Abstract
Background and objectives: In idiopathic membranous nephropathy (IMN), CD20 B-cell depletion by rituximab may induce nephrotic syndrome (NS) remission. Whether this is associated with kidney function restoration and regression of the glomerular pathology was evaluated.
Design, setting, participants, & measurements: Treatment-induced morphofunctional changes were evaluated in 7 IMN patients consenting to repeat functional and morphologic evaluations after stable disease remission achieved by four weekly rituximab (375 mg/m2) infusions.
Results: Over a median of 21 mo from rituximab administration, NS remission was associated with 8.5-fold increase versus baseline in sodium fractional clearance from 1.56 to 13.25, decrease in renal plasma flow from 440.8 to 276.6 ml/min per 1.73 m2, stable glomerular filtration rate, and increased renal vascular resistances. Changes in sodium fractional clearance and hemoglobin concentration were positively correlated (r = 0.82). Biopsy reevaluations showed complete or partial reabsorption of subepithelial deposits. Median (interquartile range) IgG4 staining score decreased from 3 (3–3) to 1 (0–2), whereas total numbers of slit diaphragms (0.27; range, 0.19 to 0.30 versus 0.86; range, 0.53 to 1.16 slits/μm glomerular basement membrane) and percentages of those with electron-dense diaphragm (55.2; range, 42.0 to 62.0 versus 78.5; range, 73.0 to 82.7 of all slits) significantly increased in parallel with amelioration of glomerular ultrastructural changes. Changes in slit frequency and albumin fractional clearance were negatively correlated (r = −0.79).
Conclusions: In human IMN, treatment-induced NS remission is associated with restoration of sodium homeostasis and kidney hemodynamics, and regression of the glomerular changes underlying proteinuria. These effects are likely to translate into long-term renoprotection.
Idiopathic membranous nephropathy (IMN), the most common cause of the nephrotic syndrome in whites, is an immune-mediated disease resulting from deposition of immunoglobulin G (IgG) and complement components on the subepithelial layer of the glomerular capillary wall (1). The production of nephritogenic immunoglobulins by B cells is one of the earliest steps in the sequence of pathogenic events underlying progressive renal dysfunction in IMN. So far, however, therapeutic approaches to IMN have mostly relied on steroids and immunosuppressant drugs, such as alkylating agents, calcineurin inhibitors, and mycophenolate mofetil, which are not fully specific and carry the risk of severe toxic effects, without appreciably affecting patient and kidney survival (2). This may explain why, over the past 30 yr, the outcome of IMN has not substantially improved. Up to 40% of patients still progress to end-stage renal failure despite treatment (3).
Conceivably, treatments targeted to specific pathogenic mechanisms might limit disease activity more effectively and with less side effects than aspecific immunosuppression. Agents that specifically interfere with B cells ideally represent the first step toward selective therapy (4). With this rationale, we first investigated the efficacy and safety profile of rituximab (Roche, Monza, Italy), a monoclonal antibody against the cell surface antigen CD20 of B cells (5), in 8 IMN patients with persistent nephrotic syndrome refractory to conservative treatment and without previous spontaneous or treatment-induced remissions (6,7). Treatment reduced urinary proteins by 60% and stabilized kidney function in all patients over 1-yr follow-up (6,7). These encouraging results, which still have to be confirmed in a randomized controlled clinical trial, stimulated studies on the use of rituximab for primary glomerular diseases, and several reports subsequently confirmed and extended our initial findings (8–10). Following this preliminary experience, we treated (with rituximab) 50 consecutive patients with IMN and long-lasting nephrotic syndrome refractory to usual treatments. Ten of these patients achieved full and persistent reduction of 24 h proteinuria to less than 0.5 g. Here we evaluated: 1) whether normalization of urinary proteins corrected the functional abnormalities of the nephrotic syndrome and 2) whether this effect could be associated with regression of abnormal IgG deposition and ultrastructural changes in repeat biopsies. Results of this analysis formed the basis of the present report.
Materials and Methods
Patients and Study Design
From May 2001 to January 2007, we treated (with rituximab) 50 consecutive patients with biopsy-proven IMN and a creatinine clearance >20 ml/min per 1.73 m2 who had no previous spontaneous or treatment-induced remissions. Patients had persistent urinary protein excretion rate greater than 3.5 g/24 h despite continued angiotensin-converting enzyme (ACE) inhibitor therapy (ramipril 5 to 10 mg/d) for at least 6 mo. All patients were on a standard low sodium (2 to 3 g/d Na+Cl−) and controlled protein (0.8 g/kg body weight /d) diet. They were treated with loop and/or thiazide diuretics to control edema, combined to ACE inhibitors (ramipril 5 to 10 mg/d according to blood pressure (BP) control and tolerability), angiotensin II receptor blocker, β-blockers, nondihydropyridine calcium channel blockers, and statins as deemed appropriate to control BP, proteinuria, and hypercholesterolemia. Following a baseline evaluation, they received four weekly intravenous infusions (375 mg/m2 each) of rituximab as described previously (6,7). An intensivist attended the start of the infusion and was on call throughout the whole infusion period. After rituximab administration, progressive down-titration in diuretic treatment and concomitant medications were allowed in parallel with proteinuria reduction and progressive amelioration of the nephrotic syndrome. On follow-up, 10 of these patients eventually achieved a complete remission of the disease with a persistent reduction of urinary proteins to less than 0.5 g/24 h for 6 mo or more. Seven of these patients provided a written informed consent to repeat the functional and morphologic evaluations performed at baseline and were therefore considered for the present analyses. The study was run in adherence to the Declaration of Helsinki.
