Abstract
Background and objectives: The objective of this study was to describe the normal range of serum cystatin C and identify factors associated with variability in serum cystatin C contrasting with factors that are known to influence creatinine levels in the general US adolescent population.
Design, setting, participants, & measurements: Serum cystatin C and creatinine were measured in 719 participants aged 12 to 19 yr in the Third National Health and Nutrition Examination Survey, a national cross-sectional survey conducted in 1988 through 1994. We calculated gender- and race/ethnicity-specific cystatin C and creatinine ranges and conducted multivariable linear regression analyses to assess factors that contribute to variability in cystatin C and creatinine levels.
Results: Overall, the mean serum cystatin C level was 0.84 mg/L and was higher in male than female individuals and higher in non-Hispanic white versus non-Hispanic black and Mexican American individuals. The mean serum creatinine was 0.71 mg/dl and was higher in male than in female individuals but lower in non-Hispanic white and Mexican American compared with non-Hispanic black individuals. Unlike creatinine, which increases with age from 12 to 19 yr, cystatin C levels decrease, particularly in female individuals. After adjustment for age, gender, and race/ethnicity, uric acid and blood urea nitrogen were significantly associated with cystatin C levels.
Conclusions: Serum cystatin C is significantly related to gender, age, race/ethnicity, uric acid, and blood urea nitrogen in adolescents.
Serum creatinine is the most common marker of kidney function used in clinical practice. Nonetheless, the limitations of using serum creatinine as a kidney function measure are well documented (1,2). In particular, the production of creatinine is highly affected by dietary intake and muscle mass, which itself varies by age, height, gender, and race/ethnicity. In children, the use of creatinine to assess kidney function is further complicated by changing muscle mass, which influences the circulating creatinine concentration (3) as children grow. Serum cystatin C, a product of an endogenous “housekeeping” gene thought to be produced at a constant rate, is a novel marker of kidney function that has been suggested to be able to overcome some of the age and body size limitations of serum creatinine as a marker of kidney function (4,5). Cystatin C has been proposed by several groups and a recent meta-analysis to be a more sensitive and specific marker of kidney function in both children and adults (6–9).
Little is known, however, about the normal ranges for cystatin C in a diverse, healthy pediatric population and which factors contribute to variability in levels. Cystatin C levels are highest within the first few days of life (presumably reflecting the physiologic low GFR in newborns) but normalize by age 1 and have been reported to remain constant throughout adulthood up to age 50 (10). In the existing literature, proposed ranges for the serum concentration of cystatin C in pediatric populations are inconsistent, with several small, single-institution, hospital- or clinic-based studies reporting reference ranges that have varied widely between 0.58 and 1.38 mg/dl (10–13). In addition, reported cystatin C ranges are affected by use of different cystatin C assays (4). These are important limitations to the use of cystatin C in routine assessment of kidney function. Furthermore, some previous studies suggested that cystatin C levels are independent of gender, age, or body composition (14–16), whereas others showed differences in cystatin C levels according to demographic characteristics, adiposity, thyroid function, and inflammation (17–20). It is unknown whether factors such as gender, age, race, adiposity, thyroid function, and inflammation affect cystatin C levels in the normal adolescent population.
The objective of this study was to establish the range of cystatin C levels in a nationally representative adolescent sample and to examine whether cystatin levels are indeed independent of age, gender, and race in a general population of adolescents. To accomplish this, we analyzed serum cystatin C and serum creatinine data from participants aged 12 to 19 yr in the Third National Health and Nutrition Examination Survey (NHANES III). We directly contrast the associations between demographic factors and serum cystatin C to the widely known associations between age, gender, and race and serum creatinine in adolescents.
Materials and Methods
Study Population
NHANES III is a cross-sectional survey conducted between 1988 and 1994 by the National Center for Health Statistics. NHANES III used a complex, multistage, clustered sampling design to provide nationally representative data on the civilian, noninstitutionalized US population (21). As part of a National Institutes of Health–funded study, serum cystatin C was measured on all NHANES III participants who were ≥60 yr old and had serum creatinine measured, as well as a 25% random sample of those aged 12 to 59 yr (22). The younger age group was supplemented by sampling all individuals with high standard serum creatinine (>1.189 mg/dl in male individuals and >0.997 mg/dl in female individuals). In this analysis, we analyzed data available from all NHANES III participants who were aged 12 to 19 yr and for whom serum cystatin C had been measured (n = 719; 337 male and 382 female individuals, 10 of whom had high standard serum creatinine [nine male individual and one female individual]).
Data Collection and Measurement
Serum samples were stored at −70°C until 2006 when cystatin C was measured at the Cleveland Clinic Research Laboratory. Cystatin C was measured using the Dade Behring N Latex Cystatin C assay, which is an automated particle-enhanced immunonephelometric assay run on the Dade Behring Nephelometer II (Dade Behring, Deerfield, IL). The interassay coefficients of variation for the assay were 5.05 and 4.87% at mean concentrations of 0.97 and 1.90 mg/L, respectively. This assay is currently the most precise automated assay across the clinical concentration range (4).
