in the study is shown in Table 2, and more detailed information is shown in Tables 3 and 4.
Table 2.
Demographic information
| Encapsulating Sclerosing Peritonitis (EPS) Patient | Peritoneal Dialysis (PD) Control | Hemodialysis (HD) Control | |
|---|---|---|---|
| Age at diagnosis (yr) [median (range)] | 51 (22–72) | 62 (37–82) | 65 (43–78) |
| Male:female | 15:12 | 13:7 | 10:5 |
| Median time on PD at time of scan (yr) | 7.0 | 4.0 | Not applicable |
Table 3.
Supplementary demographic information on PD patients
| Subject No. | Sex | Renal Diagnosis | Age (yr) at EPS Diagnosis | Duration of PD (yr) | Reason for Transfer from PD | Onset of EPS after Withdrawal from PD (yr) | Total CT Scan Score at Diagnosis | Current Status | Time to Death or Follow-up Time (yr) |
|---|---|---|---|---|---|---|---|---|---|
| EPS1 | M | APKD | 49 | 6.0 | Ultrafiltration failure | 0.4 | 2 | Died after peritoneolysis | 1.4 |
| EPS2 | F | Unknown | 26 | 6.6 | Bacterial peritonitis | 0.4 | 10 | Survived, HD | 4.6 |
| EPS3 | M | Small kidneys | 43 | 7.0 | Transplanted | −1.3 | 7 | Died, EPS | 1.5 |
| EPS4 | M | Unknown | 51 | 12.9 | Transplanted | 0.7 | 10.5 | Survived, Txp | 1.5 |
| EPS5 | M | IgA nephropathy | 68 | 10.0 | Ultrafiltration failure | 0.5 | 6.5 | Died, EPS | 0.1 |
| EPS6 | F | Reflux nephropathy | 33 | 10.5 | Bacterial peritonitis | 0.1 | 14 | Died, EPS | 0.8 |
| EPS7 | M | Membranous GN | 51 | 7.8 | Transplanted | 0.5 | 10.5 | Survived, Txp | 3.0 |
| EPS8 | M | Small kidneys | 50 | 2.1 | EPS, refused to transfer | −1.7 | 7 | Died, EPS | 1.7 |
| EPS9 | M | MCGN | 63 | 7.6 | Transplanted | 0.6 | 6.5 | Survived, Txp | 1.2 |
| EPS10 | M | Type 2 diabetes | 64 | 5.6 | Bacterial peritonitis | 0.4 | 11 | Survived, HD | 1.7 |
| EPS11 | F | Type 2 diabetes | 65 | 6.8 | Ultrafiltration failure | 3.9 | 9 | HD, then EPS diagnosed after Txp, died unrelated | 1.9 |
| EPS12 | F | Hypertension | 52 | 9.5 | Transplanted | 1.6 | 5.5 | Died, EPS | 0.9 |
| EPS13 | M | Vasculitis | 48 | 6.3 | Bacterial peritonitis | 0.3 | 9.5 | Died, EPS | 0.9 |
| EPS14 | F | Hypertension | 40 | 10.4 | Ultrafiltration failure | 0.7 | 12.5 | Survived, HD | 1.1 |
| EPS15 | M | IgA nephropathy | 53 | 8.7 | EPS | 0.1 | 16 | Survived, HD | 1.8 |
| EPS16 | F | FSGS | 41 | 5.1 | Transplanted | 0.2 | 9.5 | Survived, Txp | 1.0 |
| EPS17 | M | HIVAN | 49 | 3.8 | Bacterial peritonitis | 0.3 | 4.5 | Survived, Txp | 3.9 |
| EPS18 | F | Hypertension | 70 | 9.6 | Poor ultrafiltration | 0.5 | 5.5 | Died, unrelated | 1.3 |
| EPS19 | M | Alport syndrome | 22 | 7.0 | Poor ultrafiltration | 0.3 | 16 | Survived, HD | 3.3 |
| EPS20 | F | Reflux | 65 | 6.9 | EPS | 0.0 | 5 | Died, EPS | 0.2 |
| EPS21 | F | Type 2 diabetes | 72 | 4.3 | Transplanted | 0.1 | 10.5 | Survived, Txp | 1.1 |
| EPS22 | F | Unknown | 35 | 9.2 | EPS | 0.0 | 15.5 | Died, EPS | 0.1 |
| EPS23 | M | Reflux | 40 | 5.7 | Transplanted | 0.6 | 3 | Survived, Txp | 8.1 |
| EPS24 | F | Eclampsia | 54 | 10.0 | Bacterial peritonitis | 3.0 | 9 | Survived, HD | 2.5 |
| EPS25 | M | Renal carcinoma | 412 | 8.0 | Transplanted | 0.3 | 7.5 | Died, EPS | 0.6 |
| EPS26 | M | Hypertension | 62 | 5.7 | Bacterial peritonitis | 0.1 | 6 | Survived, HD | 2.2 |
| EPS27 | F | Unknown | 52 | 4.3 | Transplanted | 0.1 | 9 | Survived, Txp | 2.4 |
APKD, adult polycystic kidney disease; FSGS, focal and segmental glomerulosclerosis; HIVAN, HIV nephropathy; Txp, renal transplant.