Clinical and Laboratory Evaluations
Before rituximab administration, body weight and BP were measured and blood and urine samples were collected to evaluate renal function parameters, such as 24-h urinary protein excretion (mean of three measurements in three consecutive urine collections), albuminuria and albumin fractional clearance, serum creatinine and creatinine clearance; routine hematochemistry including serum lipids (total and HDL cholesterol, and triglycerides), serum albumin and electrolytes, white blood cell, red blood cell, and platelet count; and lymphocyte subpopulations, including CD20 and CD19 B cells, CD3, CD4, CD8 T cells, natural killer (NK) cells, and CD4/CD8 ratio. Lymphocyte subpopulations were assessed by fluorescence-activated cell sorting. CD19 was used as B-cell marker that, in contrast to CD20, is detected by flow cytometry regardless of rituximab binding to its receptor (11).
The above parameters were then evaluated every month during the first year after therapy, and at least every 3 mo thereafter up the repeat biopsy (study end).
Clearance Studies
Before rituximab infusion and at the time of the repeat biopsy, consenting patients were admitted at the Department of Renal Medicine of the Clinical Research Center for Rare Disease of the Mario Negri Institute for Pharmacologic Research to have their glomerular filtration rate (GFR) and renal plasma flow (RPF) measured by inulin and para-aminohippuric acid (PAH) renal clearances (12), respectively. Data were expressed per 1.73 m2 of body surface area. During the clearance studies, plasma and urine samples were collected to measure albumin and sodium fractional clearance. Inulin and PAH concentrations in plasma and urine samples were determined using previously described colorimetric assays (13,14). GFR and RPF were calculated as the average of three inulin and PAH clearances, filtration fraction and renal vascular resistances by standard formula.
Histology Evaluation
Renal tissue specimens were obtained at basal evaluation (before rituximab treatment) and at the time of the repeat biopsy. Histology samples were processed for light microscopy, immunofluorescence, and electron microscopy analysis by using standard techniques. Sections of paraffin-embedded specimens were stained with hematoxylin and eosin, Masson's trichrome, periodic acid-Schiff, and silver stain.
Immunofluorescence analysis.
Total IgG, IgG subclasses, and C3 were analyzed by direct immunofluorescence technique. For the detection of total IgG, a fluorescein isothiocyanate (FITC)-conjugated purified Ig fraction of rabbit antiserum against normal human IgG (not reacting with IgA and IgM or with IgG-deprived plasma) was used (DakoCytomation, Glostrup, Denmark). IgG2 and IgG4 were detected using FITC-conjugated, subclass-specific mouse monoclonal antibodies (Sigma, St Louis, MO). Complement C3 was detected using FITC-conjugated purified immunoglobulins of rabbit antiserum against human C3c (reacting with C3c complement and with the C3c part of C3 and C3b; DakoCytomation). Fragments of renal tissues were embedded in OCT medium and frozen in liquid nitrogen. Tissue sections (3 μm thick) were cut using a Mikrom 500 O cryostat (Mikrom, Walldorf, Germany) and either stained immediately or stored at −20°C until further processing. Nonspecific binding of antibodies was blocked with 1% phosphate-buffered saline/bovine serum albumin for 30 min at room temperature. The sections were fixed with acetone and incubated with antibody for 30 min at room temperature. Primary antibodies were omitted in control experiments. An average of seven glomeruli was analyzed in each sample by an investigator unaware of biopsy groups. Sections were examined with a Leika DM-R microscope equipped with epifluorescence and appropriate filters. Signal intensity was graded on a scale of 0 to 3 (0, no staining; 1, weak; 2, moderate; 3, strong intensity).
Transmission electron microscopy.
Biopsy specimens were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 4 h at 4°C, washed in cacodylate buffer, and then postfixed with a solution of tannic acid-glutaraldehyde (1% tannic acid and 1% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4) for 2 h to increase the contrast and definition of extracellular structures in the glomerulus and reveal the isoporous substructure of the slit diaphragm (15). The kidney fragments were then postfixed in 1% osmium tetroxide for 1 h, dehydrated through ascending grades of alcohol, and embedded in Epon resin. Semithin sections were stained with toluidine blue in borax and examined by light microscopy. Ultrathin sections (60 to 100 nm) were cut on an ultramicrotome (LKB Instruments, Milan, Italy), collected on copper grids, and stained with uranyl acetate and lead citrate. The evaluation of the subepithelial deposits distribution together with the morphometric analysis of the ultrastructural changes of the epithelial layer of glomerular barrier were performed by transmission electron microscopy (Morgagni 268D, Philips, Brno, Czech Republic).
