Abstract
Background and objectives: Patients with chronic kidney disease (CKD) have an elevated cardiovascular risk. This study was designed to understand better the presence and strength of the relationship between physical activity and BP and to explore determinants of hemodynamic reactivity.
Design, setting, participants, & measurements: Twenty-four patients with CKD (mean age 69.5 yr; 3.1 antihypertensive drugs; estimated GFR 47 ml/min per 1.73 m2, albumin/creatinine ratio 403 mg/g) were studied on three occasions during a 6-wk period with 24-h ambulatory BP monitoring and simultaneous activity monitoring with wrist actigraphy.
Results: Nondippers were found have a greater level of sleep activity compared with dippers, although the awake activity level was similar (7.06 versus 6.73) between groups (P = 0.042 for interaction). In 3587 BP activity pairs, hemodynamic reactivity was variable between individuals (systolic BP reactivity 1.06 [SD 10.50]; diastolic BP reactivity 0.89 [SD 7.80] heart rate reactivity 1.18 [SD 11.00]); those who were more sedentary had a greater increment in systolic BP compared with those who were less sedentary. Antihypertensive drugs blunted hemodynamic reactivity. Hemodynamic reactivity was greatest between 12 a.m. and 8 a.m., making this a vulnerable period for cardiovascular events.
Conclusions: Greater hemodynamic reactivity in sedentary people with CKD offers a possible and thus far unrecognized mechanism of cardiovascular damage. Besides reducing BP, antihypertensive drugs reduce hemodynamic reactivity, which offers another plausible mechanism of cardiovascular protection with their use.
In health, BP falls during sleep and rises in the waking hours. This circadian variation in BP is determined in large part by the level of physical activity, which increases both BP and heart rate (HR) in proportion to the vigor of activity (1–10). The diagnosis and treatment of hypertension, however, are based almost exclusively on seated, resting BP that is obtained in physician offices. Because physical activity increases BP, it follows that if BP were to be measured over 24 h during the course of usual activity, people with higher intensity or duration of physical activity may have a higher BP level. Higher BP in turn is related to poorer outcomes. Herein lies a paradox. We know that increased physical activity and fitness level are associated with lower cardiovascular risk (11) and that 24-h ambulatory BP is a prognostically superior marker of cardiovascular outcomes compared with clinic-obtained BP recordings in patients with hypertension (12). In fact, in otherwise healthy individuals, the extent of increase in BP with physical activity is an independent predictor of incident hypertension (13). In those who are referred for exercise testing, BP reactivity is a marker of cardiovascular outcomes (14,15). Thus, it is logical to postulate that a higher level of physical activity over the long-term would foster a greater level of cardiovascular health. Better cardiovascular health in turn may be associated with less increment in hemodynamic reactivity. According to this paradigm, people with sedentary habits would be exposed to greater increments in BP than their more physically active peers. If this in fact is true, then it would be a worthy explanation of the paradox noted.
Another reason that physical activity is a potent determinant of the circadian variation in BP is because active individuals are more likely to raise their BP when active and therefore are more likely to manifest a fall in BP when sleeping. The presence of BP “dipping” is also associated with a better prognosis (16); however, it is equally likely that patients who do not demonstrate dipping are nocturnally active (17). Whether nondipping in patients with chronic kidney disease (CKD) is due to poor daytime physical activity (and therefore lack of rise in daytime BP) or to increased nighttime physical activity is unclear. Patients with CKD generally have a low level of physical activity often as a result of cardiopulmonary disease or concomitant illnesses that may restrict activity. Sedentary people are less likely to achieve a restful sleep, which in turn may be associated with less dipping; however, patients with CKD also experience nocturia, which may increase their nighttime activity. In patients with CKD, dipping is impaired, but the factors that are associated with impaired circadian variation in BP are poorly understood (18–20).
The relationship between activity and hemodynamic responses in patients with CKD has not been studied. It is important to study this relationship because it may offer clues to why patients with CKD are nondippers. We performed this study to understand better the presence and strength of the relationship between activity and BP so that we may explore the provenance of nondipping in free-living patients with CKD. Another aim of our study was to explore factors that are associated with hemodynamic reactivity and whether hemodynamic reactivity depends on the time of the day.