Serum creatinine was measured by the modified kinetic Jaffe reaction using a Hitachi 737 analyzer (Roche Diagnostics, Indianapolis, IN). Serum creatinine values were calibrated to standard creatinine using an enzymatic creatinine assay traceable to liquid chromatography-isotope dilution gas chromatography mass spectrometry reference method at the Cleveland Clinic Research Laboratory using the following equation: Standardized serum creatinine (mg/dl) = −0.184 + 0.96 * [original NHANES III serum creatinine (mg/dl)] (23–25).
Detailed information regarding data collection in NHANES III has been published previously (26). Ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican American, and other. Estimates for the last category are not presented because of small sample size and heterogeneity within this group (27). Measured weight and height were used to generate categories of underweight (<5% body mass index [BMI] for age), normal weight (5 to 85% BMI for age), at risk for overweight (85 to 95% BMI for age), and overweight (>95% BMI for age) using gender-specific BMI-for-age growth percentiles (28). The percentage of body fat (%BF) and free fat mass (FFM) were calculated using data obtained from bioelectric impedance analysis, gender, and weight (29). Serum C-reactive protein (CRP) was categorized (<0.22, 0.22 to 1.0, or >1 mg/dl) as normal, borderline elevated, or elevated (30). Tanner stage of pubic hair development was available only for male and female individuals who were 8 to 18 yr of age and available for 75.5% of the study population.
Statistical Analysis
For accounting for the complex sampling design of NHANES III and for generation of estimates that are representative of the US adolescent population aged 12 to 19 yr, all analysis were conducted using the Stata svy commands (StataCorp release 10.0, College Station, TX), which incorporated the appropriate sampling weights for the cystatin C subpopulation (22). For continuous variables, the mean ± SEM is reported; for categorical variables, we examined the proportion of individuals in each category. The adjusted Wald test was used to compare means. P < 0.05 was considered statistically significant.
We determined the distribution of serum cystatin C and creatinine in the overall study population and generated age-, race-, and gender-specific percentiles. We conducted univariable and multivariable linear regression analyses to evaluate factors influencing cystatin C and creatinine levels. In multivariable modeling, biologically plausible variables, including gender, age, and race/ethnicity, as well as potential correlates previously described (16–19) as associated with serum cystatin C levels were maintained in the model when they were significant with at P < 0.05.
The protocols for conducting this study were approved by the institutional review boards of the National Center for Health Statistics and the Johns Hopkins Bloomberg School of Public Health. Informed consent was obtained from all participants during the survey.
Results
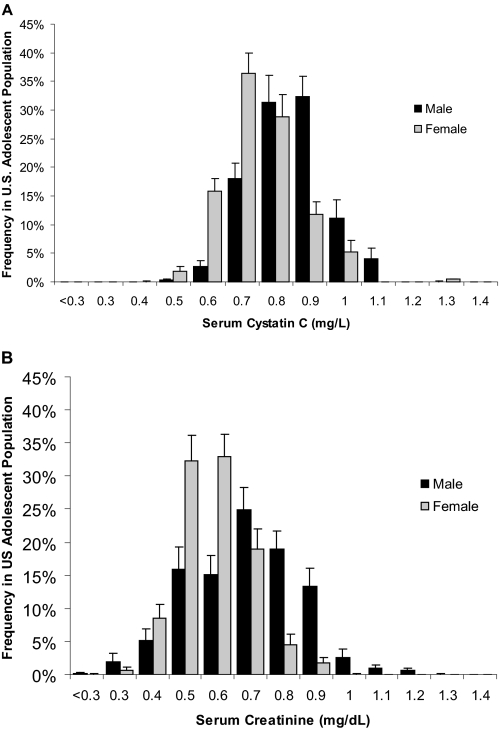
Table 1 shows the demographics of the sample and nationally representative estimates of selected characteristics, overall and by gender for the 12- to 19-yr-old US population included in NHANES III. The study population represents a generally healthy adolescent US population with <3% having self-reported comorbidities such as hypertension or hyperlipidemia or laboratory evidence of inflammation with elevated CRP or white blood cell count. The distributions of serum cystatin C and standardized serum creatinine, stratified by gender, are shown in Figure 1. The distributions seem close to normally distributed, but cystatin C has a narrower distribution than serum creatinine and creatinine is slightly more skewed toward higher values. For both cystatin C and serum creatinine, the overall distribution is shifted to lower values for female compared with male individuals. In the US adolescent population 12 to 19 yr of age, the mean serum cystatin C level was 0.84 mg/L (95% confidence interval [CI] 0.83 to 0.86) and was significantly higher in male compared with female individuals (0.89 versus 0.79 mg/L; P < 0.001). On average, male individuals had serum cystatin C levels that were 12.7% higher than those for female individuals. The mean serum creatinine level was 0.72 mg/dl (95% CI 0.70 to 0.73) and was also significantly higher in male compared with female individuals (0.77 versus 0.66 mg/dl; P < 0.001). On average, male individuals had serum creatinine levels that were 16.7% higher than those for female individuals.
Table 1.