Morphometric analysis.
An average of 50 images of three glomeruli per patient was digitized (final magnification, ×56,000) and processed with the image-processing software (NIH Image, version 1.6.). For each image, the length of the glomerular basement membrane (GBM) was calculated using computer-assisted morphometric unit. GBM profile was manually outlined using a line automatically measured in screen pixels. Exact enlargement was calculated using digitized images of a calibration grid (Ernest F. Fullam, Latham, NY). Epithelial filtration slit frequency was evaluated as number of slits observed per micrometer of GBM length. The presence of the filamentous structure in the slits observed in each patient was evaluated and expressed as a percentage.
Statistical Analysis
Results are reported as N (%) or mean ± SD or median (interquartile range) as appropriate. Between-groups comparisons were performed by means of unpaired t test of Wilcoxon rank sum test. Within-group comparisons were performed by paired t test or Wilcoxon signed rank test. Multiple logistic regression analysis was used to explore whether selected baseline characteristics were associated with remission. The statistical significance level was defined as P < 0.05.
Results
Baseline Characteristics
At the time of rituximab administration, patients had a relatively well-controlled BP, normal or moderately reduced kidney function, dyslipidemia, and heavy proteinuria (Table 1). Compared with those who failed to achieve complete disease remission, the 10 patients achieving remission following rituximab administration were more frequently female and had lower serum creatinine levels and less severe proteinuria to start with. Multiple logistic regression showed that female gender (P < 0.05) independently predicted the remission of the disease. Baseline characteristics of the 7 patients (six females and one male) achieving remission and consenting to the repeat functional and histology evaluations (Table 2) were similar to those of nonconsenting patients.
Table 1.
Clinical and laboratory parameters at the time of rituximab therapy in the study group as a whole and in patients who achieved or did not achieve stable remission of proteinuria considered separately
| All Patients (n = 50) | Patients with Remission (n = 10) | Patients without Remission (n = 40) | |
|---|---|---|---|
| Demographics | |||
| age (yr) | 53.2 ± 16.5 | 46.6 ± 20.1 | 54.9 ± 15.3 |
| male gender | 31 (62%) | 1 (10%) | 30 (75%)d |
| Clinical parameters | |||
| body weight (kg) | 74.0 ± 18.2 | 60.3 ± 28.7 | 77.8 ± 12.0b |
| systolic BP (mmHg) | 133.9 ± 17.7 | 127.0 ± 11.9 | 135.6 ± 18.6 |
| diastolic BP (mmHg) | 81.5 ± 11.7 | 78.3 ± 7.5 | 82.3 ± 12.5 |
| mean BP (mmHg) | 98.6 ± 12.4 | 94.6 ± 8.3 | 99.6 ± 13.2 |
| Laboratory parameters | |||
| Hb concentration (g/dl) | 12.7 ± 1.8 | 11.4 ± 1.2 | 13.0 ± 1.8a |
| hematocrit (%) | 37.1 ± 5.9 | 34.4 ± 3.2 | 37.9 ± 6.3a |
| cholesterol (mg/dl) | 277.5 ± 65.7 | 273.1 ± 71.3 | 278.6 ± 65.1 |
| triglycerides (mg/dl) | 175.1 ± 81.0 | 112.5 ± 56.3 | 192.4 ± 78.7b |
| serum creatinine (mg/dl) | 1.4 ± 0.6 | 1.0 ± 0.2 | 1.5 ± 0.6d |
| serum albumin (g/dl) | 2.2 ± 0.7 | 2.4 ± 0.5 | 2.2 ± 0.7 |
| proteinuria (g/24 h) | 10.1 ± 6.9 | 6.1 ± 2.3 | 11.1 ± 7.3c |
| 8.4 (5.7–12.7) | 5.5 (4.8–6.3) | 9.5 (6.2–13.3) |
Data are no. (%), mean ± SD, or mean ± SD and median (interquartile range).
P < 0.05, versus patients with remission.
P < 0.01, versus patients with remission.
P < 0.001, versus patients with remission.
P < 0.0001, versus patients with remission.
Table 2.
