Abstract
Background and objectives: Hyperphosphatemia is an independent risk factor for mortality in ESRD, but factors regulating phosphate clearance on peritoneal dialysis (PD) are incompletely understood. The objective of this study was to test the hypothesis that peritoneal phosphate clearance is better with continuous ambulatory PD (CAPD) as compared with continuous cyclic PD (CCPD) after adjusting for membrane transport status.
Design, setting, participants, & measurements: In this cross-sectional and retrospective study, measurements of peritoneal phosphate clearance of 129 prevalent PD patients were reviewed. Patients were divided according to membrane transport status (high, high average, low average-low categories) and PD modality (CAPD or CCPD).
Results: Among high transporters, peritoneal phosphate clearances were comparable in both modalities. However, treatment with CAPD was associated with increased peritoneal phosphate clearance compared with CCPD among high-average transporters (42.4 ± 11.4 versus 36.4 ± 8.3 L/wk/1.73 m2, P = 0.01), and low-average-low transporters (35.6 ± 5.9 versus 28.9 ± 11 L/wk/1.73 m2, P = 0.034). On multivariate linear regression, PD modality, membrane transport category, and peritoneal creatinine clearance, but not Kt/V urea, were independently associated with peritoneal phosphate clearance.
Conclusions: Peritoneal phosphate clearance is determined by PD modality and membrane transport category, suggesting that PD regimes with longer dwell times may help control hyperphosphatemia in lower transporters.
Hyperphosphatemia is a strong predictor of overall and cardiovascular mortality in ESRD patients treated with peritoneal dialysis (PD) therapy (1,2). Despite this well established risk, approximately 40% of PD patients have serum phosphate concentrations above levels recommended by current guidelines (2). Control of hyperphosphatemia is difficult to achieve for several reasons. First, dietary phosphate restriction is characterized by poor adherence and if achieved can result in protein malnutrition (3). Second, the use of phosphate binders, although effective, is associated with secondary effects, including hypercalcemia, gastrointestinal disturbances, and poor adherence (4,5). Finally, in PD patients phosphate control is known to deteriorate as residual renal function declines, which may contribute to sustained hyperphosphatemia (6). In addition to these factors, peritoneal phosphate clearance plays a role in achieving adequate phosphate homeostasis, although this component of phosphate balance has not been well studied.
In PD patients, diffusive and convective transport of phosphate across the peritoneal membrane contributes to overall phosphate homeostasis. Phosphate is surrounded by an aqueous layer because of its hydrophilic properties and may behave like a larger molecule with respect to solute clearance (7). Because peritoneal phosphate clearance is time-dependent, it may be altered with different peritoneal dialysis modalities and across different peritoneal membrane transport categories (8). These clearance properties have not been well characterized because previous studies were limited by small sample size and no adjustment for peritoneal membrane transport category (9–11). Accordingly, the objective of this study was to determine the effect of membrane transport characteristics and PD modality on peritoneal phosphate clearance in a prevalent PD population. We hypothesized that peritoneal phosphate clearance would be inferior in patients with lower transport membranes when treated with PD modalities with shorter dwell times such as continuous cyclic PD (CCPD) compared with those treated with continuous ambulatory PD (CAPD).
Materials and Methods
Study Population
This was a cross-sectional and retrospective study conducted at The Ottawa Hospital home dialysis unit. The Ottawa Hospital is a tertiary care center providing regional dialysis services to a population of 1.5 million. The study protocol was approved by The Ottawa Hospital Research Ethics Board. Patients included in this study were on CAPD or CCPD for a minimum of 3 mo and were followed between January 1, 2005 and December 31, 2006. We excluded patients who were treated with nocturnal intermittent PD.
Data Collection
Baseline patient demographic characteristics were collected and peritoneal membrane transport category was determined by the closest peritoneal equilibration test (PET) to the time of measurement of dialysis adequacy. PET tests were performed in the morning with 2L of 2.5% dextrose solution (Dianeal 2.5%, Baxter Healthcare, Deerfield, Illinois, USA). The long overnight dwell before the PET was performed with 2.5% Dianeal solution. Dialysate/plasma (D/P) creatinine ratio was measured at 4 h and used to classify patients as low (D/P creatinine ≤0.49), low average (D/P creatinine 0.50 to 0.64), high average (D/P creatinine 0.65 to 0.80) or high (D/P creatinine ≥0.81) transporters according to the criteria defined by Twardowski et al. (12). CAPD was defined as three or more manual twin bag exchanges per day and CCPD was defined as three or more exchanges using a cycler with at least one daytime dwell. There was no restriction on maximum number of exchanges or volumes. Cycler patients with more than one daytime exchange were classified as CCPD.