Characteristics of US Adolescents 12 to 19 yr by gender, NHANES IIIa
| Characteristic | All(n = 719)b | Male(n = 337)b | Female(n = 382)b |
|---|---|---|---|
| Age (yr; mean [SE]) | 15.2 (0.2) | 15.2 (0.2) | 15.2 (0.2) |
| Race/ethnicity (% [SE])c | |||
| non-Hispanic white | 69.4 (3.6) | 71.5 (4.4) | 67.2 (3.9) |
| non-Hispanic black | 15.5 (2.1) | 15.2 (2.5) | 15.9 (2.4) |
| Mexican American | 8.3 (1.0) | 8.2 (1.3) | 8.4 (1.3) |
| Serum creatinine (mg/dl; mean [SE]) | 0.72 (0.01) | 0.77 (0.01) | 0.66 (0.01) |
| Cystatin C (mg/L; mean [SE]) | 0.84 (0.01) | 0.89 (0.01) | 0.79 (0.01) |
| BUN (mg/dl; mean [SE]) | 11.00 (0.23) | 13.00 (0.21) | 10.00 (0.34) |
| Uric acid (mg/dl; mean [SE]) | 5.00 (0.06) | 5.60 (0.07) | 4.40 (0.04) |
| CRP (mg/dl) | |||
| <0.22 | 88.9 (2.4) | 88.3 (3.6) | 89.5 (2.1) |
| 0.22 to 1.00 | 7.8 (1.5) | 7.1 (1.9) | 8.6 (1.8) |
| >1.00 | 3.3 (1.4) | 4.6 (2.6) | 1.9 (0.7) |
| %BF (mean [SE]) | 23.10 (0.80) | 18.60 (0.01) | 28.00 (0.80) |
| FFM (mean [SE]) | 47.20 (0.70) | 52.80 (1.10) | 41.00 (0.40) |
| BMI (kg/m2; mean [SE]) | 22.40 (0.45) | 22.50 (0.77) | 22.30 (0.40) |
| Weight categories (% [SE])d | |||
| underweight | 3.2 (0.8) | 2.0 (0.8) | 4.5 (1.6) |
| normal weight | 72.4 (2.9) | 74.2 (4.9) | 70.5 (3.3) |
| at risk | 15.0 (2.4) | 14.1 (3.6) | 16.0 (3.0) |
| overweight | 9.4 (1.8) | 10.0 (3.0) | 8.9 (1.8) |
| Tanner stage (pubic hair; % [SE]) | |||
| I | 3.9 (1.2) | 5.6 (2.1) | 1.9 (1.5) |
| II | 5.8 (1.4) | 7.4 (2.0) | 4.0 (1.9) |
| III | 11.5 (1.9) | 10.6 (3.3) | 12.5 (3.0) |
| IV | 36.3 (3.1) | 33.7 (4.6) | 39.2 (4.5) |
| V | 42.5 (3.6) | 42.7 (4.7) | 42.4 (5.4) |
| Hyperlipidemia (% [SE])e | 1.10 (0.80) | 1.50 (1.20) | 0.70 (0.01) |
| Hypertension (% [SE])e | 2.70 (1.90) | 3.70 (3.40) | 1.70 (1.50) |
BMI, body mass index; BUN, blood urea nitrogen; CRP, C-reactive protein; FFM, free-fat mass; NHANES III, Third National Health and Nutrition Examination Survey; %BF, percentage of body fat.
n represents the sample size of individuals used to derive nationally representative estimates of US adolescents 12 to 19 yr of age.
Other race/ethnicity category not shown.
As defined by the Centers for Disease Control and Prevention. See the Materials and Methods section for further details and reference.
Self-reported condition. See the Materials and Methods section for further details.\
Figure 1.
Weighted frequency distribution of values of serum cystatin C (mg/L; A) and standardized serum creatinine (mg/dl; B) for male and female individuals, representative of US adolescents 12 to 19 yr of age.
Table 2 shows the distribution of serum cystatin C and creatinine for US adolescents aged 12 to 19 yr by gender and race/ethnicity. The mean cystatin C level was 0.86 mg/L in non-Hispanic white individuals (95% CI 0.84 to 0.88), 0.80 mg/L in non-Hispanic black individuals (95% CI 0.79 to 0.82), and 0.80 mg/L in Mexican American individuals (95% CI 0.79 to 0.82). Mean serum cystatin C levels were significantly lower in non-Hispanic black and Mexican American individuals compared with non-Hispanic white individuals (P = 0.003 and P = 0.001, respectively), although the magnitude of the difference was relatively small. The mean serum creatinine level was 0.71 mg/dl in non-Hispanic white individuals (95% CI 0.69 to 0.73), 0.76 mg/dl in non-Hispanic black individuals (95% CI 0.74 to 0.79), and 0.67 mg/dl in Mexican American individuals (95% CI 0.64 to 0.70). Non-Hispanic black individuals had significantly higher mean serum creatinine values compared with non-Hispanic white and Mexican American individuals (P = 0.004 and P = 0.001, respectively).
Table 2.