Clinical, laboratory, and clearance parameters before rituximab therapy (baseline) and at the time of repeated biopsy
| Baseline | Repeat Biopsy | |
|---|---|---|
| Clinical parameters | ||
| body weight (kg) | 67.1 ± 10.2 | 69.1 ± 12.3 |
| systolic BP (mmHg) | 123.4 ± 9.3 | 126.6 ± 7.7 |
| diastolic BP (mmHg) | 76.9 ± 8.9 | 76.3 ± 7.1 |
| mean BP (mmHg) | 92.4 ± 8.5 | 93.0 ± 6.0 |
| Laboratory parameters | ||
| Hb concentration (g/dl) | 11.4 ± 1.2 | 12.4 ± 1.4 |
| hematocrit (%) | 34.3 ± 3.5 | 37.8 ± 2.4 |
| cholesterol (mg/dl) | 254.4 ± 58.6 | 192.7 ± 56.4b |
| triglycerides (mg/dl) | 107.3 ± 57.4 | 63.1 ± 35.4a |
| proteinuria (g/24 h) | 5.5 [4.8 to 6.32] | 0.3 [0.2 to 0.3]c |
| serum creatinine (mg/dl) | 1.0 ± 0.3 | 1.0 ± 0.3 |
| serum albumin (g/dl) | 2.5 ± 0.4 | 3.8 ± 0.4b |
| Clearance parameters | ||
| GFR (ml/min per 1.73 m2) | 45.5 [37.3 to 82.5] | 52.5 [37.1 to 56.5] |
| RPF (ml/min per 1.73 m2) | 440.8 [378.6 to 652.4] | 276.6 [258.8 to 360.1]a |
| RVR (mmHg/(ml/min per 1.73 m2)) | 14.8 [9.5 to 16.0] | 21.1 [16.8 to 22.9] |
| filtration fraction | 0.12 [0.09 to 0.14] | 0.16 [0.15 to 0.20] |
| albumin fractional clearance | 1.50 [0.62 to 3.38] | 0.07 [0.02 to 0.10]b |
| sodium fractional clearance | 1.56 [1.18 to 1.90] | 13.25 [8.90 to 20.60]b |
Data are mean ± SD or median [interquartile range].
P < 0.05, versus at the time of repeat biopsy.
P < 0.01, versus at the time of repeat biopsy.
P < 0.0001, versus at the time of repeat biopsy.
Clinical Outcomes
Repeat biopsies were performed after a median time of 21 mo (range, 7 to 59 mo) from rituximab therapy and 11 mo (range, 6 to 47 mo) from persistent reduction of 24-h proteinuria to less than 0.5 g. In all patients, proteinuria reduction to normal range was paralleled by a full remission of the signs of the nephrotic syndrome, with normalization of serum albumin and cholesterol levels, and a significant increase in body weight, hematocrit, and hemoglobin concentration. BP and serum creatinine were stable over time (Table 2). CD20 and CD19 B cells were fully cleared from the circulation just after the first rituximab administration. In all patients, CD20 and CD19 counts progressively increased toward normal range, starting from 6 to 9 mo after infusion, but in no case this was followed by recurrence of proteinuria.
Functional Evaluations
GFR was stable over time, whereas the RPF significantly declined (Table 2). Consequently, the filtration fraction decreased and renal vascular resistance increased at the time of the repeat biopsy compared with baseline.
Albumin fractional clearance significantly decreased and sodium fractional clearance significantly increased (by 8.5-fold) compared with baseline (Table 2). Percent changes in sodium fractional excretion and hemoglobin concentration were positively correlated (r = 0.82, P < 0.05). The correlation between changes in sodium fractional clearance and hematocrit showed a similar trend that, however, failed to achieve the statistical significance.
Morphologic Studies
Light microscopy and immunostaining.
Basal histology samples showed a typical pattern of IMN. At basal evaluation, all of the tissue samples showed diffuse, granular immunostaining for total IgG, IgG4, and C3 along glomerular capillary walls. At repeat biopsy, no change was observed in staining for total IgG, whereas three tissue samples had no staining for IgG4 and the remaining four samples had a remarkably reduced staining compared with baseline (Table 3). Thus, in the study group as a whole, the IgG4 staining score significantly decreased from baseline to repeat evaluation (P < 0.01). C3 staining was no longer detectable in two repeat biopsies and, in the study group as a whole, it showed a trend to decrease compared with baseline that, however, was not statistically significant. There was no IgG2 staining at baseline as well as at repeat biopsy (Table 3).
Table 3.
Immunostaining scores (ranging from 0 = no staining to 3 = maximum staining) for total IgG, IgG2, IgG4, and C3 in kidney biopsies obtained from seven patients before rituximab administration (Pre) and after remission of the nephrotic syndrome (Post)
| Patient No. | Stage
|
Total IgG
|
IgG2
|
IgG4
|
C3
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | III | III/IV | 3 | 3 | 0 | 0 | 3 | 1 | 2 | 2 |
| 2 | III | III/IV | 3 | 3 | 0 | 0 | 3 | 1 | 1 | 3 |
| 3 | I | no deposits | 3 | 2 | 0 | 0 | 3 | 0 | 2 | 0 |
| 4 | III | III/IV | 3 | 3 | 0 | 0 | 3 | 1 | 1 | 0 |
| 5 | II | III/IV | 3 | 3 | 0 | 0 | 3 | 2 | 3 | 1 |
| 6 | I | no deposits | 3 | 3 | 0 | 0 | 3 | 0 | 3 | 2 |
| 7 | II | III/IV | 3 | 3 | 0 | 0 | 3 | 0 | 3 | 2 |
Electron microscopy.