In our program, all PD patients undergo measurements of dialysis adequacy and phosphate clearance 4 wk after initiation, and then every 6 mo. Additional adequacy testing is performed 1 mo after any change in the dialysis prescription or when indicated clinically. If patients had more than one measurement of dialysis adequacy and phosphate clearance, the first one was included in the analysis. Measurements of both peritoneal and renal Kt/V urea and creatinine clearances, GFR, body surface area (BSA), and protein catabolic rate were calculated by using PD Adequest 2.0 for Windows software (Baxter Healthcare, Deerfield, Illinois, USA). Presence of residual renal function was defined as GFR ≥1 ml/min/1.73 m2. Phosphate concentration in serum and urine were measured using Ortho Vitros 250 Analyser (Ortho-Clinical Diagnostics Inc., Raritan, New Jersey), dialysate phosphate concentration was measured using a Beckman Coulter LX20 (Beckman Coulter Inc., Mississauga, Canada). Coefficient of variation was between 1 and 2% for all phosphate measurements. Phosphate clearances were calculated as follows: (13)
Peritoneal phosphate clearance (L/wk/1.73 m2) = (dialysate phosphate in mmol/L ÷ plasma phosphate in mmol/L) × 24 h effluent dialysate volume (L) × 7 (corrected for 1.73 m2 BSA).
Renal phosphate clearance (L/wk/1.73 m2) = (urine phosphate in mmol/d ÷ plasma phosphate in mmol/L) × 7 (corrected for 1.73 m2 BSA).
Statistical Analyses
Results were expressed as frequencies and percentages for categorical variables, mean ± SD for continuous variables, and median and interquartile range for nonparametric data. Patients were divided according to PD modality (CAPD or CCPD) as well as by peritoneal membrane transport status. Low and low-average transporters were combined because of the small number of low transporters in our population. Differences between the two PD modalities were compared using the t test where data were parametric and the Mann-Whitney U test if nonparametric. Distributions of categorical variables across the three transport groups were compared by means of χ2 test, continuous variables by one-way ANOVA if data were parametric, and Kruskal-Wallis test if nonparametric. Correlation between two continuous variables was expressed as Pearson's correlation coefficient. Simple linear regression was used to analyze the determinants of peritoneal phosphate clearance. Categorical variables entered in the regression included gender, diabetes, PD modality, and peritoneal membrane transport status. The following continuous variables were used in the model: age, time spent on PD, body mass index, protein catabolic rate, serum albumin, GFR, serum phosphate, dialysis volume, ultrafiltration volume, peritoneal creatinine clearance, and peritoneal Kt/V. P values ≤0.05 were considered statistically significant. Statistical analyses were performed using Stata/SE 9.2 (College Station, TX, USA) statistical software.
Results
Patient Characteristics
During the 2-yr study period, a total of 194 PD patients underwent at least one measurement of dialysis adequacy. Of these 194 patients, 27 patients were excluded because they had not completed 3 mo on PD, 24 automated PD (APD) patients were excluded because of treatment with nocturnal intermittent PD, 4 were excluded for incomplete data collection, and 10 were excluded because of incremental PD with fewer than three exchanges per day. The remaining 129 patients were included in this analysis. Characteristics of the population by modality and membrane transport category are described in Tables 1a and 1b respectively. Of the 129 patients, 62 (48%) patients were treated with CAPD, and 67 (52%) with CCPD. When classified by membrane transport category, 22 (17%) were high transporters, 76 (59%) were high-average transporters, and 31 (24%) were low-average or low transporters. Patients treated with CCPD were more likely to be male, and tended to have lower residual GFR and higher transporting membranes. When classified according to the membrane transport category, the only significant baseline difference was the higher serum albumin in lower transporters.
Table 1a.