Nationally representative percentiles of the serum cystatin C and serum creatinine distribution in NHANES III adolescents aged 12 to 19 yr overall, as well as by gender and ethnicity
| Parameter | na | 1st | 2.5th | 5th | 25th | 50th | 75th | 95th | 97.5th | 99th |
|---|---|---|---|---|---|---|---|---|---|---|
| Serum cystatin C (mg/L) weighted percentiles | ||||||||||
| overall | 719 | 0.59 | 0.61 | 0.64 | 0.75 | 0.83 | 0.93 | 1.05 | 1.11 | 1.12 |
| male | 337 | 0.63 | 0.66 | 0.71 | 0.81 | 0.88 | 0.98 | 1.07 | 1.12 | 1.15 |
| female | 382 | 0.55 | 0.60 | 0.62 | 0.71 | 0.79 | 0.86 | 1.00 | 1.02 | 1.08 |
| non-Hispanic white | 184 | 0.60 | 0.63 | 0.69 | 0.79 | 0.85 | 0.94 | 1.05 | 1.11 | 1.12 |
| male | 82 | N/A | 0.70 | 0.71 | 0.83 | 0.89 | 0.98 | 1.08 | 1.12 | N/A |
| female | 102 | N/A | 0.60 | 0.64 | 0.74 | 0.81 | 0.88 | 1.00 | 1.01 | N/A |
| non-Hispanic black | 255 | 0.58 | 0.61 | 0.63 | 0.70 | 0.78 | 0.89 | 1.03 | 1.08 | 1.09 |
| male | 119 | N/A | 0.63 | 0.67 | 0.78 | 0.86 | 0.94 | 1.05 | 1.09 | N/A |
| female | 136 | N/A | 0.60 | 0.62 | 0.68 | 0.73 | 0.8 | 0.96 | 0.99 | N/A |
| Mexican American | 244 | 0.51 | 0.55 | 0.59 | 0.72 | 0.79 | 0.88 | 1.03 | 1.07 | 1.17 |
| male | 122 | N/A | 0.57 | 0.69 | 0.78 | 0.84 | 0.94 | 1.07 | 1.12 | N/A |
| female | 122 | N/A | 0.51 | 0.59 | 0.65 | 0.74 | 0.81 | 0.89 | 0.92 | N/A |
| Serum creatinine (mg/dl) weighted percentiles | ||||||||||
| overall | 719 | 0.39 | 0.49 | 0.49 | 0.58 | 0.68 | 0.78 | 0.97 | 1.06 | 1.06 |
| male | 337 | 0.39 | 0.39 | 0.49 | 0.68 | 0.78 | 0.87 | 0.97 | 1.06 | 1.16 |
| female | 382 | 0.49 | 0.49 | 0.49 | 0.58 | 0.68 | 0.78 | 0.87 | 0.87 | 0.97 |
| non-Hispanic white | 184 | 0.39 | 0.49 | 0.49 | 0.58 | 0.68 | 0.78 | 0.97 | 0.97 | 1.06 |
| male | 82 | N/A | 0.39 | 0.49 | 0.58 | 0.78 | 0.87 | 0.97 | 1.06 | N/A |
| female | 102 | N/A | 0.49 | 0.49 | 0.58 | 0.68 | 0.78 | 0.87 | 0.87 | N/A |
| non-Hispanic black | 255 | 0.49 | 0.49 | 0.54 | 0.68 | 0.78 | 0.87 | 1.06 | 1.16 | 1.16 |
| male | 119 | N/A | 0.49 | 0.58 | 0.68 | 0.87 | 0.97 | 1.16 | 1.16 | N/A |
| female | 136 | N/A | 0.49 | 0.49 | 0.58 | 0.68 | 0.78 | 0.87 | 0.87 | N/A |
| Mexican American | 244 | 0.30 | 0.39 | 0.49 | 0.58 | 0.68 | 0.78 | 0.97 | 0.97 | 1.06 |
| male | 122 | N/A | 0.39 | 0.49 | 0.68 | 0.78 | 0.87 | 0.97 | 1.06 | N/A |
| female | 122 | N/A | 0.39 | 0.39 | 0.49 | 0.58 | 0.68 | 0.87 | 0.87 | N/A |
Sample size (n) denotes the number of individuals used to obtain the nationally representative estimates presented here. Gender- and ethnicity-specific estimates for the 1st and 99th percentiles of the serum cystatin C distribution are not available (N/A) because there are too few individuals for reliable estimation.
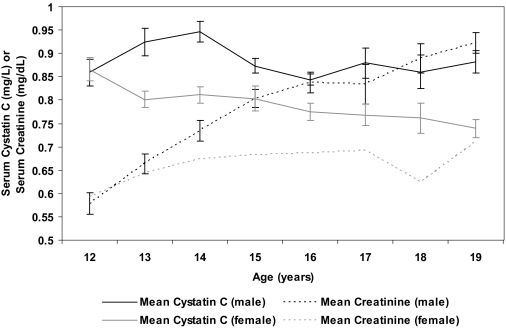
Figure 2 shows the mean cystatin C and creatinine levels for male and female individuals by age. Cystatin C levels decrease from a peak at age 12 yr in female individuals through age 19 yr, whereas in male individuals, cystatin C levels were highest at age 14 yr. When examined by Tanner stage, cystatin C levels were highest in Tanner stage II for female individuals (0.83 mg/L) and highest in Tanner stage IV for male individuals (0.92 mg/L), consistent with the levels by age. Mean creatinine levels sharply increased with age for both male and female individuals between ages 12 and 19 yr.