Table 4.
Demographic information for control patients at time of scan
| Control No. | Dialysis Modality | Age (yr) | Sex | Renal Diagnosis | Duration of PD (yr) | Indication for CT Scan | Total CT Scan Score |
|---|---|---|---|---|---|---|---|
| 1 | HD | 64 | M | Unknown | 0 | Weight loss | 0 |
| 2 | HD | 45 | F | Unknown | 0 | Back pain | 0 |
| 3 | HD | 71 | F | Unknown | 0 | Weight loss, fever | 0 |
| 4 | HD | 77 | F | Type 2 diabetes | 0 | Abdominal pain | 0 |
| 5 | HD | 65 | M | Type 2 diabetes | 0 | After carcinoma screening | 0 |
| 6 | HD | 55 | M | Renal tumors | 0 | After carcinoma screening | 0 |
| 7 | HD | 69 | M | Type 2 diabetes | 0 | Erythropoietin resistance | 0 |
| 8 | HD | 78 | M | Scleroderma | 0 | Erythropoietin resistance | 0 |
| 9 | HD | 77 | M | Renal vascular | 0 | Abdominal aneurysm | 0 |
| 10 | HD | 67 | F | Renal vascular | 0 | Fever of unknown origin | 0 |
| 11 | HD | 58 | M | Type 2 diabetes | 0 | Carcinoma screening | 0 |
| 12 | HD | 44 | M | Papillary necrosis | 0 | Lung lesion? TB | 0 |
| 13 | HD | 43 | M | Renal vascular | 0 | Weight loss | 0 |
| 14 | HD | 53 | F | Adult polycystic | 0 | Lung lesion, screening | 0 |
| 15 | HD | 68 | M | Reflux | 0 | Cystic kidneys | 0 |
| 16 | PD | 67 | M | Glomerulonephritis | 5.7 | Liver hemangioma | 1 |
| 17 | PD | 78 | M | Unknown | 1.8 | Erythropoietin resistance | 0 |
| 18 | PD | 66 | M | Light chain disease | 0.8 | Screening for hematologic disease | 0 |
| 19 | PD | 75 | M | Renal vascular | 5.8 | Abdominal lymph nodes on ultrasound | 1 |
| 20 | PD | 46 | M | Type 2 diabetes | 6.7 | Sarcoidosis | 3 |
| 21 | PD | 82 | F | Renal vascular | 2.2 | Cystic pancreas | 0.5 |
| 22 | PD | 58 | F | Scleroderma | 0.8 | Upper abdominal pain | 0 |
| 23 | PD | 53 | M | Unknown | 3.5 | Change in bowel habit | 1.5 |
| 24 | PD | 37 | F | Unknown | 5.9 | Hypercalcemia, previous ovarian cancer | 2.5 |
| 25 | PD | 42 | F | Adult polycystic | 3.9 | Gallstones | 0 |
| 26 | PD | 55 | M | Type 2 diabetes | 1.7 | Fever, cough? TB | 0 |
| 27 | PD | 55 | F | Renal vascular | 4.0 | Weight loss | 0 |
| 28 | PD | 71 | M | Malignant hypertension | 7.7 | Screening for long-term PD patient | 1 |
| 29 | PD | 58 | F | Glomerulonephritis | 3.2 | ?CMV after transplant | 2 |
| 30 | PD | 75 | M | Unknown | 5.3 | Screening for long-term PD patient | 0 |
| 31 | PD | 69 | M | Renal vascular | 3.0 | Upper abdominal pain | 1.5 |
| 32 | PD | 58 | M | Membranous GN | 5.8 | Prostate cancer screening | 1 |
| 33 | PD | 44 | F | Type 1 diabetes | 6.5 | Screening for long-term PD patient | 1 |
| 34 | PD | 72 | M | IgA nephropathy | 6.1 | Screening for long-term PD patient | 0 |
| 35 | PD | 68 | M | Type 2 diabetes | 3.4 | Fever of unknown origin | 2 |
EPS patients.