Patient characteristics, comparison across peritoneal dialysis (PD) modality
| Variable | Total | CAPD | CCPD | P Value |
|---|---|---|---|---|
| N | 129 | 62 (48.1%) | 67 (51.9%) | |
| Male gender | 70 (54.3%) | 26 (41.9%) | 44 (65.7%) | 0.007 |
| Age (years) | 60.3 ± 15.9 | 60.7 ± 15.7 | 59.8 ± 16.3 | 0.753 |
| Diabetes | 40 (31%) | 20 (32.3%) | 20 (29.9%) | 0.768 |
| Time spent on PD (months)a | 9.1 (6.4-21.5) | 8.4 (6.6-14.6) | 10.1 (6.1-28.8) | 0.278 |
| BMI (kg/m2) | 27.1 ± 5.4 | 27 ± 5.1 | 27.2 ± 5.8 | 0.853 |
| RRF present | 89 (68.9%) | 52 (83.9%) | 37 (55.2%) | < 0.001 |
| GFR (ml/min)a | 2.6 (0.6-4.5) | 3.9 (2.3-5.8) | 1.3 (0-3.6) | < 0.001 |
| Urine output (L/day)a | 0.49 (0.11-0.88) | 0.69 (0.35-1.22) | 0.26 (0-0.65) | < 0.001 |
| D/P creatinine | 0.71 ± 0.11 | 0.68 ± 0.1 | 0.73 ± 0.11 | 0.009 |
| Membrane status | 0.028 | |||
| High | 22 (17.1%) | 6 (9.7%) | 16 (23.9%) | |
| High-average | 76 (58.9%) | 36 (58.1%) | 40 (59.7%) | |
| Low-average & low | 31 (24%) | 20 (32.3%) | 11 (16.4%) | |
| PCR (g/kg/day) | 0.81 ± 0.22 | 0.83 ± 0.22 | 0.80 ± 0.24 | 0.564 |
| Serum urea (mmol/L) | 17.2 ± 5.6 | 16.9 ± 5.5 | 17.5 ± 5.6 | 0.616 |
| Plasma creatinine (μmol/L) | 784.3 ± 277.4 | 699.7 ± 249.8 | 862.5 ± 280.5 | < 0.001 |
| Serum albumin (g/L) | 36.4 ± 4.3 | 36.2 ± 4.3 | 36.6 ± 4.4 | 0.631 |
| Serum phosphate (mmol/L) | 1.62 ± 0.5 | 1.55 ± 0.4 | 1.69 ± 0.5 | 0.091 |
Median (interquartile range). CAPD, continuous ambulatory peritoneal dialysis; CCPD, continuous cyclic peritoneal dialysis; NIPD, nocturnal intermittent peritoneal dialysis; BMI, body mass index; RRF, residual renal function; D/P creatinine, dialysate plasma creatinine ratio at 4 h; PCR, protein catabolic rate.
Table 1b.
Patient characteristics, comparison across peritoneal membrane transport status
| Variable | High | High-Average | Low-Average and Low | P Value |
|---|---|---|---|---|
| N | 22 (17.1%) | 76 (58.9%) | 31 (24%) | |
| Male gender | 16 (72.7%) | 41 (53.9%) | 13 (41.9%) | 0.085 |
| Age (years) | 61.8 ± 16.3 | 60.8 ± 15.4 | 57.8 ± 17.3 | 0.599 |
| Diabetes | 5 (22.7%) | 26 (34.2%) | 9 (29%) | 0.569 |
| Time spent on PD (months)a | 7.8 (4.7-12.2) | 9.4 (6.5-22) | 10.4 (6.9-29.4) | 0.339 |
| BMI (kg/m2) | 26.1 ± 6.2 | 27.2 ± 5.1 | 27.7 ± 5.8 | 0.583 |
| RRF present | 14 (63.6%) | 55 (72.4%) | 20 (64.5%) | 0.609 |
| GFR (ml/min)a | 2.1 (0-4.3) | 2.6 (0.8-4.7) | 3.2 (0.5-3.2) | 0.659 |
| Urine output (L/day)a | 0.41 (0-0.66) | 0.51 (0.12-0.99) | 0.5 (0.13-0.88) | 0.322 |
| D/P creatinine | 0.87 ± 0.05 | 0.72 ± 0.04 | 0.57 ± 0.06 | < 0.001 |
| PD modality | 0.028 | |||
| CAPD | 6 (27.3%) | 36 (47.4%) | 20 (64.5%) | |
| CCPD | 16 (72.7%) | 40 (52.6%) | 11 (35.5%) | |
| PCR (g/kg/day) | 0.81 ± 0.22 | 0.84 ± 0.25 | 0.77 ± 0.2 | 0.366 |
| Urea (mmol/L) | 15.9 ± 7.7 | 17.7 ± 5.3 | 17.1 ± 4.3 | 0.435 |
| Creatinine (μmol/L) | 769.1 ± 343.6 | 758.7 ± 233.6 | 857.6 ± 319.3 | 0.239 |
| Albumin (g/L) | 35.2 ± 4.6 | 36 ± 4.2 | 38.4 ± 4 | 0.013 |
| Phosphate (mmol/L) | 1.59 ± 0.4 | 1.60 ± 0.4 | 1.70 ± 0.6 | 0.605 |
Median (interquartile range).