Materials and Methods
Data were gathered from patients who participated in a randomized, controlled trial to test the utility of a vitamin D analog, paricalcitol, on endothelial function and inflammation (http://clinicaltrials.gov registration number NCT00428246). Patients were randomly assigned in three equal proportions to receive a placebo or 1 or 2 μg of paricalcitol.
Participants
Between November 2006 and June 2007, we recruited patients from the renal clinics at Wishard Memorial Hospital and Richard L. Roudebush VA Medical Center (Indianapolis, IN). Patients were considered eligible for the study when they were older than 18 yr and had CKD (21) with an estimated GFR (22) >30 ml/min and were on a stable dosage of an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) for at least 1 mo. Patients with poorly controlled hypertension (≥180/110 mmHg), unstable BP control (change in BP medication within 1 mo), poorly controlled diabetes (hemoglobin A1c of >11%), hyperphosphatemia (>6 mg/dl), or hypercalcemia (>10 mg/dl) or those who were taking vitamin D or its analogs were excluded. The study protocol was approved by the institutional review boards and the VA Research and Development Committee, and all patients provided written informed consent.
Study Procedures
Baseline measurements were performed on 2 consecutive days in a research laboratory. After obtaining a medical history and performing a physical examination, we measured seated clinic BP. We then placed an ambulatory BP monitor on the nondominant arm for BP measurement and simultaneous activity monitor as described next. After 1 mo of exposure to the drug and 2 wk after withdrawal of the drug, actigraphy-guided BP monitoring was repeated, thus, each participant had actigraphy-guided BP monitoring performed at baseline, 1 mo, and 6 wk.
Ambulatory BP Monitoring
Ambulatory BP were recorded every 20 min during the day (6 a.m. to 10 p.m.) and every 30 min during the night (10 p.m. to 6 a.m.) using a SpaceLab 90207 ABP monitor (SpaceLabs Medical, Redmond, WA) in the nondominant arm. Accuracy of ambulatory BP recordings was confirmed against auscultated BP. Hourly averages were calculated, and the average of these averages represented the mean systolic (SBP) and diastolic BP (DBP). Dipping was defined as asleep/awake SBP ratio of ≤0.9. Patients were asked to keep a diary of sleep and awake times including naps. One patient on one occasion failed to keep a sleep diary; for this recording, we assumed midnight to 5 a.m. to be sleep time and 8 a.m. to 10 p.m. to be awake time.
Actigraphy
Actigraphy is a technique that allows quantitative evaluation of physical activity throughout a 24-h period (23). When ambulatory BP monitoring (ABPM) is combined with concomitant actigraphy, we can explore the provenance of circadian rhythms. We measured the activity level with an actigraph (Actiwatch 64; Mini Mitter, Bend, OR), a watch-sized device worn on the dominant wrist for the duration of ABPM. The internal clocks of the ABPM and actigraph were synchronized, and activity was assessed in 15-s epochs throughout the 24-h period. The units of activity are arbitrary but have been calibrated to metabolic equivalents. Data were exported to a custom-designed relational database. Mean awake and asleep activities were calculated in 5-min periods before each ambulatory BP measurement.
Statistical Analysis
The relationship between actimetry and hemodynamics is nonlinear. The square root of activity best describes the relationship of activity and HR. Accordingly, we took the square root transform of the actigraph data for descriptive and analytical evaluation.
A mixed model was used to allow for correlated data at three visits within individuals. A multilevel modeling technique was used (24). The effect of visit was modeled as a nested effect within patients to account for the random variation. Dipping status of systolic ambulatory BP, activity (awake or sleep), and their interaction were tested on actigraphically recorded activity (square root transformed) using this mixed-effects model using the full maximal-likelihood approach. Means and 95% confidence intervals (CI) derived from this model are reported. We also used a random coefficient model with maximal likelihood to calculate the physical activity–induced changes in HR and BP. Visits were nested within patients for this analysis. The random effects of slopes and intercepts of activity were allowed to be correlated by using an unstructured covariance matrix. To explore determinants of BP reactivity, we tested the fixed effect of number of BP medications and their interaction (as nominal factors) with activity as well as overall level of activity and its interaction with activity preceding the BP measurement.