Thirty patients diagnosed with EPS were retrospectively identified during the study period (June 1, 1997 to December 1, 2006; 9.5 yr). The diagnosis of EPS was made on clinical grounds, supported by imaging investigations. Typical CT scan features of EPS are shown in Figure 1. Twelve of 30 EPS patients (40%) died of EPS, and a further six required long-term total parenteral nutrition (TPN) and/or peritoneolysis surgery; this group was classed as having a “severe” course (total 18 of 30, i.e., 60%). The other 40% had several episodes of subacute bowel obstruction and/or blood-stained ascites but improved after several months or years and now require only oral nutritional supplements or no nutritional support. Three patients in the EPS cohort have died of unrelated causes. Ten patients were diagnosed with EPS after renal transplantation, and the outcomes of these patients were similar to the overall series (3 died of EPS and 3 prolonged TPN). Abdominopelvic CT scans from the time of diagnosis of EPS were available in 27 of 30 EPS patients.
Figure 1.
Cross-sectional abdominal CT images of patients diagnosed with EPS. (A and B) Extensive peritoneal calcification (arrowheads) as well as thick-walled loops of small bowel (arrows), which are being tethered to the mesentery (*). Note also the surrounding ascites in this patient who had ceased peritoneal dialysis. (C and D) This postcontrast enhanced CT demonstrates subtle peritoneal thickening and enhancement (arrows) with a marked amount of ascites in this patient who developed EPS following renal transplantation.
Control patients.
Fifteen CT scans of hemodialysis patients (HD controls) and 20 scans of PD patients (PD controls) were randomly selected from our dialysis populations as controls. The control scans had been taken for various indications, including weight loss, erythropoietin resistance, fever of unknown origin, and screening for various pathologies. PD patients with infective peritonitis at the time of the CT scan were not included. None of the PD control patients has developed symptoms of EPS subsequent to the CT scans. The median time on PD for the EPS patients was 7.0 yr compared with 4.0 yr for the PD controls. None of the HD controls had previously been on PD.
Interobserver Reliability
Kappa testing was used to assess inter-rater agreement in scores for each parameter between the three radiologists in this study. The scoring criteria are shown in Table 1. The kappa coefficient measures agreement beyond that expected by chance. Pairwise kappa testing was undertaken between radiologists 1 and 2, 1 and 3, and 2 and 3. Table 5 shows the kappa scores for each parameter grouped as normal (score 0) or abnormal (scores 1 to 4). Agreement was moderate to substantial (0.40 to 0.75) for peritoneal calcification, bowel wall thickening, bowel tethering, and bowel dilation between all three radiologists. Radiologists 2 and 3 agreed more about peritoneal thickening (kappa = 0.59) compared with observer 1 (kappa score = 0.32 and 0.27 versus observers 2 and 3, respectively). This suggests systematic differences in the way observer 1 rated the scans for this particular parameter. The agreement between radiologists for loculation of ascites was fair to poor (kappa range, 0.21 to 0.4), showing that this is a less reliable parameter.
Table 5.
Kappa statistics for interobserver variation (normal versus abnormal scan)
| Parameter | Comparison between Observers | Kappa Score | Probability > Z |
|---|---|---|---|
| Peritoneal calcification | Observer 1 versus 2 | 0.66 | 0.00001 |
| Observer 1 versus 3 | 0.75 | 0.00001 | |
| Observer 2 versus 3 | 0.54 | 0.00001 | |
| Bowel thickening | Observer 1 versus2 | 0.46 | 0.00001 |
| Observer 1 versus 3 | 0.42 | 0.00001 | |
| Observer 2 versus 3 | 0.71 | 0.00001 | |
| Bowel tethering | Observer 1 versus 2 | 0.72 | 0.00001 |
| Observer 1 versus3 | 0.57 | 0.00001 | |
| Observer 2 versus 3 | 0.57 | 0.00001 | |
| Loculations | Observer 1 versus 2 | 0.33 | 0.0009 |
| Observer 1 versus 3 | 0.21 | 0.01 | |
| Observer 2 versus 3 | 0.40 | 0.00006 | |
| Peritoneal thickening | Observer 1 versus2 | 0.32 | 0.004 |
| Observer 1 versus 3 | 0.27 | 0.008 | |
| Observer 2 versus 3 | 0.59 | 0.00001 | |
| Bowel dilatation | Observer 1 versus3 | 0.63 | 0.00001 |
CT Scan Scores
A total CT score was calculated for each scan by summing the median scores for each of the six parameters. The highest possible score was 22 (Table 1, the scoring criteria). Total scores for EPS patients, PD controls, and HD controls are shown in Figure 2A. The median total score was 9 of 22 (range, 2 to 16) for EPS patients, 1 of 22 (range, 0 to 3) for the PD controls, and 0 of 22 for the HD controls. There was a significant difference in total score between EPS patients and PD controls (P < 0.00001) and EPS patients and HD controls (P < 0.00001; Wilcoxon rank-sum test). Interestingly, there was also a significant difference between PD controls versus HD controls, with PD controls having significantly higher scores (P = 0.0004).