Peritoneal Ultrafiltration and Urea and Creatinine Clearances
There were no significant differences in peritoneal ultrafiltration between the two PD modalities or across the membrane transport categories (Tables 2a, 2b). The CCPD group had higher peritoneal Kt/V urea than the CAPD group (1.86 ± 0.4 versus 1.62 ± 0.3 P < 0.001), but there was no difference in peritoneal creatinine clearance between the two modalities (47 ± 12.8 versus 44 ± 8.1 L/wk/1.73 m2 BSA, P = 0.11) (Table 2a).
Table 2a.
Fluid and solute clearances, comparison across PD modality
| Variable | Total | CAPD | CCPD | P Value |
|---|---|---|---|---|
| Dialysate volume (L/day) | 11.4 ± 4.2 | 8.2 ± 1.6 | 14.4 ± 3.5 | < 0.001 |
| Ultrafiltration on PD (L/day)a | 1.04 (0.67–1.43) | 1.16 (0.63–1.45) | 0.99 (0.67–1.36) | 0.478 |
| Peritoneal Kt/V | 1.74 ± 0.4 | 1.62 ± 0.3 | 1.86 ± 0.4 | < 0.001 |
| Peritoneal creatinine clearance (L/wk/1.73 m2 BSA) | 45.6 ± 10.8 | 44 ± 8.1 | 47 ± 12.8 | 0.11 |
| Peritoneal P/phosphate clearance (L/wk/1.73 m2 BSA) | 39.5 ± 11.3 | 40.9 ± 10.4 | 38.3 ± 12 | 0.199 |
Median (interquartile range). P value by ANOVA if parametric variable, and by Kruskal-Wallis test if nonparametric. BSA, body surface area.
Table 2b.
Fluid and solute clearances, comparison across peritoneal membrane transport status
| Variable | High | High-Average | Low-Average and Low | P Value |
|---|---|---|---|---|
| Dialysate volume (L/day) | 12.7 ± 4 | 11.4 ± 4.5 | 10.6 ± 3.5 | 0.213 |
| Ultrafiltration on PD (L/day)a | 1.07 (0.69–1.5) | 1.03 (0.62–1.40) | 1.23 (0.86–1.45) | 0.468 |
| Peritoneal Kt/V | 1.94 ± 0.5 | 1.71 ± 0.4 | 1.68 ± 0.3 | 0.035 |
| Peritoneal creatinine clearance (L/wk/1.73 m2 BSA) | 53.9 ± 14.2 | 45.8 ± 8.8 | 39.2 ± 8.5 | < 0.001 |
| Peritoneal phosphate clearance (L/wk/1.73 m2 BSA) | 49.6 ± 11.4 | 39.2 ± 10.3 | 33.2 ± 8.5 | < 0.001 |
Median (interquartile range). P value by ANOVA if parametric variable, and by Kruskal-Wallis test if nonparametric.
Patients with high-transport membranes had higher peritoneal Kt/V (1.94 ± 0.5 versus 1.71 ± 0.4 versus 1.68 ± 0.3, for high, high-average, and low-average-low membranes respectively, P = 0.035) and higher peritoneal creatinine clearance (53.9 ± 14.2 versus 45.8 ± 8.8 versus 39.2 ± 8.5 L/wk/1.73 m2, for high, high-average, and low-average-low membranes respectively, P < 0.001) (Table 2b).