To determine the impact of time of day on hemodynamic reactivity, we divided the 24-h period into 4-h intervals starting at midnight. The interaction effect of square root of activity and period was tested on BP and HR using a mixed-effects model allowing for repeated measures. Statistical analysis was performed using Stata 10.0 (Stata Corp., College Station, TX). Significance was set at two-sided P < 0.05.
Results
Twenty-four patients were recruited, 22 of whom completed the entire study. All 24 patients had at least one actigraphy-guided ambulatory BP recording. Of a total of 72 expected ambulatory BP recordings, 66 were available for final analysis. Taken together, 3587 pairs of activity and BP measurements were available for analysis. Paricalcitol did not influence ambulatory BP or the level of activity (data not shown); therefore, drug assignment was not used as an explanatory variable.
Baseline characteristics of the patients are listed in Table 1. Each patient was taking an ACEI or an ARB. Estimated GFR ranged from 32 to 74 ml/min per 1.73 m2. Median 24-h urine albumin/creatinine ratio was 81 mg/g creatinine (interquartile range 22 to 233; range 8 to 3406 mg/g).
Table 1.
Baseline characteristics of the study populationa
| Characteristic | Value |
|---|---|
| No. of patients | 24 |
| Age (yr; mean ± SD) | 69.5 ± 10.1 |
| Male (n [%]) | 20 (83) |
| BMI (kg/m2; mean ± SD) | 34.9 ± 7.5 |
| Race (n [%]) | |
| white | 16 (67) |
| black | 7 (29) |
| Asian | 1 (4) |
| Tobacco use (n [%]) | |
| current | 12 (50) |
| past | 4 (17) |
| never | 8 (33) |
| CKD duration (yr; mean ± SD) | 5.1 ± 4.3 |
| Cardiovascular disease (n [%]) | 13 (54) |
| Diabetes (n [%]) | 17 (71) |
| Cause of CKD (n [%]) | |
| diabetes | 13 (54) |
| hypertension | 6 (24) |
| glomerulonephritis | 1 (4) |
| other | 4 (17) |
| Hemoglobin (g/dl; mean ± SD) | 12.8 ± 1.6 |
| Albumin (g/dl; mean ± SD) | 4.1 ± 0.3 |
| Calcium (mg/dl; mean ± SD) | 9.4 ± 0.3 |
| Phosphorus (mg/dl; mean ± SD) | 3.3 ± 0.6 |
| Estimated GFR (ml/min per 1.73 m2; mean ± SD) | 46.7 ± 10.4 |
| 24-h urine albumin/creatinine (mg/g; mean ± SD) | 403.0 ± 856.0 |
| BP medications (mean ± SD) | 3.1 ± 1.2 |
| β blockers (n [%]) | 10 (42) |
| α blocker (n [%]) | 7 (29) |
| ACEI (n [%]) | 18 (75) |
| ARB (n [%]) | 8 (33) |
| non–loop diuretics (n [%]) | 4 (17) |
| loop diuretics (n [%]) | 12 (50) |
| dihydrodipine CCB (n [%]) | 7 (29) |
| clonidine (n [%]) | 2 (8) |
| vasodilators (n [%]) | 2 (8) |
BMI, body mass index; CCB, calcium channel blocker; CKD, chronic kidney disease.
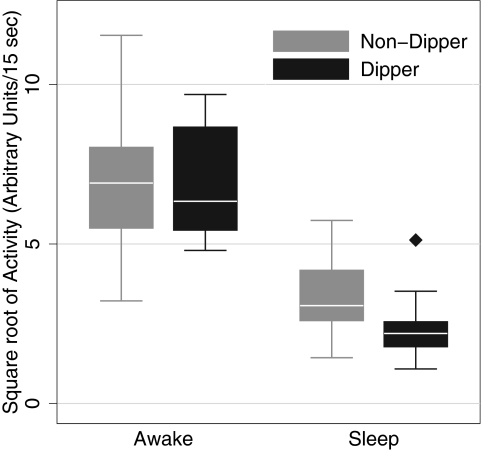
Table 2 shows the relationship between dipping status and physical activity. Awake activity level between dippers and nondippers was similar. As expected, the level of activity was lower during sleeping periods; however, nondippers had a higher level of sleep activity compared with dippers. On average, dippers had 1.03-unit greater decline in activity from awake to asleep level compared with the decline in dippers (P = 0.042 for interaction effect; Figure 1).