Figure 2.
CT scan scores for EPS patients, HD and PD controls. (A) Total score (sum of scores for each parameter out of a maximum of 22). (B) Peritoneal calcification. (C) Bowel tethering. (D) Bowel wall thickening. (E) Loculation. (F) Peritoneal thickening. (G) Bowel dilation. **P < 0.01, ***P < 0.0001, ****P < 0.00001. (A) Wilcoxon rank-sum test. (B–G) Fisher's exact test. Horizontal lines indicate median scores.
In addition to differences in total score, there were significant differences between the CT scans of EPS patients at diagnosis compared with HD and PD controls for all individual parameters assessed (P = 0.0001 for all parameters except peritoneal calcification, P = 0.006, Fisher's exact test; Figure 2B–G). Small bowel distribution was abnormal (in-drawing to the center of the abdomen) in 20 of 27 EPS patients at diagnosis, compared with 0 of 35 controls. Peritoneal calcification was also specific, but not as sensitive, being abnormal in 9 of 27 cases but only 1 of 35 controls. Loculations were the least specific abnormality, being present in 6 of 20 (30%) of the PD controls. Most of the EPS cases demonstrated bowel wall thickening (23 of 27) and bowel dilation (26 of 27), consistent with the presence of clinical or subclinical subacute bowel obstruction at diagnosis. Two EPS patients had relatively low total scores at diagnosis (2 of 22 and 3 of 22). However, postdiagnostic CT scans in both of these patients 3 to 6 mo later showed higher scores (7 of 22 and 4.5 of 22, respectively).
There were minor abnormalities in 12 of 20 of the PD control CT scans, which was significantly different from the HD controls (P < 0.0004, Wilcoxon rank-sum test). These included mild loculation of ascites (4 scans), mild bowel wall thickening (3 scans), small degree of localized peritoneal thickening (3 scans), and mild localized bowel dilation (4 scans). The indications for the PD control scans included change in bowel habit, previous ovarian carcinoma, sarcoidosis, cystic pancreas, and routine screening when on PD for more than 4 yr; as already stated, no control patients have subsequently developed EPS.
EPS CT Scan Score at Diagnosis and Clinical Outcome
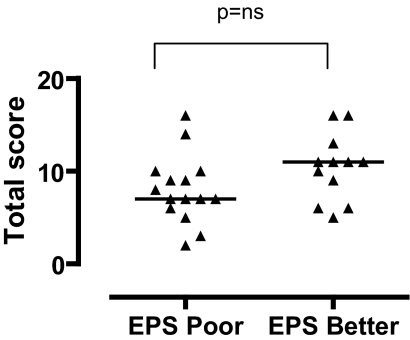
Fifteen of 27 of the EPS patients in the CT scan cohort had a poor outcome (9 deaths, 6 prolonged TPN and /or peritoneolysis surgery). The median score at diagnosis was 7 for the poor outcome group and 10.5 for the better outcome group (Figure 3). This was not statistically significant (P = 0.08, Wilcoxon rank-sum test).
Figure 3.
Total CT scan scores at diagnosis in EPS patients divided into those with poor final outcome (death or prolonged TPN) and those with better outcome. There was no significant difference in the CT scan scores at diagnosis between the poor and better outcome groups. Horizontal lines indicate median scores.