Peritoneal Phosphate Clearance
When examined by PD modality alone, there was no significant difference in peritoneal phosphate clearance between those treated with CCPD or CAPD (38.3 ± 12 versus 40.9 ± 10.4 L/wk/1.73 m2, P = 0.199).
When examined by membrane transport category alone, the patients in the low-average and low membrane transport categories had lower peritoneal phosphate clearance than the high-average or the high membrane transport categories (33.2 ± 8.5 versus 39.2 ± 10.3 versus 49.6 ± 11.4 L/wk/1.73 m2, respectively, P < 0.001).
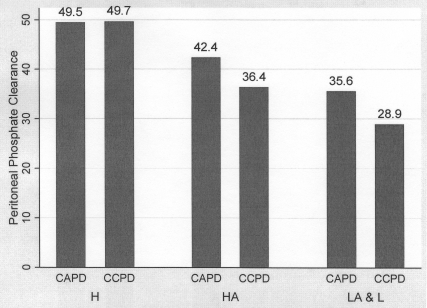
When peritoneal phosphate clearance by modality was examined for each membrane category, there was no significant difference in phosphate clearance between the modalities in the high transport category (Figure 1 and Table 3). In the high average category, however, there was significantly higher phosphate clearance among those treated with CAPD compared with CCPD (42.4 ± 11.4 versus 36.4 ± 8.3 L/wk/1.73 m2, P = 0.01). In the low-average and low membrane category, treatment with CAPD was associated with significantly higher phosphate clearance than CCPD (35.6 ± 5.9 versus 28.9 ± 11 L/wk/1.73 m2, P = 0.034). There was no significant difference in peritoneal creatinine clearance between the two modalities across any of the membrane transport categories (Table 3).
Figure 1.
Mean peritoneal phosphate clearance (L/wk/1.73 m2 BSA) according to peritoneal membrane transport category and peritoneal dialysis modality. CAPD, continuous ambulatory peritoneal dialysis; CCPD, continuous cyclic peritoneal dialysis; H, high transport category; HA, high-average transport category, LA & L: combined low-average and low transport category.
Table 3.
Peritoneal creatinine and phosphate clearances according to peritoneal membrane transport characteristic and PD modality
| Peritoneal Membrane Transport | CAPD | CCPD | P Value |
|---|---|---|---|
| Peritoneal creatinine clearance | |||
| High | 52.6 ± 6 | 54.4 ± 16.4 | 0.792 |
| High-average | 44.5 ± 7.9 | 46.9 ± 9.5 | 0.248 |
| Low-average and low | 40.5 ± 6.7 | 36.9 ± 10.9 | 0.266 |
| Peritoneal phosphate clearance | |||
| High | 49.5 ± 7.6 | 49.5 ± 7.6 | 0.972 |
| High average | 42.4 ± 11.4 | 36.4 ± 8.3 | 0.01 |
| Low-average and low | 35.6 ± 5.9 | 28.9 ± 10.9 | 0.034 |
Determinants of Peritoneal Phosphate Clearance
There was a strong correlation between mean peritoneal phosphate clearance and mean peritoneal creatinine clearance (r = 0.67, P < 0.001). On simple linear regression, peritoneal creatinine clearance and Kt/V directly correlated with peritoneal phosphate clearance whereas serum albumin, high-average transporter category, and low-average -low transporter category were negatively correlated with peritoneal phosphate clearance (Table 4). Multivariate linear regression confirmed that both membrane transport category and PD modality were independently associated with peritoneal phosphate clearance. It is important to note that these findings were independent of peritoneal creatinine clearance. With CAPD as the reference, multivariate regression showed that CCPD had inferior peritoneal phosphate clearance. With high transport status as the reference, high-average and low-average-low transporters both had inferior peritoneal phosphate clearance. Peritoneal creatinine clearance was positively associated with peritoneal phosphate clearance but peritoneal Kt/V urea did not remain significant on multivariate regression analysis.
Table 4.