Table 2.
Relationship between dipping and activitya
| Parameter | Activity/Factor | Mean | 95% CI | P |
|---|---|---|---|---|
| Square root of activityb | ||||
| dipper | Awake | 7.06 | 6.19 to 7.93 | |
| Asleep | 2.58 | 1.71 to 3.45 | ||
| nondipper | Awake | 6.73 | 6.24 to 7.21 | |
| Asleep | 3.28 | 2.80 to 3.76 | ||
| Effect size of factors | Dipper status | 0.33 | −0.61 to 1.27 | 0.490 |
| Activity | −3.44 | −3.90 to −2.99 | <0.001 | |
| Dipper status × activity | −1.03 | −2.03 to −0.04 | 0.042 |
CI, confidence interval.
Arbitrary units per 15-s epoch.
Figure 1.
Relationship between circadian activity and dipping status of systolic BP (SBP). Awake activity was similar between dippers and nondippers, but dippers had a greater decline in sleep activity compared with nondippers (P = 0.042 for interaction effect; see Table 2 for details).
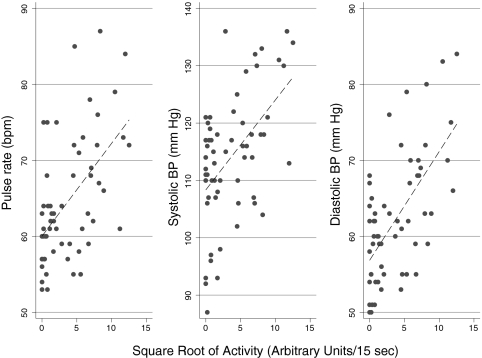
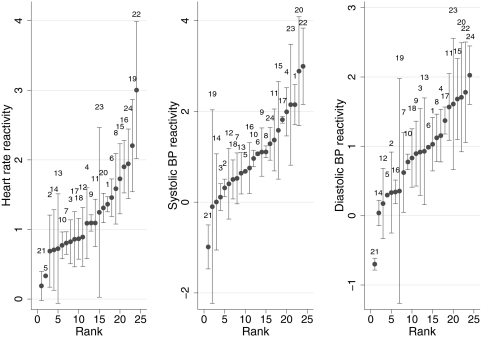
Figure 2 shows a representative example of the relationship of activity and hemodynamic changes collected during a 24-h period from one patient. A direct relationship between square root of the physical activity recorded by the actigraph and hemodynamic parameters such as HR, SBP, and DBP is seen. This relationship is variable between individuals as depicted in Figure 3 and Table 3. The rank order of the changes in HR, SBP, and DBP for each patient is shown in Figure 3; the error bars represent SD of repeated measurements within patients. HR reactivity varied from nearly 0 to approximately 3 beats per minute. One patient (patient 21) had negative BP reactivity, which is fall in both SBP and DBP with activity. For some patients, the error bars are large. The SD between individuals in SBP reactivity slopes was 0.78 and within individuals between visits was 0.42, yielding an intraclass correlation coefficient of 0.78 (Table 3). Similarly, the intraclass correlation coefficient was 0.75 for DBP and 0.78 for HR.
Figure 2.
Relationship of activity and hemodynamic changes collected during a 24-h period from a single patient. A direct relationship between square root of the actigraph-recorded physical activity and hemodynamic parameters such as heart rate, SBP, and diastolic BP (DBP) is seen. The slope of the line reflects the hemodynamic reactivity.
Figure 3.
Rank order of hemodynamic reactivity or the changes in heart rate, SBP, and DBP per square root of activity measured during a 15-s epoch. The error bars represent 1 SD. The numbers above the error bars represent the patient number.
Table 3.
Partitioning of variability of reactivity slopesa
| Parameter | Pulse Rate | SBP | DBP |
|---|---|---|---|
| Fixed effect | |||
| mean slope | 1.18 | 1.06 | 0.89 |
| 95% CI | 0.94 to 1.41 | 0.71 to 1.42 | 0.65 to 1.14 |
| Random effect | |||
| between-subject SD in slopes | 0.51 | 0.78 | 0.53 |
| between-visit SD in slopes | 0.27 | 0.42 | 0.31 |
| −log likelihood | −12899.60 | −14115.40 | −13061.10 |
| AIC | 25815.10 | 28246.80 | 26138.20 |
| BIC | 25864.60 | 28296.20 | 26187.70 |
AIC, Akaike information criteria; BIC, Bayesian information criteria; DBP, diastolic BP; SBP, systolic BP.