At basal evaluation, all biopsy samples showed stage I to III IMN lesions (Table 3) with diffuse subepithelial electron-dense deposits and diffuse effacement of foot processes (Figure 1, A). At repeat biopsy, electron-dense deposits almost completely disappeared and the podocyte changes were remarkably less severe compared with baseline (Figure 1, C). In glomeruli of 2 of the 7 tissue samples, the deposits were no longer detectable. In the remaining 5 cases, the deposits were replaced by electron-lucent material, reflecting almost complete reabsorption of deposits as seen in stage IV IMN, although mixed patterns were found (Table 3).
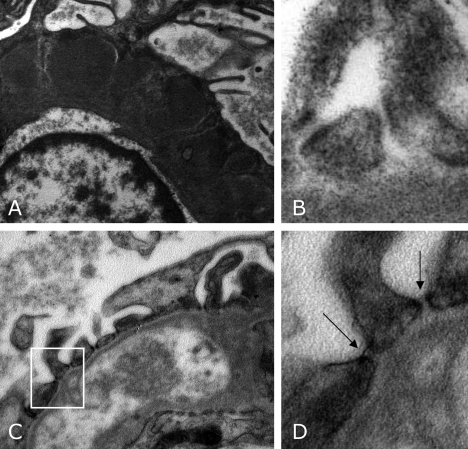
Figure 1.
Representative transmission electron micrographs of the capillary wall in a patient before (A, B) and after rituximab therapy (C, D). (A) Diffuse subepithelial electron-dense immune deposits at baseline. They almost completely disappeared after rituximab therapy (C). Slit diaphragm ultrastructure between neighboring podocytes is shown in panels B and D. Before rituximab therapy, a high percentage of slit pores did not show intact slit diaphragms (B). The electron-dense filamentous images were instead detectable after treatment (D). (D) Enlarged detail of panel C. Arrows indicate electron-dense diaphragms in the filtration slits. Original magnifications: A, C, ×18,000; B, D, ×56,000.
Electron microscopy analysis of filtration slits.
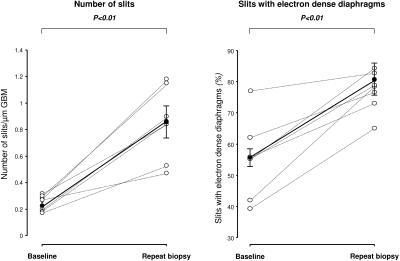
At basal evaluation, diffuse effacement of foot processes was associated with a remarkable reduction in the frequency of filtration slit pores (0.27 slits/μm GBM; range, 0.19 to 0.30 slits/μm GBM) compared with that found in a group of nonproteinuric controls (2.04 slits/μm GBM; range, 1.80 to 2.15 slits/μm GBM). At repeat biopsy, slit pore frequency significantly increased compared with basal biopsy in all patients (0.86 slits/μm GBM; range, 0.53 to 1.16 slits/μm GBM, P < 0.05) (Figure 2, left panel).
Figure 2.
Number of slits per μm GBM (left) and percentage of slits with electron-dense diaphragms (right) before rituximab administration (baseline) and after persistent disease remission (repeat biopsy).
At baseline, the percentage of pores spanned by the linear image of slit diaphragm (55.2%; range, 42.0% to 62.0%) was low in respect to the percent observed in controls (71.5%; range, 56.0% to 75.0%). At repeat biopsy, the proportion of slit pores showing electron-dense diaphragms (Figure 1D, arrows, for comparison with baseline in 1B) significantly increased (78.5%; range, 73.0% to 82.7%, P < 0.05) in all patients to levels similar to those observed in healthy subjects (Figure 2, right panel).
Morphofunctional Correlations
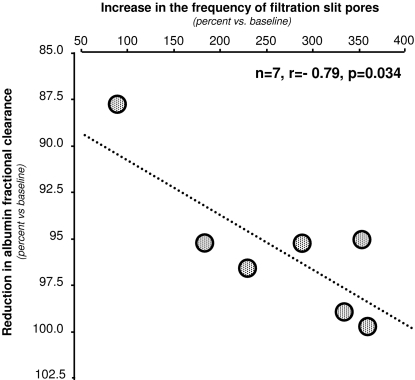
Percent changes (versus baseline) in albumin fractional clearance and frequency of filtration slit pores were negatively correlated (r = −0.79), and the correlation was statistically significant (P < 0.05, Figure 3). A similar correlation was observed between changes in albumin fractional clearance and changes in the percentage of slit diaphragms displaying filamentous image that, however, was only marginally significant (r = −0.70, P = 0.08).
Figure 3.
Correlation between the percent changes in albumin fractional clearance and the concomitant percent changes in the frequency of filtration slit pores at repeat biopsies versus baseline.