Determinants of peritoneal phosphate clearance, by linear regression
| Variable | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| Standardized Beta | Regression Coefficient | P value | Standardized Beta | Regression Coefficient | P value | |
| Age | 0.14 | 0.10 | 0.103 | 0.02 | 0.01 | 0.835 |
| Female gender | −0.13 | −2.92 | 0.143 | −0.13 | −3.01 | 0.100 |
| Diabetes | 0.04 | 0.91 | 0.674 | −0.08 | −1.89 | 0.229 |
| Time on PD | 0.01 | 0.01 | 0.881 | −0.10 | −0.12 | 0.073 |
| BMI | 0.09 | 0.20 | 0.287 | 0.11 | 0.22 | 0.139 |
| PCR | −0.06 | −3.14 | 0.472 | 0.09 | 4.51 | 0.225 |
| Serum albumin | −0.18 | −0.46 | 0.042 | −0.10 | −0.26 | 0.168 |
| GFR | −0.16 | −0.59 | 0.063 | −0.08 | −0.29 | 0.330 |
| Peritoneal membrane transport status | ||||||
| High | Reference | Reference | ||||
| High-average | −0.46 | −10.39 | < 0.001 | −0.25 | −5.80 | 0.005 |
| Low-average and low | −0.62 | −16.42 | < 0.001 | −0.31 | −8.27 | 0.003 |
| Dialysate volume | 0.09 | 0.24 | 0.306 | |||
| PD modality | ||||||
| CAPD | Reference | Reference | ||||
| CCPD | −0.11 | −2.55 | 0.2 | −0.30 | −6.65 | < 0.001 |
| Ultrafiltration on PD | 0.15 | 2.47 | 0.087 | 0.10 | 1.68 | 0.146 |
| Peritoneal creatinine clearance | 0.67 | 0.70 | < 0.001 | 0.55 | 0.57 | < 0.001 |
| Peritoneal Kt/V | 0.39 | 11.11 | < 0.001 | 0.06 | 1.58 | 0.652 |
Discussion
The major novel finding in this study is that PD modality is a significant determinant of peritoneal phosphate clearance with superior peritoneal phosphate clearance associated with treatment with CAPD except among high transporters. This clinical study is the first to address the determinants of peritoneal phosphate clearance in a large, prevalent PD population with standard dialysis prescriptions. These findings are important considering the fact that in the Unites States about two-thirds and globally about one-third of PD patients are treated with automated peritoneal dialysis (APD) (14–15). Patient preference and lifestyle are the most common indications to choose APD as a PD modality (16), and many low average and low transporters are treated with APD and achieve adequate Kt/V urea and ultrafiltration with this modality. Our data suggest that peritoneal phosphate clearance should also be considered when choosing PD modality.
Modality choice may be of particular significance to anuric PD patients because these patients are known to have worse phosphate control than those with residual GFR (6). For an anuric patient with a constant daily phosphate load and no net flux of phosphate from bone, the serum phosphate is an inverse function of the peritoneal phosphate clearance. Thus, our finding that treatment with CAPD was associated with an increase in peritoneal phosphate clearance of 16% among high-average transporters and 23% among low-average and low transporters compared with CCPD suggests that PD modality may play an important role in control of serum phosphate among anuric patients. Among anuric patients, this difference in phosphate clearance between the two modalities could result in differences in serum phosphate of 0.2 to 0.4 mmol/L, depending on baseline levels. In patients with well preserved residual renal function these differences in peritoneal clearances are unlikely to be of clinical significance because they are minor in comparison to renal phosphate clearance (6).
We observed a strong correlation between peritoneal phosphate and creatinine clearance in both of the PD modalities. In our multivariate regression analysis, peritoneal phosphate clearance was independently associated with peritoneal creatinine clearance and not with Kt/V urea. These findings confirm that peritoneal creatinine clearance can be used as a surrogate marker for peritoneal phosphate clearance and highlight the peril of relying solely on Kt/V urea as a measure of PD adequacy. The recently published International Society for Peritoneal Dialysis guidelines on PD adequacy recommend a separate target of at least 45 L/wk/1.73 m2 of creatinine clearance, but the 2006 National Kidney Foundation-Kidney Disease Outcomes Quality Initiative guidelines on PD adequacy recommend only a target for Kt/V (17,18). Our data suggest that focusing only on urea for assessment of dialysis adequacy may result in inadequate clearance of phosphate (19).