The composite model examining the impact of the number of BP medications and the overall level of activity on hemodynamics and hemodynamic reactivity is shown in Table 4. Using the number of medications as a proxy for the severity of hypertension, we found that patients who used a greater number of antihypertensive medications were more likely to have a higher SBP; however, increasing number of antihypertensive medications blunted the activity-induced increase in BP. Although the average overall activity level was not associated with overall BP or HR changes, those with greater overall activity had blunted hemodynamic reactivity.
Table 4.
Effects of antihypertension medications and overall activity on hemodynamic reactivitya
| Coefficient | HR
|
SBP
|
DBP
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | P | Mean | 95% CI | P | Mean | 95% CI | P | |
| No. of BP medications | 0.060b | 0.025b | >0.200b | ||||||
| 2 | 4.80 | −9.90 to 19.50 | 14.00 | −0.40 to 28.30 | 5.30 | −7.30 to 18.00 | |||
| 3 | −3.40 | −17.70 to 10.90 | 18.20 | 4.20 to 32.20 | 4.30 | −8.00 to 16.60 | |||
| 4 | −7.20 | −22.30 to 7.80 | 22.20 | 7.40 to 36.90 | 4.70 | −8.20 to 17.70 | |||
| 5 | −10.10 | −28.00 to 7.90 | 27.70 | 10.30 to 45.10 | 9.60 | −5.80 to 25.10 | |||
| 6 | −21.00 | −42.70 to 1.20 | 19.00 | −2.20 to 40.10 | −2.70 | −21.50 to 16.10 | |||
| Sqrt activity | 2.54 | 1.63 to 3.46 | <0.001b | 2.51 | 1.18 to 3.84 | <0.001b | 2.26 | 1.39 to 3.13 | <0.001b |
| Interactions | 0.001c | 0.005c | <0.001c | ||||||
| 2 BP medications × Sqrt activity | −0.40 | −1.11 to 0.36 | −0.17 | −1.24 to 0.90 | −0.30 | −0.97 to 0.36 | |||
| 3 BP medications × Sqrt activity | −0.50 | −1.17 to 0.26 | −0.05 | −1.10 to 0.99 | −0.30 | −0.99 to 0.31 | |||
| 4 BP medications × Sqrt activity | −0.70 | −1.43 to 0.08 | −0.61 | −1.72 to 0.50 | −0.70 | −1.41 to −0.03 | |||
| 5 BP medications × Sqrt activity | −0.80 | −1.68 to 0.07 | −1.20 | −2.49 to 0.08 | −1.30 | −2.1 to −0.52 | |||
| 6 BP medications × Sqrt activity | −0.40 | −1.48 to 0.65 | 0.71 | −0.86 to 2.28 | −0.40 | −1.35 to 0.58 | |||
| Average activity Sqrt | 0.58 | −0.33 to 1.50 | >0.200c | −0.67 | −2.26 to 0.91 | >0.200c | 0.21 | −0.76 to 1.19 | >0.200c |
| Sqrt activity × average activity Sqrt | −0.20 | −0.25 to −0.05 | 0.003c | −0.19 | −0.33 to −0.05 | 0.007c | −0.20 | −0.25 to −0.05 | 0.002c |
| Constant | 69.90 | 55.80 to 84.00 | <0.001c | 106.00 | 89.50 to 121.70 | <0.001c | 58.20 | 45.5 to 70.8 | <0.001c |
HR, heart rate; Sqrt, square root.
Test equality of coefficients for number of medications.
Test equality of slopes for number of medications.