Figure 2.
Mean serum cystatin C (mg/L) and mean serum creatinine (mg/dl) for male and female individuals by age groups in Third National Health and Nutrition Examination Survey, representative of US adolescents 12 to 19 yr of age.
We conducted univariable and multivariable linear regression analyses to evaluate factors potentially associated with serum cystatin C levels (Table 3). Corresponding regression models are shown for serum creatinine. In unadjusted analysis, gender, age, race/ethnicity, serum creatinine, uric acid, blood urea nitrogen (BUN), albumin, hemoglobin, thyroid-stimulating hormone (TSH), %BF, and FFM all were statistically significantly associated with cystatin C levels; however, the magnitude of the change in cystatin level per unit change in the covariate, especially with TSH and FFM, was small. CRP and BMI, reported to be associated with cystatin C levels in adults, were not statistically significantly associated with cystatin C levels in adolescents. Not surprising, cystatin C and creatinine were moderately correlated (r = 0.3036), because each represents a measure of kidney function. Increasing %BF was negatively correlated with both creatinine and cystatin C, as has been reported in adults.
Table 3.
Linear regression models of serum cystatin C (mg/L) and creatinine (mg/dl) in US adolescents aged 12 to 19 yr, NHANES IIIa
| Dependent Variable | Covariate | Unadjusted Association β (95% CI) | Adjustedb for Demographics Model β (95% CI) | Fully Adjusted Modelc,d β (95% CI) |
|---|---|---|---|---|
| Cystatin C (mg/L) | Gendere | −0.092 (−0.122 to −0.063)g | −0.091 (−0.12 to −0.06)g | −0.039 (−0.061 to −0.016)g |
| Age (yr) | −0.009 (−0.015 to −0.003)g | −0.008 (−0.014 to −0.002)g | −0.015 (.−0.022 to −0.008)g | |
| Non-Hispanic blackf | −0.054 (−0.081 to −0.027)g | −0.049 (−0.075 to −0.022 )g | −0.045 (−0.070 to −0.021)g | |
| Mexican Americanf | −0.056 (−0.083 to −0.029)g | −0.050 (−0.075 to −0.025)g | −0.020 (−0.066 to −0.014)g | |
| Serum creatinine (mg/dL) | .203 (.127 to .279 )g | .160 (.077 to .25)g | ||
| Uric acid (mg/dL) | .040 (.025 to .054)g | .021 (.005 to .037)h | ||
| BUN (mg/dL) | .010 (.006 to .015)g | .004 (.001 to .008)h | ||
| Albumin (g/dL) | .030 (.019 to .082)g | |||
| Hemoglobin (g/dL) | .026 (.015 to .037)g | |||
| CRP (mg/dL) | .011 (−0.010 to .032) | |||
| TSH (μIU/mL) | .008 (.001 to .015)h | |||
| %BF | −0.350 (−0.500 to −0.190)g | |||
| FFM (kg) | .003 (.002 to −0.004)g | |||
| BMI (kg/m2) | −0.000 (−0.003 to .003) | |||
| R2 | N/A | .208 | .309 | |
| Creatinine (mg/dl) | Gendere | −0.100 (−0.140 to −0.068)g | −0.100 (−0.130 to −0.080)g | −0.030 (−0.067 to −0.002)h |
| Age (yr) | .029 (.021 to .037)g | .030 (.022 to .036)g | .030 (.021 to .036)g | |
| Non-Hispanic blackf | −0.039 (−0.077 to −0.002)h | .050 (.014 to .079)g | .070 (.042 to .100)g | |
| Mexican American f | −0.000 (−0.057 to .058) | −0.050 (−0.080 to −0.020)g | −0.040 (−0.068 to −0.006)h | |
| Serum cystatin C (mg/L) | .308 (.20 to .41)g | .190 (.09 to .30)g | ||
| Uric acid (mg/dL) | .053 (.041 to .065)g | .029 (.016 to .042)g | ||
| BUN (mg/dL) | .014 (.010 to .019)g | .008 (.004 to .010)g | ||
| Albumin (g/dL) | .083 (.030 to .140)g | |||
| Hemoglobin (g/dL) | .048 (.036 to .060)g | |||
| CRP (kg/m2) | .010 (−0.001 to .030) | |||
| TSH (μIU/mL) | .006 (−0.001 to .013) | |||
| %BF | −0.290 (−0.470 to −0.110)g | |||
| FFM (kg) | .007 (.005 to .009)g | |||
| BMI (kg/m2) | .003 (.0004 to .066)g | |||
| R2 | N/A | .350 | .462 |
CI, confidence interval; BUN, blood urea nitrogen; CRP, C-reactive protein; TSH, thyroid-stimulating hormone; %BF, percentage of body fat; FFM, free-fat mass; BMI, body mass index.
Adjusted for age, gender, and race/ethnicity.
Fully adjusted model for cystatin C: adjusted model + creatinine + uric acid + BUN.
Fully adjusted model for creatinine: adjusted model + cystatin C + BUN.
Male = reference population.
Non-Hispanic white = reference population.