CT Scan Scores before Diagnosis of EPS
Thirteen EPS patients had 25 CT scans more than 4 mo before the diagnosis of EPS. The 4-mo cut-off was selected to avoid the peridiagnostic period. Where more than one scan was taken (7 patients), the scan closest to 4 mo before the diagnosis of EPS was considered (median, 1.47 yr before diagnosis; range, 0.41 to 3.1 yr). As can be seen from Figure 4A, only 4 of 13 patients had significantly abnormal prediagnostic CT scans (total score >2.5). One of these patients had an abnormal scan 0.62 yr before the diagnosis (total score 9), but the total score had been 1 of 22 on an earlier scan 1.3 yr before diagnosis. This patient was asymptomatic, and scans were done for screening purposes. All of the other three patients with significantly abnormal prediagnostic scans had symptoms at the time of the scan consistent in retrospect with EPS. The remaining 9 EPS patients had prediagnostic scans, which showed only mild abnormalities (Figure 4A).
Figure 4.
Longitudinal changes in CT scans in EPS patients. (A) CT scan scores of prediagnostic scans taken more than 4 mo before diagnosis, compared with CT scans taken at the time of diagnosis of EPS. Nine of 13 patients had near-normal prediagnostic CT scans. (B) Diagnostic and postdiagnostic scans in EPS patients with poor outcome (postdiagnostic scans 0.32 to 1.93 yr after diagnosis). There was little change between these scans. (C) Diagnostic and postdiagnostic scans in patients with better outcome (postdiagnostic scans taken between 0.46 and 2.5 yr after diagnosis). Only half (4 of 8) of the better outcome patients showed improvements in their postdiagnostic scans. (D) Peritoneal calcification scores tended to increase with time in the EPS patients.
CT Scan Scores after Diagnosis of EPS
Fifteen patients had CT scans more than 4 mo after the diagnosis of EPS (30 scans). Where patients had more than one scan, the scan furthest in time from diagnosis was considered. Of those EPS patients with poor outcomes, seven had postdiagnostic scans between 0.34 and 1.93 yr postdiagnosis (median, 1.2 yr postdiagnosis). There was little change in the overall scores in this group (median total score at diagnosis, 7 of 22; postdiagnosis, 5.5 of 22; Figure 4B). In the better outcome group, 4 of 8 scans improved while 4 of 8 scans showed the same or worse scores (Figure 4C). Thus, even in the patients with a better clinical outcome, 50% of the scans continued to show marked abnormalities.
As may be expected, the degree of peritoneal calcification tended to increase from the prediagnostic to the diagnostic and postdiagnostic scans (Figure 4D). Changes in other individual parameters with time were too variable among different patients to draw any firm conclusions.
Discussion
This study was designed to determine whether CT scanning could reliably be used to make a diagnosis of EPS and to resolve which CT parameters indicative of EPS could be consistently interpreted by different radiologists. The observation that three radiologists reviewing anonymized scans in random order all found that there were significant changes in abdominopelvic CT scans at the time of diagnosis of EPS compared with control scans in HD and PD patients, confirms that CT scanning is a useful adjunct in the diagnosis of EPS in the correct clinical setting. Interobserver reliability was good for peritoneal calcification, small bowel tethering to the mesentary, bowel wall thickening, and bowel dilation (kappa >0.4 for comparisons between each of the radiologists). Interobserver reliability was poorer for peritoneal thickening, but radiologists 2 and 3 agreed more strongly for this parameter (kappa = 0.59) than with observer 1. This suggests that peritoneal thickening is less reliable but also that there may have been systematic differences between the radiologists in scoring this parameter. Loculation of ascites was not a very reliable indicator, both in terms of the fact that it was not reliably scored and it was also present in several of the control PD patients. As would be expected, the agreement was stronger when scores were merged as either normal (score 0) or abnormal (score 1 to 4), than when there were three variables (score normal, mild changes, or severe changes). While scoring was independent and blinded, the radiologists and clinicians involved agreed to scoring criteria at two meetings before the study, which may have been critical in improving the agreement in this study. This highlights the importance of education and training for radiologists involved in analyzing CT scans in PD patients.
Having determined which CT scan changes are suggestive of a diagnosis of EPS, we reviewed prediagnostic scans to determine whether CT scanning could be useful as a screening tool in long-term PD patients for EPS. Prediagnostic scans were available for 13 of 27 of the EPS patients (>4 mo before the diagnosis of EPS). Interestingly, the prediagnostic scans were normal or near-normal in 9 of 13 patients. Of the 4 patients with abnormal prediagnostic scans, 3 had symptoms at the time of the prediagnostic scans that suggest delayed diagnosis rather than preclinical changes detectable by CT scanning. Only one patient had abnormalities detected on prediagnostic CT scanning that were not clinically apparent. Thus it appears that many patients may have a fulminant course of EPS, where the CT and clinical changes occur over a relatively short space of time in susceptible individuals, perhaps triggered by bacterial peritonitis, or change of dialysis modality. This might make a system of screening PD patients with CT scans less useful, although clearly the index of suspicion should be especially high after cessation of PD. This finding needs to be evaluated further in a prospective study of screening CT scans in patients on long-term PD.