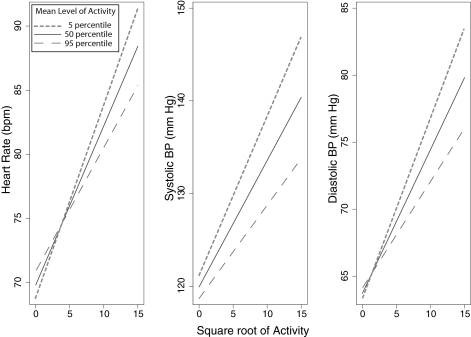
The effect of sedentary versus active lifestyle on hemodynamic reactivity is shown in Figure 4. Those with a sedentary lifestyle had a greater hemodynamic reactivity compared with their more active peers. As shown in Table 4, the coefficients of average activity × square root of activity all were <0.01, indicating that there is a high degree of statistical significance for the difference between slopes for hemodynamic reactivity shown in Figure 4.
Figure 4.
Relationship of hemodynamic reactivity with activity as a function of mean level of activity. The solid line presents the hemodynamic change with activity in those who had median level of activity and were taking on average three antihypertension drugs. The short dashed line represents sedentary individuals, and the long dashed line represents active individuals. The hemodynamic reactivity slopes are blunted in those who were active and heightened in those who were sedentary (P < 0.01; see Table 4 for coefficients of slopes).
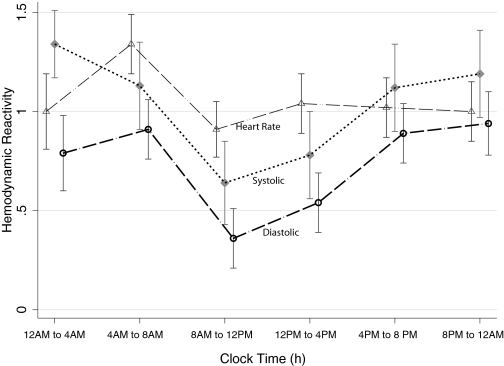
Tables 5 and 6 show the relationship of the time of the day, BP, HR, and hemodynamic reactivity. As expected, HR and BP varied with the time of day. Hemodynamic reactivity also varied with the time of the day. The greatest reactivity was seen between 12 a.m. and 8 a.m., and least reactivity was seen between 8 a.m. and 12 p.m. (Figure 5).
Table 5.
Effects of time of day on hemodynamics
| Time | HR
|
SBP
|
DBP
|
|||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| 12 a.m. to 4 a.m. | 68.2 | 63.6 to 72.7 | 115.9 | 111.2 to 120.5 | 60.9 | 57.5 to 64.2 |
| 4 a.m. to 8 a.m. | 69.3 | 64.7 to 73.8 | 120.1 | 115.4 to 124.8 | 64.2 | 60.8 to 67.6 |
| 8 a.m. to 12 p.m. | 71.7 | 67.0 to 76.3 | 122.5 | 117.7 to 127.4 | 68.7 | 65.2 to 72.2 |
| 12 p.m. to 4 p.m. | 72.0 | 67.3 to 76.6 | 119.6 | 114.8 to 124.4 | 66.0 | 62.5 to 69.4 |
| 4 p.m. to 8 p.m. | 72.3 | 67.6 to 76.9 | 119.7 | 115.0 to 124.5 | 64.5 | 61.0 to 67.9 |
| 8 p.m. to 12 a.m. | 71.4 | 66.8 to 76.0 | 120.6 | 115.8 to 125.3 | 63.6 | 60.2 to 67.1 |
| P for equality of time factor | <0.001 | <0.001 | <0.001 | |||
Table 6.
Effects of time of day on hemodynamic reactivitya
| Time | HR Reactivity
|
SBP Reactivity
|
DBP Reactivity
|
|||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| 12 a.m. to 4 a.m. | 1.00 | 0.63 to 1.37 | 1.34 | 0.81 to 1.87 | 0.79 | 0.41 to 1.17 |
| 4 a.m. to 8 a.m. | 1.34 | 1.05 to 1.63 | 1.13 | 0.70 to 1.56 | 0.91 | 0.61 to 1.21 |
| 8 a.m. to 12 p.m. | 0.91 | 0.63 to 1.19 | 0.64 | 0.22 to 1.06 | 0.36 | 0.07 to 0.65 |
| 12 p.m. to 4 p.m. | 1.04 | 0.75 to 1.32 | 0.78 | 0.35 to 1.20 | 0.54 | 0.25 to 0.84 |
| 4 p.m. to 8 p.m. | 1.02 | 0.74 to 1.30 | 1.12 | 0.70 to 1.55 | 0.89 | 0.60 to 1.19 |
| 8 p.m. to 12 a.m. | 1.00 | 0.70 to 1.30 | 1.19 | 0.74 to 1.63 | 0.94 | 0.63 to 1.25 |
| P for equality of time factor | 0.055 | 0.007 | <0.001 | |||
Reactivity is measured in change in HR or BP per square root of activity per 15-s epoch.