P < .01.
P < .05.
Female individuals had a mean cystatin C that was 0.092 mg/L lower than that of male individuals (95% CI β −0.122 to −0.063), and gender accounted for approximately 14.8% of the variability in serum cystatin C levels. This is similar to the effect of gender on creatinine. On average, non-Hispanic black individuals had serum cystatin C levels 0.054 mg/L lower than those of non-Hispanic white individuals, and Mexican American individuals had serum cystatin C levels 0.056 mg/L lower than those of non-Hispanic white individuals. Age was positively and strongly associated with creatinine, whereas cystatin C was more weakly and negatively correlated with age. In unadjusted analysis, each 1-yr increase in age was associated with a 0.029-mg/dl increase in serum creatinine (95% CI β 0.021 to 0.037), and age accounted for approximately 19.7% of the variation of serum creatinine levels.
In a multivariable model adjusted only for demographic factors (gender, age, and race/ethnicity; Table 3), female gender, increasing age, and non-Hispanic black and Mexican American race/ethnicity were still associated with lower cystatin C levels after adjustment. Female gender, lower age, and non-Hispanic white and Mexican American race/ethnicity were also associated with lower serum creatinine levels after adjustment. The fully adjusted model in Table 3 presents the clinical variables that remain significant sources of variation in serum levels of cystatin C or creatinine after adjustment for demographic factors. In this analysis, the effect of gender on creatinine and cystatin C is comparable, whereas creatinine increases with age and cystatin C decreases slightly. Non-Hispanic black individuals have lower cystatin C, whereas they have higher serum creatinine as compared with non-Hispanic white individuals. Overall, the clinical variables included in the multivariable analyses accounted for 30.9% of the variation in serum cystatin C and 46.2% of the variation in serum creatinine. After adjustment for demographic factors, CRP, hemoglobin, serum albumin, TSH, and BMI were no longer statistically significant sources of variation for cystatin C.
Discussion
To our knowledge, this study represents the first evaluation of the distribution of serum cystatin C and comparison with serum creatinine in a nationally representative adolescent population, including individuals of different race/ethnicities. The estimates from this investigation are representative of the civilian, noninstitutionalized population of the United States aged 12 to 19 yr in 1988 through 1994. Previous studies of cystatin levels in adolescents have been conducted in relatively small, selected study populations or study populations limited to one institution or ethnicity (7,8,11–13). Thus, one of the largest advantages to this study is the generalizability of the results, presenting the distribution of cystatin C levels in a diverse population of race and ethnicity from across the United States. In this broad sample of US adolescents, contrary to reports of cystatin C levels’ being independent of age and gender in adolescents, female gender, increasing age, and non-Hispanic black and Mexican American race/ethnicity were significantly associated with lower cystatin C levels after adjustment.
Earlier reports provided conflicting evidence regarding whether cystatin C levels vary by gender. Some investigators found statistically significant differences between genders in adults (31–34), with men having higher cystatin C levels than women, whereas others did not (10,13). Even among those who did find a statistically significant difference between serum cystatin C concentrations in men and women, some authors suggested that the difference was small enough to justify a universal reference range. Only two studies suggested that gender-specific reference ranges might be appropriate (34,35). In our study, male adolescents had on average 12.7% higher mean serum cystatin C level compared with female adolescents, and this difference was constant across race/ethnicity groups. Creatinine showed a similar gender difference, with the mean male serum creatinine levels 16.7% higher than those in female individuals. This result is consistent with other studies that examined creatinine levels in pediatric populations (36). Considering that in US adolescents 12 to 19 yr old the effect of gender on cystatin C levels is nearly equivalent to the effect of gender on serum creatinine levels, it seems reasonable to conclude that gender-specific cystatin C reference intervals may be appropriate in this population.
It is unclear why female individuals have lower cystatin C levels than male individuals. Male and female individuals in this population were similar in age and distribution of race/ethnicity, and adjustment for these factors did not account for the gender difference. Young male adults have had GFR reported to be 127 ml/min per 1.73 m2 compared with 118 ml/min per 1.73 m2 in women (37), perhaps explaining the lower cystatin levels in female individuals. Differences in cystatin C production rather than kidney function may also account for the lower serum cystatin C levels in girls. Higher extrarenal elimination, different hormonal variations, or factors that are present in female individuals and interfere with the cystatin C assay may also account for the difference by gender.
Serum cystatin C and creatinine levels also differed significantly by race/ethnicity. The higher creatinine levels in non-Hispanic black compared with non-Hispanic white individuals are well reported and are thought to be explained by racial differences in muscle mass (38); however, little information exists regarding cystatin C generation, extrarenal elimination, or potential assay interference by race/ethnicity. It is possible that non-Hispanic black and Mexican American individuals have underlying genetic factors that alter the physiologic production and/or elimination of cystatin C. It may also be that this represents differences in physiologic GFR by race/ethnicity.