It is known that experimentally, peritoneal urea and creatinine clearances can be maximized on APD through manipulation of the number of cycles, total cycler volume, and the amount of tidal volume (20,21). Juergensen and colleagues have reported that increasing the number of APD cycles from 7 to 12 and the total cycler volume by 70% to 24 L improved peritoneal phosphate clearance by 19% (20). The effect of decreasing the number of cycles and increasing dwell time on peritoneal phosphate clearance on APD has not been systematically studied. Our findings of increased peritoneal phosphate clearance among lower transporters with CAPD suggest that a strategy of increasing cycler dwell times may be effective in the patients with lower membrane transport status. For those with high transporting membranes more frequent cycling as described by Jurgensen et al. may be an effective strategy. This highlights the importance of individualizing PD prescriptions on the basis of membrane transport status.
Our findings expand upon the work of other groups who have compared phosphate clearance between CAPD and CCPD by adjusting for peritoneal membrane transport category. Sedlacek et al. reported that phosphate clearance is increased in higher transporters compared with lower transporters in CAPD as well as CCPD groups (9). Prior direct comparisons of phosphate clearance between CAPD and CCPD, however, have not shown significant differences in peritoneal phosphate clearance (9,10), or shown worse peritoneal phosphate clearance on CAPD (11). The lack of separate analysis of membrane and modality effect in these studies may have obscured the finding that CAPD produces superior peritoneal phosphate clearance than CCPD among lower transport groups with standard PD prescriptions. In our population, lower transporters were significantly more likely to be treated with CAPD and higher transporters more likely to be treated with CCPD. High transporters have more rapid equilibration of phosphate across the peritoneal membrane, which likely accounts for the lack of modality effect that we observed in this group. Thus, a propensity to treat higher transporters with CCPD and lower transporters with CAPD may mask the effect of modality when membrane status is not controlled for.
The strengths of our study include a large study population and adjustment by multivariate linear regression analysis of the known variables that could influence peritoneal phosphate clearance. Moreover, we excluded all of the measurements of dialysis dose and phosphate clearance done in the first 3 mo of PD to remove the possible bias of incident patients with inadequate dialysis. We reported peritoneal phosphate clearance instead of peritoneal phosphate removal because it is assumed that phosphate removal is indicative merely of phosphate intake in the steady state and is not a measure of dialysis dose with respect to phosphate. One limitation is that this is a cross-sectional and observational study. We assessed a cross-section of the PD population who were receiving standard prescriptions and adequate dialysis on CAPD or APD, but did not attempt to assess the effect of tidal regimens, dwell times, or number of cycles on phosphate clearance.
Conclusions
This study contributes to our understanding of peritoneal phosphate clearance by highlighting the importance of peritoneal membrane transport status and PD modality. Peritoneal creatinine clearance, but not Kt/V urea, is a strong determinant of peritoneal phosphate clearance and may be used as a surrogate. In patients with inadequate phosphate control on APD, it is logical to consider PD prescription modifications to maximize dwell time and increase peritoneal creatinine clearance rather than an immediate increase in phosphate binder prescription.
Disclosures.
None.
Acknowledgments
We are grateful to the late Dr. Denis Pagé for his role in establishing the Ottawa Hospital Home Dialysis program and for his invaluable guidance in this and other academic endeavors. We gratefully acknowledge the substantial assistance by the PD nurses at the Home Dialysis unit of the Ottawa Hospital.