Figure 5.
Hemodynamic reactivity varies with the time of the day. Error bars represent the SEM. The greatest reactivity is seen during the hours of sleep and the least between 8 a.m. and 12 p.m.
Discussion
The major findings of our study are as follows: (1) Nondippers had a greater level of sleep activity compared with dippers, although the awake activity level was similar between the two groups; (2) activity-induced changes in BP were heterogeneous but had good test–retest reliability within individuals; sedentary patients had greater hemodynamic reactivity; (3) antihypertensive drugs blunted the physical activity-induced rise in BP, which may confer an additional mechanism of cardiovascular protection; and (4) hemodynamic reactivity varied with the time of day, with 12 a.m. to 8 a.m. being the most vulnerable period and 8 a.m. to 12 p.m. being the least. Our study is unique because this is the first study to report the hemodynamic reactivity in patients with CKD and also the first study to report the test–retest reliability of hemodynamic reactivity.
Clark et al. (1) were among the first to demonstrate that diary-recorded physical activity could explain much of the circadian variation in BP. Subsequent investigators using actigraphy could extend these observations to demonstrate that between 20 and 60% of variation in daytime BP could be explained by physical activity (2–6). Kario et al. (9) proposed the BP reactivity index and also found a strong relationship between BP and physical activity in working adults who had seated BP of <160/105 mmHg and were not on antihypertensive drugs. Our study extends the observations generated in either healthy people (2,3) or those with treated (4) or untreated hypertension (1,9) to those with CKD and treated hypertension. Preceding physical activity strongly influenced both BP and HR. The test–retest reliability of hemodynamic reactivity was good.
Importantly, it was the sedentary individuals who experienced the steepest increments in BP and HR with physical activity compared with their less sedentary peers. This observation is particularly important because it offers another mechanism of cardiovascular damage in sedentary individuals. Studies using invasive cardiovascular hemodynamics during exercise for patients with hypertension demonstrated that there is an increase in peripheral vascular resistance during exercise compared with healthy peers; our study is consistent with the hypothesis that this increment in BP may be heightened in sedentary individuals (25). Increasing the fitness level may reduce the impact of exercise-induced hemodynamic reactivity (26). Besides the use of cardiovascular reactivity to gauge the level of physical and cardiovascular fitness, cardiovascular reactivity seems to be of prognostic importance. In a population-based sample of 1033 normotensive Japanese men, for example, exercise-induced increase in BP was a strong determinant of future hypertension (13). Both HR reactivity (27–29) and BP reactivity (14,15) in response to graded exercise are potent indicators of cardiovascular outcomes. Taken together, these data suggest that performing actigraphy and ABPM concomitantly may further enhance the prognostic information already contained in ambulatory BP recordings. The use of actigraphy, therefore, in free-living individuals may offer a simple and inexpensive screening test to assess hemodynamic reactivity and offer insights into cardiovascular health. Future studies should assess the relationship between actigraphically assessed hemodynamic reactivity and that assessed by graded exercise testing.
Previous long-term studies have demonstrated that even long-term treatment with antihypertensive drugs does not correct the aberrant increase in peripheral vascular resistance with exercise (25). We found that antihypertensive drugs blunt hemodynamic reactivity. Cardiovascular remodeling is directly related to the wall stress that when accentuated during exercise may instigate left ventricular hypertrophy. In fact, BP obtained during submaximal exercise is a better predictor of left ventricular hypertrophy compared with clinic or even ambulatory BP (30). Thus, we speculate that blunting of hemodynamic reactivity may be another mechanism of cardiovascular protection induced by antihypertensive therapy.