In contrast to the positive correlation of age with serum creatinine corresponding to adolescent maturation and increase in muscle mass, even within the relatively tight age range of 7 yr in this study population, age was a modest but significant negative predictor for serum cystatin C levels. This was especially true in female individuals after the age of 12 and in male individuals after the age of 14 yr. This contrasts to previous work that concluded that cystatin C levels were independent of age in pediatric populations (11,14). Several authors noted an age-related rise in cystatin C levels after the age of 50 to 60 yr (15,32,39), which presumably reflects a decline of kidney function. It is known that there are no changes in GFR during adolescent maturation (40); thus, in this population of generally healthy adolescents aged 12 to 19 yr, the age-related variation in cystatin C levels is likely related to variation in cystatin production. It is interesting that these age-related changes in cystatin levels parallel peak height velocity, which also varies by gender (41) with girls reaching peak height velocity earlier in development (Tanner stage II) than boys (Tanner stage IV); this correlates with our analysis that found that the highest median cystatin C level was in Tanner stage II for female individuals and Tanner stage IV for male individuals. Perhaps the variation in cystatin C levels is affected by growth and metabolic demands in adolescents.
Another notable finding in this study was the independent association of higher uric acid with higher levels of cystatin C. Other authors noted an association between hyperuricemia and renal disease, but most attributed the association to a simple clustering of hyperuricemia with other well-established cardiovascular and renal risk factors (e.g., insulin resistance or type 2 diabetes, high BP, central obesity), and mild hyperuricemia is generally regarded as insignificant. In this large, generally healthy population with overall normal uric acid levels and a low prevalence of renal disease, it is unlikely that renal disease is responsible for this association. Serum uric acid has been reported to be correlated with the prevalence of the metabolic syndrome, particularly abdominal obesity, in NHANES participants (42). Although the association of uric acid with serum cystatin C levels has not been previously described, many other groups have noted the effect of body composition (including BMI and %BF) on serum cystatin C levels (16). Although in our analyses BMI and %BF were not statistically significantly associated with serum cystatin C levels after adjustment for demographic factors, perhaps the association of uric acid with cystatin C is related to other anthropomorphic factors not measured in the NHANES III survey.
Our study has several limitations, particularly its cross-sectional design and our retrospective use of the data; therefore, we cannot assess longitudinal changes in cystatin C levels with age, height velocity, or changes in body habitus. In addition, a number of measures that are available for adults in NHANES III are not measured in the adolescent age range, such as dual-energy x-ray absorptiometry scans to assess body composition further. Serum samples were available only for individuals who were ≥12 yr of age; therefore, our results cannot be generalized to children who are younger than 12. In addition, formal GFR measurements are not performed in NHANES III; therefore, we also cannot distinguish intrinsic renal factors from extrarenal causes of variation in cystatin C levels. Despite these limitations, this is the largest nationally representative study of serum cystatin C levels in a pediatric population to date, demonstrating previously unreported variation in cystatin C levels according to age, race, and gender in the 12- to 19-yr age group.
Conclusions
Our study provides gender- and race/ethnicity-specific normal values of cystatin C and creatinine for the general population of US adolescents aged 12 to 19 yr. We observed a clear gender difference in serum cystatin C levels with female individuals having lower levels than male individuals. We conclude that serum cystatin C, in conjunction with serum creatinine, is a useful clinical tool for physicians in the routine assessment of renal function, particularly in the adolescent population, in which rapidly changing serum creatinine levels may present a confusing clinical picture. Our study cannot directly address whether serum cystatin C is a better clinical marker of kidney function compared with creatinine, because we have no direct measures of GFR; however, in a comparison of the effects of age, gender, and race on cystatin C levels with creatinine, gender and race have comparable magnitude effects on cystatin C and creatinine, whereas age has a much smaller effect on cystatin C than on creatinine. Clinicians should bear in mind that the variation pattern in cystatin C levels is in many ways opposite to creatinine; namely, that cystatin C levels decrease slightly with age and are slightly lower in non-Hispanic black compared with non-Hispanic white adolescents. The tables herein present normal ranges for cystatin C in US adolescents as measured by the Dade Behring N Latex Cystatin C assay. Age-, race-, and gender-specific ranges for cystatin C presented here can be used as another tool for the clinician to assess kidney function in adolescents.
Disclosures
None.