References
- 1.Ansell D: Serum phosphate and outcomes in PD patients.Nephrol Dial Transplant 22:667–668,2007 [DOI] [PubMed] [Google Scholar]
- 2.Noordzij M, Korevaar JC, Bos WJ, Boeschoten EW, Dekker FW, Bossuyt PM, Krediet RT: Mineral metabolism and cardiovascular morbidity and mortality risk: peritoneal dialysis patients compared with haemodialysis patients.Nephrol Dial Transplant 21:2513–2520,2006 [DOI] [PubMed] [Google Scholar]
- 3.Rufino M, de Bonis E, Martin M, Rebollo S, Martin B, Miguel R, Cobo M, Hernandez D, Torres A, Lorenzo V: Is it possible to control hyperphosphataemia with diet, without inducing protein malnutrition?Nephrol Dial Transplant 13[Suppl 3]:65–67,1998 [DOI] [PubMed] [Google Scholar]
- 4.Sperschneider H, Gunther K, Marzoll I, Kirchner E, Stein G: Calcium carbonate (CaCO3): An efficient and safe phosphate binder in haemodialysis patients? A 3-year study.Nephrol Dial Transplant 8:530–534,1993 [DOI] [PubMed] [Google Scholar]
- 5.Curtin RB, Svarstad BL, Keller TH: Hemodialysis patients’ noncompliance with oral medications.ANNA J 26:307–316,1999 [PubMed] [Google Scholar]
- 6.Wang AY, Woo J, Sea MM, Law MC, Lui SF, Li PK: Hyperphosphatemia in Chinese peritoneal dialysis patients with and without residual kidney function: What are the implications?Am J Kidney Dis 43:712–720,2004 [PubMed] [Google Scholar]
- 7.Zehnder C, Gutzwiller JP, Renggli K: Hemodiafiltration–a new treatment option for hyperphosphatemia in hemodialysis patients.Clin Nephrol 52:152–159,1999 [PubMed] [Google Scholar]
- 8.Nolph KD, Twardowski ZJ, Popovich RP, Rubin J: Equilibration of peritoneal dialysis solutions during long-dwell exchanges.J Lab Clin Med 93:246–256,1979 [PubMed] [Google Scholar]
- 9.Sedlacek M, Dimaano F, Uribarri J: Relationship between phosphorus and creatinine clearance in peritoneal dialysis: Clinical implications.Am J Kidney Dis 36:1020–1024,2000 [DOI] [PubMed] [Google Scholar]
- 10.Evenepoel P, Bammens B, Verbeke K, Vanrenterghem Y: Superior dialytic clearance of [beta]2-microglobulin and p-cresol by high-flux hemodialysis as compared to peritoneal dialysis.Kidney Int 70:794–799,2006 [DOI] [PubMed] [Google Scholar]
- 11.Gallar P, Ortega O, Gutierrez M, Munoz M, Hilara L, Oliet A, Rodriguez I, Gimenez E, Vigil A: Influencing factors in the control of phosphorus in peritoneal dialysis. Therapeutic options. [Spanish].Nefrologia 20:355–361,2000 [PubMed] [Google Scholar]
- 12.Twardowski Z, Nolph KO, Khanna R, Prowant BF, Ryan LP, Moore HL, Neilsen MP: Peritoneal equilibration test.Perit Dial Int 7:138–148,1987 [Google Scholar]
- 13.Twardowski ZJ, Nolph KD, Khanna R, Gluck Z, Prowant BF, Ryan LP: Daily clearances with continuous ambulatory peritoneal dialysis and nightly peritoneal dialysis.ASAIO Trans 32:575–580,1986 [DOI] [PubMed] [Google Scholar]
- 14.Venkataraman V, Nolph KD: Utilisation of PD modalities: Evolution.Semin Dial 15:380–384,2002 [DOI] [PubMed] [Google Scholar]
- 15.Mujais S, Story K: Peritoneal dialysis in the US: Evaluation of outcomes in contemporary cohorts.Kidney Int 70:S21–S26,2006 [DOI] [PubMed] [Google Scholar]
- 16.Wilson J, Nissenson AR: Determinants in APD selection.Semin Dial 15:388–392,2002 [DOI] [PubMed] [Google Scholar]
- 17.Lo WK, Bargman JM, Burkart J, Krediet RT, Pollock C, Kawanishi H, Blake PG, ISPD Adequacy of Peritoneal Dialysis Working Group: Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis.Perit Dial Int 26:520–522,2006 [PubMed] [Google Scholar]
- 18.National Kidney Foundation. Clinical practice recommendations for peritoneal dialysis adequacy.Am J Kidney Dis 48:S130–S158,2006 [DOI] [PubMed] [Google Scholar]
- 19.Kim DJ, Do JH, Huh W, Kim YG, Oh HY: Dissociation between clearances of small and middle molecules in incremental peritoneal dialysis.Perit Dial Int 21:462–466,2001 [PubMed] [Google Scholar]
- 20.Juergensen P, Eras J, McClure B, Kliger AS, Finkelstein FO: The impact of various cycling regimens on phosphorus removal in chronic peritoneal dialysis patients.Int J Artif Organs 28:1219–1223,2005 [DOI] [PubMed] [Google Scholar]
- 21.Juergensen PH, Murphy AL, Kliger AS, Finkelstein FO: Increasing the dialysis volume and frequency in a fixed period of time in CPD patients: The effect on Kpt/V and creatinine clearance.Perit Dial Int 22:693–697,2002 [PubMed] [Google Scholar]