Discussion
Here we found that the normalization of proteinuria after rituximab treatment in 7 patients with IMN and long-lasting nephrotic syndrome refractory to RAS inhibitor treatment was associated with both restored sodium homeostasis and kidney hemodynamics and regression or amelioration of immunopathologic changes of active glomerular disease. As expected, the female gender independently predicted an increased chance of remission.
All of the typical features of the nephrotic syndrome recovered at repeat evaluation after rituximab treatment. The most striking functional change was the almost nine-fold increase in sodium fractional clearance observed after the induction of disease remission compared with baseline. The concomitant increase in hemoglobin concentration and resolution of edema most likely reflected the correction of the sodium retentive status (16), which allowed withdrawing diuretic therapy in all patients. In most adult patients with the nephrotic syndrome, increased renal sodium reabsorption, possibly sustained by decreased GFR or protein-induced interstitial inflammation (16) and tubular resistance to atrial natriuretic peptide (17), results in expanded plasma volumes with secondary fluid leakage into the interstitium, a process that can be amplified by hypoalbuminemia and decreased plasma oncotic pressure (18). Thus, extracellular volume expansion upon sodium retention might explain the relatively high renal plasma flow we observed in our patients with overt nephrotic syndrome. Conversely, the restored circulating volumes would account for the decrease in kidney perfusion, with stable GFR, that paralleled the normalization of proteinuria.
Of note, no change in dietary protein or sodium intake was introduced during the study period. Down titration of treatment with diuretics, ACE inhibitors, or angiotensin II receptor blockers was allowed in parallel with reduction of proteinuria and amelioration of the nephrotic syndrome. These changes in concomitant treatment would have been expected to translate into reduced sodium fractional clearance and increased blood volume, with secondary increase in renal plasma flow. Thus, the increase in sodium fractional clearance and the decrease in kidney perfusion we observed after rituximab therapy cannot be explained by changes in diet or concomitant therapy, and were most likely to reflect the remission of the nephrotic syndrome.
Immune-mediated damage to the podocyte has been suggested to play a central role in the pathogenesis of proteinuria of IMN. Data from rat models (Heymann nephritis) that closely simulate human disease (19) and the case of infants with a rare form of membranous nephropathy secondary to immunization with maternal antibodies (20) suggest that damage to the podocyte is initiated by IgG binding to native antigens expressed on the membrane of podocyte foot processes (21). These antibody-antigen complexes that are typically detectable as immune deposits in the subepithelial space are likely to activate complement by the recruitment of later-acting C5, C6, and C5b-9 and insertion of sublytic amounts of the membrane attack complex into podocytes, with cell injury and loss of the glomerular barrier to protein filtration (22–24). Conceivably, rituximab might interrupt this process upstream to the synthesis of pathogenic autoantibodies (5–7). This would explain the extensive reabsorption of immune deposits we observed with rituximab-induced remission, leading to the appearance of electron-lucent areas within the GBM. These changes were similar to those interpreted by Cameron as sites of leached-out deposits (25). In this respect, one major finding of the present study was the complete or almost complete disappearance from repeat biopsies of the strong glomerular IgG4 staining that, instead, was found in all baseline samples. Of note, the IgG4 subclass is a typical component of subepithelial immune-deposits in IMN (26–28), and IgG4 have been detected in circulating immune complexes from patients with IMN (29). Thus, IMN has been suggested to affect individuals who generate a low-affinity IgG4 response to certain antigens (30). The possibility of a pathogenic role for IgG4 in IMN is also suggested by the evidence that, unlike other IgG subclasses (31), IgG4 may fix complement via alternative pathway. Although other complex interactions with the complement system have been described (32,33), the activation of the alternative pathway might specifically explain why at baseline histology evaluation IgG4 glomerular deposition was associated with intense C3 staining and why disappearance of glomerular IgG4 deposits from repeat biopsies was associated with reduced staining for C3 and amelioration of complement-mediated glomerular injury (34).
We found that the decrease in IgG4 staining we observed with disease remission was not paralleled by a concomitant decrease in total IgG. In this regard, it is interesting to note that in other immune-mediated diseases rituximab selectively reduced circulating autoantibodies without substantially affecting total IgG levels (35,36), an effect that was possibly mediated by a selective inhibition of memory B cells (37) and of generation of autoantibody-forming cells. A plausible speculation is that, through depletion of proliferating CD20 B cells, rituximab might promote a progressive depletion of autoreactive plasma cell clones, which have a much faster turnover compared with inactive clones that may proliferate only after contact with exogenous antigens (3). However, autoantibodies produced by autoreactive plasma cells may represent a small proportion of the whole amount of circulating immunoglobulins, and inhibited autoantibody may not translate into appreciable changes in total IgG. Alternative B-cell-mediated effects, such as inhibition of cytokine production, antigen presentation, and T-cell-mediated responses (38–40), might also play a role.