Acknowledgments
The project is funded by grants UO1 DK 053869, UO1 DK 067651, and UO1 DK 35073 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). A.K. was supported by a German Research Foundation fellowship. E.S. was supported by National Institutes of Health (NIH)/NIDDK grant KO1 DK076595. G.J.S. was partly supported by NIH/NIDDK grant UO1 DK66116. J.C. was partly supported by NIH/NCRR grant MO1 RR000052. S.F. was partly supported by NIH/NIDDK grant UO1 DK66174.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function: Measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Perrone RD, Madias NE, Levey AS: Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 38: 1933–1953, 1992 [PubMed] [Google Scholar]
- 3.Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 4.Newman DJ. Cystatin C. Ann Clin Biochem 39: 89–104, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Madero M, Sarnak MJ, Stevens LA: Serum cystatin C as a marker of glomerular filtration rate. Curr Opin Nephrol Hypertens 15: 610–616, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Dharnidharka VR, Kwon C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 40: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Roos JF, Doust J, Tett SE, Kirkpatrick CM: Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children: A meta-analysis. Clin Biochem 40: 383–391, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Ylinen EA, la-Houhala M, Harmoinen AP, Knip M: Cystatin C as a marker for glomerular filtration rate in pediatric patients. Pediatr Nephrol 13: 506–509, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Grubb A, Nyman U, Bjork J, Lindstrom V, Rippe B, Sterner G, Christensson A: Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem 51: 1420–1431, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Finney H, Newman DJ, Thakkar H, Fell JM, Price CP: Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Intern Med 82: 71–75, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Brodehl J: Reference values for cystatin C serum concentrations in children. Pediatr Nephrol 12: 125–129, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Galteau MM, Guyon M, Gueguen R, Siest G: Determination of serum cystatin C: Biological variation and reference values. Clin Chem Lab Med 39: 850–857, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Takuwa S, Ito Y, Ushijima K, Uchida K: Serum cystatin-C values in children by age and their fluctuation during dehydration. Pediatr Int 44: 28–31, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J: Cystatin C: A new marker of glomerular filtration rate in children independent of age and height. Pediatrics 101: 875–881, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Norlund L, Fex G, Lanke J, Von SH, Nilsson JE, Leksell H, Grubb A: Reference intervals for the glomerular filtration rate and cell-proliferation markers: Serum cystatin C and serum beta 2-microglobulin/cystatin C-ratio. Scand J Clin Lab Invest 57: 463–470, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Vinge E, Lindergard B, Nilsson-Ehle P, Grubb A: Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 59: 587–592, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Macdonald J, Marcora S, Jibani M, Roberts G, Kumwenda M, Glover R, Barron J, Lemmey A: GFR estimation using cystatin C is not independent of body composition. Am J Kidney Dis 48: 712–719, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Fricker M, Wiesli P, Brandle M, Schwegler B, Schmid C: Impact of thyroid dysfunction on serum cystatin C. Kidney Int 63: 1944–1947, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE: Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65: 1416–1421, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Risch L, Huber AR: Glucocorticoids and increased serum cystatin C concentrations. Clin Chim Acta 320: 133–134, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: Programs and collection procedures. Vital Health Stat 1 32: 1–407, 1994 [PubMed] [Google Scholar]
- 22.Köttgen A, Selvin E, Stevens LA, Levey AS, Van LF, Coresh J: Serum cystatin C in the United States: The Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 51: 385–394, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Selvin E, Manzi J, Stevens LA, Van LF, Lacher DA, Levey AS, Coresh J: Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 50: 918–926, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS: Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 39: 920–929, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics: NHANES III Laboratory Data File Documentation, Ages One Year and Older [Catalog 76300], Hyattsville, MD, National Center for Health Statistics Centers for Disease Control and Prevention, 1996
- 27.National Center for Health Statistics: Analytic and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988–94), Hyattsville, MD, National Center for Health Statistics Centers for Disease Control and Prevention, 1996
- 28.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL: CDC growth charts: United States. Adv Data 314: 1–27, 2000 [PubMed] [Google Scholar]
- 29.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, Lukaski HC, Friedl K, Hubbard VS: Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord 26: 1596–1609, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB: Elevated C-reactive protein levels in overweight and obese adults. JAMA 282: 2131–2135, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Croda-Todd MT, Soto-Montano XJ, Hernandez-Cancino PA, Juarez-Aguilar E: Adult cystatin C reference intervals determined by nephelometric immunoassay. Clin Biochem 13: 1084–1087, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Finney H, Newman DJ, Price CP: Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem 37: 49–59, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Ichihara K, Saito K, Itoh Y: Sources of variation and reference intervals for serum cystatin C in a healthy Japanese adult population. Clin Chem Lab Med 45: 1232–1236, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Pergande M, Jung K: Sandwich enzyme immunoassay of cystatin C in serum with commercially available antibodies. Clin Chem 39: 1885–1890, 1993 [PubMed] [Google Scholar]
- 35.Ognibene A, Mannucci E, Caldini A, Terreni A, Brogi M, Bardini G, Sposato I, Mosconi V, Salvadori B, Rotella CM, Messeri G: Cystatin C reference values and aging. Clin Biochem 39: 658–661, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, Balk E, Lau J, Levin A, Kausz AT, Eknoyan G, Levey AS, National Kidney Foundation's Kidney Disease Outcomes Quality Initiative: National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: Evaluation, classification, and stratification. Pediatrics 111: 1416–1421, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Smith HW: Comparative physiology of the kidney. In: The Kidney: Structure and Function in Health and Disease, edited by Smith HW, New York, Oxford University Press, 1951, pp 520–574
- 38.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S: Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37: 478–494, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Fliser D, Ritz E: Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis 37: 79–83, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Kher K, Makker SP, Schnaper HW. Clinical assessment of renal function. In: Clinical Pediatric Nephrology, 2nd Ed., edited by Kher K, Makker SP, Schnaper HW, New York, Informa Healthcare, 2006, pp 71–93
- 41.Tanner JM: Normal growth and techniques of growth assessment. Clin Endocrinol Metab 15: 411–451, 1986 [DOI] [PubMed] [Google Scholar]
- 42.Ford ES, Li C, Cook S, Choi HK: Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 115: 2526–2532, 2007 [DOI] [PubMed] [Google Scholar]