The total CT scan score at the time of diagnosis of EPS did not correlate with the clinical outcome (death, peritoneolysis surgery, or prolonged TPN) in this study. Similarly when postdiagnostic scans were considered, it was not possible to differentiate those patients with a better clinical outcome from those with a worse outcome dependent on improvements in CT scan scores. It is interesting to note that 4 of 8 patients in the good outcome group with postdiagnostic scans available improved clinically with marked changes still present on their CT scans.
Since this is a retrospective study, the CT scanning protocols used were not identical between individual subjects. However, the scans were all considered by the radiologists to be of diagnostic quality. Because most of the CT scans of the control patients were taken for clinical indications rather than as a screening procedure, the number of abnormalities in the control scans may have been higher than would be the case in a screening study. We did find significantly more abnormalities in the CT scans of the PD control patients than the HD controls. This is an interesting finding but would need to be confirmed in a prospective study. Despite these limitations, there was a clear separation between the CT abnormalities in the EPS patients compared with the controls. The fact that the scans were done as part of the clinical service, and yet CT scan changes in EPS were readily apparent, makes this study more clinically relevant.
Conclusion
This is the first systematic analysis of a CT scan scoring system in EPS. We have demonstrated that there are significant abnormalities detectable at the time of diagnosis of EPS and that these may be reliably scored by independent radiologists with the appropriate training. This study also suggests that CT scanning may not be useful as a screening tool for EPS as the scan is frequently normal even a few months before the fulminant illness. This observation needs to be confirmed in a larger prospective study.
Disclosures
None.
Acknowledgments
The authors thank Joseph Eliahoo, Statistical Consultant, Imperial College Statistical Advisory service, for performing the statistical analyses, and Terry Solomon for anonymizing the CT scans. This work was supported by the Imperial College Kidney and Transplant Institute. The authors have no competing financial interests to declare.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gandhi VC, Humayun HM, Ing TS, Daugirdas JT, Jablokow VR, Iwatsuki S, Geis WP, Hano JE: Sclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patients. Arch Intern Med 140: 1201–1203, 1980 [PubMed] [Google Scholar]
- 2.Bradley JA, McWhinnie DL, Hamilton DN, Starnes F, Macpherson SG, Seywright M, Briggs JD, Junor BJ: Sclerosing obstructive peritonitis after continuous ambulatory peritoneal dialysis. Lancet 2: 113–114, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Oules R, Challah S, Brunner FP: Case-control study to determine the cause of sclerosing peritoneal disease. Nephrol Dial Transplant 3: 66–69, 1988 [PubMed] [Google Scholar]
- 4.Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, Kubo H, Kin M, Nakamoto M, Ohira S, Shoji T: Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 44: 729–737, 2004 [PubMed] [Google Scholar]
- 5.Rigby RJ, Hawley CM: Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant 13: 154–159, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Stafford-Johnson DB, Wilson TE, Francis IR, Swartz R: CT appearance of sclerosing peritonitis in patients on chronic ambulatory peritoneal dialysis. J Comput Assist Tomogr 22: 295–299, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Krestin GP, Kacl G, Hauser M, Keusch G, Burger HR, Hoffmann R: Imaging diagnosis of sclerosing peritonitis and relation of radiologic signs to the extent of the disease. Abdom Imaging 20: 414–420, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Korzets A, Korzets Z, Peer G, Papo J, Stern D, Bernheim J, Blum M: Sclerosing peritonitis: possible early diagnosis by computerized tomography of the abdomen. Am J Nephrol 8: 143–146, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Summers AM, Clancy MJ, Syed F, Harwood N, Brenchley PE, Augustine T, Riad H, Hutchison AJ, Taylor P, Pearson R, Gokal R: Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failure. Kidney Int 68: 2381–2388, 2005 [DOI] [PubMed] [Google Scholar]
- 10.George C, Al-Zwae K, Nair S, Cast JE: Computed tomography appearances of sclerosing encapsulating peritonitis. Clin Radiol 62: 732–737, 2007 [DOI] [PubMed] [Google Scholar]