At baseline evaluation heavy proteinuria was associated with extensive foot process effacement and loss of intact slit diaphragms in a high percentage of filtration pores. Finding that these changes largely recovered at repeat biopsies suggests that preventing the immunologically mediated injury allowed progressive restoration of the glomerular epithelial layer (41). The correlation between treatment-induced changes in albumin fractional clearance and number of intact slit diaphragms reinforces a causal link between restoration of glomerular sieving and recovery of podocyte dysfunction. Similarly, podocyte injury/dysfunction was attenuated following transplantation of kidneys from rats with Heymann nephritis into syngenic animals without disease (42). By abrogating kidney exposure to pathogenic antibodies, transplantation also allowed progressive resolution of subepithelial deposits and remission of proteinuria over about 4 wk.
A potential limitation of the present study is the small number of patients, owing to the fact that IMN is a relatively rare disease, and we had to focus on those who had a severe form and at the same time benefited the most from lymphocytolytic therapy. The Ethical Committee did not approve the inclusion of matched controls with proteinuria refractory to rituximab administration, to avoid invasive evaluations that were a priori expected to show no appreciable changes compared with baseline. However, fully consistent findings in repeat biopsies among patients under consideration here substantially overcame this problem.
Conclusion
The morphofunctional abnormalities of IMN can be reverted. Amelioration of renal hemodynamics and normalization of the functional and biologic abnormalities of the nephrotic syndrome are associated with regression of lesions of the glomerular capillary barrier. Whether this may apply to other glomerulopathies and may translate into long-term protection from renal function loss and the potentially life-threatening complications of the nephrotic syndrome remains to be established in properly powered prospective trials.
Disclosures
The authors have no conflicts of interest. This was a fully academic and internally funded study without company involvement.
Acknowledgments
The authors thank Annalisa Perna for performing the statistical analyses, Silvia Ferrari, Nadia Stucchi, Clara Petrò, Antonio Cannata, Fabiola Carrara, Cristian Locatelli for contributing to laboratory evaluations, the nurses of the Clinical Research Center and of the Unit of Nephrology for patient care and monitoring, and Manuela Passera for help in preparing the manuscript.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Glassock RJ, Cohen AH, Adler SG: Primary glomerular diseases. In: The Kidney, 5th ed, edited by Brenner BM, Philadelphia, Saunders, 1996, p 1392
- 2.Perna A, Schieppati A, Zamora J, Giuliano GA, Braun N, Remuzzi G: Immunosuppressive treatment for idiopathic membranous nephropathy: a systematic review. Am J Kidney Dis 44: 385–401, 2004 [PubMed] [Google Scholar]
- 3.Ruggenenti P, Cravedi P, Remuzzi G: Latest treatment strategies for membranous nephropathy. Expert Opin Pharmacother 8: 3159–3171, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Biancone L, Andres G, Ahn H, DeMartino C, Stamenkovic I: Inhibition of the CD40-CD40ligand pathway prevents murine membranous glomerulonephritis. Kidney Int 48: 458–468, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Johnson PW, Glennie MJ: Rituximab: mechanisms and applications. Br J Cancer 85: 1619–1623, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggenenti P, Chiurchiu C, Brusegan V, Abbate M, Perna A, Filippi C, Remuzzi G: Rituximab in idiopathic membranous nephropathy: a one-year prospective study. J Am Soc Nephrol 14: 1851–1857, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P: Rituximab for idiopathic membranous nephropathy. Lancet 360: 923–924, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Rossi P, Demoux AL, Granel B, Bagneres D, Bonin-Guillaume S, Frances Y: Anti-CD20 monoclonal antibody for the treatment of refractory autoimmune haemolytic anaemia associated with idiopathic membranous nephropathy. Rheumatology (Oxf) 44: 403–405, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Quartuccio L, Soardo G, Romano G, Zaja F, Scott CA, De Marchi G, Fabris M, Ferraccioli G, De Vita S: Rituximab treatment for glomerulonephritis in HCV-associated mixed cryoglobulinaemia: efficacy and safety in the absence of steroids. Rheumatology (Oxf) 45: 842–846, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Gallon L, Chhabra D: Anti-CD20 monoclonal antibody (rituximab) for the treatment of recurrent idiopathic membranous nephropathy in a renal transplant patient. Am J Transplant 6: 3017–3021, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Anolik JH, Campbell D, Felgar RE, Young F, Sanz I, Rosenblatt J, Looney RJ: The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum 48: 455–459, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Perico N, Remuzzi A, Sangalli F, Azzollini N, Mister M, Ruggenenti P, Remuzzi G: The antiproteinuric effect of angiotensin antagonism in human IgA nephropathy is potentiated by indomethacin. J Am Soc Nephrol 9: 2308–2317, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Remuzzi A, Perticucci E, Ruggenenti P, Mosconi L, Limonta M, Remuzzi G: Angiotensin converting enzyme inhibition improves glomerular size-selectivity in IgA nephropathy. Kidney Int 39: 1267–1273, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Remuzzi A, Ruggenenti P, Mosconi L, Pata V, Viberti G, Remuzzi G: Effect of low-dose enalapril on glomerular size-selectivity in human diabetic nephropathy. J Nephrol 6: 36–43, 1993 [Google Scholar]
- 15.Rodewald R, Karnovsky MJ: Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 60: 423–433, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Iturbe B, Herrera-Acosta J, Johnson RJ: Interstitial inflammation, sodium retention, and the pathogenesis of nephrotic edema: a unifying hypothesis. Kidney Int 62: 1379–1384, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Perico N, Delaini F, Lupini C, Benigni A, Galbusera M, Boccardo P, Remuzzi G: Blunted excretory response to atrial natriuretic peptide in experimental nephrosis. Kidney Int 36: 57–64, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Perico N, Remuzzi G: Edema of the nephrotic syndrome: the role of the atrial peptide system. Am J Kidney Dis 22: 355–366, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Andres G, Brentjens JR, Caldwell PR, Camussi G, Matsuo S: Formation of immune deposits and disease. Lab Invest 55: 510–520, 1986 [PubMed] [Google Scholar]
- 20.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschenes G, Ronco PM: Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346: 2053–2060, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Couser WG, Salant DJ: In situ immune complex formation and glomerular injury. Kidney Int 17: 1–13, 1980 [DOI] [PubMed] [Google Scholar]
- 22.Cybulsky AV, Quigg RJ, Salant DJ: The membrane attack complex in complement-mediated glomerular epithelial cell injury: formation and stability of C5b-9 and C5b-7 in rat membranous nephropathy. J Immunol 137: 1511–1516, 1986 [PubMed] [Google Scholar]
- 23.Couser WG: Membranous nephropathy: a long road but well traveled. J Am Soc Nephrol 16: 1184–1187, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Cybulsky AV, Quigg RJ, Salant DJ: Experimental membranous nephropathy redux. Am J Physiol Renal Physiol 289: F660–F671, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron JS: Pathogenesis and treatment of membranous nephropathy. Kidney Int 15: 88–103, 1979 [DOI] [PubMed] [Google Scholar]
- 26.Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB: IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int 51: 270–276, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Noel LH, Aucouturier P, Monteiro RC, Preud'Homme JL, Lesavre P: Glomerular and serum immunoglobulin G subclasses in membranous nephropathy and anti-glomerular basement membrane nephritis. Clin Immunol Immunopathol 46: 186–194, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Bannister KM, Howarth GS, Clarkson AR, Woodroffe AJ: Glomerular IgG subclass distribution in human glomerulonephritis. Clin Nephrol 19: 161–165, 1983 [PubMed] [Google Scholar]
- 29.Doi T, Kanatsu K, Mayumi M, Hamashima Y, Yoshida H: Analysis of IgG immune complexes in sera from patients with membranous nephropathy: role of IgG4 subclass and low-avidity antibodies. Nephron 57: 131–136, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Oliveira DB: Membranous nephropathy: an IgG4-mediated disease. Lancet 351: 670–671, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Lucisano Valim YM, Lachmann PJ: The effect of antibody isotype and antigenic epitope density on the complement-fixing activity of immune complexes: a systematic study using chimaeric anti-NIP antibodies with human Fc regions. Clin Exp Immunol 84: 1–8, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao MH, Smith RI, Morrison SL: Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. J Exp Med 178: 661–667, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Zee JS, van Swieten P, Aalberse RC: Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol 64: 415–422, 1986 [PMC free article] [PubMed] [Google Scholar]
- 34.Schulze M, Pruchno CJ, Burns M, Baker PJ, Johnson RJ, Couser WG: Glomerular C3c localization indicates ongoing immune deposit formation and complement activation in experimental glomerulonephritis. Am J Pathol 142: 179–187, 1993 [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards JC, Cambridge G: Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxf) 40: 205–211, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Keogh KA, Wylam ME, Stone JH, Specks U: Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 52: 262–268, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Roll P, Dorner T, Tony HP: Anti-CD20 therapy in patients with rheumatoid arthritis: predictors of response and B cell subset regeneration after repeated treatment. Arthritis Rheum 58: 1566–1575, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Martin F, Chan AC: Pathogenic roles of B cells in human autoimmunity: insights from the clinic. Immunity 20: 517–527, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Tsokos GC: B cells, be gone: B-cell depletion in the treatment of rheumatoid arthritis. N Engl J Med 350: 2546–2548, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Spicer ST, Tran GT, Killingsworth MC, Carter N, Power DA, Paizis K, Boyd R, Hodgkinson SJ, Hall BM: Induction of passive Heymann nephritis in complement component 6-deficient PVG rats. J Immunol 179: 172–178, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Shemesh O, Ross JC, Deen WM, Grant GW, Myers BD: Nature of the glomerular capillary injury in human membranous glomerulopathy. J Clin Invest 77: 868–877, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makker SP, Kanalas JJ: Course of transplanted Heymann nephritis kidney in normal host: implications for mechanism of proteinuria in membranous glomerulonephropathy. J Immunol 142: 3406–3410, 1989 [PubMed] [Google Scholar]