Patients with CKD often have a nondipping BP pattern. The causes of nondipping in these patients are not clear, but many factors have been proposed, such as sodium sensitivity, autonomic activation, and endocrine dysfunction (31,32). We found that sleep activity was increased in patients with nondipping, which raises the hypothesis that patients with nondipping have poor sleep quality. Although sleep disturbances are common in patients with CKD and may be associated with nondipping, documentation of poor sleep quality would require polysomnographic study because actigraphy alone is insufficient to diagnose sleep disorders. Patients with CKD often have poor renal tubular concentrating ability, which may result in nocturia. Such patients would require walking to the bathroom, an activity that would also be captured by actigraphy. Tubulointerstitial damage is related to sodium sensitivity (33) and may also be associated with nocturia; therefore, common pathways that lead to both nondipping and increased nocturnal activity may falsely give the sense that nocturnal activity and nondipping are causally related. Our results in patients with CKD support the observations of Mansoor et al. (8) in patients with untreated hypertension. These investigators also found that nondippers have similar awake activity but higher sleep activity (44 U/min) compared with dippers (25 U/min), which is similar to our results (43 U/min sleep activity in nondippers and 27 U/min sleep activity in dippers).
In a study performed of patients to assess ambulatory BP for clinical reasons, Jones et al. (34) reported the impact of the time of day on hemodynamic reactivity. They found a significant 24-h variation in hemodynamic reactivity. Both SBP and DBP reactivities were maximum between 8 a.m. and 10 a.m. and fell significantly between 10 a.m. and 12 p.m. The maximal BP and HR reactivity in our study was during the hours of sleep generally between 12 a.m. and 8 a.m., and the lowest reactivity was seen between 8 a.m. and 12 p.m. Thus, patients with CKD may differ from the general population in the circadian variation of hemodynamic reactivity. Accumulating data suggest that cardiovascular deaths are frequently triggered by daily activities (35). In the general population, the frequencies of onset of myocardial infarction, sudden cardiac death, and stroke show marked circadian variations with parallel increases in the period from 6 a.m. to 12 p.m. (35). Our data suggest that in patients with CKD, the most vulnerable period may be between 12 a.m. and 4 a.m. That nondipping BP pattern is associated with greater nocturnal activity and also with poorer cardiovascular survival raises the hypothesis that increased BP reactivity during nocturnal hours may mediate worse outcomes.
The strengths of our study are measurements of ambulatory BP and concomitant activity on multiple predetermined occasions in a population of patients who had CKD known to have high rates of cardiovascular disease. Furthermore, all patients were on ACEI or ARB, as is the standard of care. The repeated measurements on the same patient allowed the assessment of test–retest reliability, which has not previously been reported. A limitation of our work was the small sample size, which restricted our ability to discover further determinants of hemodynamic reactivity. It is possible that ABPM may restrict physical activity and actigraphy may therefore underestimate the general level of physical activity; however, we were interested in detecting the relationship of activity just before the BP measurement, which should not be influenced by simply wearing an ambulatory BP monitor. Because all participants wore ambulatory BP monitors at the time of actigraphy, it is still possible to grade the relative level of activity in all patients. The case-control approach of our study does not permit us to draw a cause-and-effect relationship between activity and hemodynamic reactivity. Furthermore, the combination therapy used for most patients does not allow evaluation of the unique effect of a class of antihypertensive agents on the hemodynamic reactivity. For example, β blockers afford a cardioprotective effect. Whether these drugs have a unique effect on hemodynamic reactivity would be only hypothesis generating given the small sample size of our study. Finally, our patients were generally obese; whether similar results would be obtained in lean individuals is unclear.
Activity-induced increase in BP is related to cardiovascular events (14,15,27–29); therefore, interventions to reduce BP reactivity may be of prognostic importance. Because sedentary individuals seem to have greater hemodynamic reactivity, it follows that regular exercise may blunt hemodynamic reactivity, as can antihypertensive drugs. Because BP variation in associated with increased cardiovascular damage, attenuation of hemodynamic reactivity with antihypertensive drugs may be an additional mechanism of cardiovascular protection. Other, unmeasured effects, such as dietary sodium intake, may also be important (36). Because hemodynamic reactivity is greatest between the hours of 12 a.m. and 8 a.m., it suggests that the most vulnerable period for cardiovascular events in patients with CKD may be during this period. Given that nondippers have greater physical activity at night, the heightened hemodynamic reactivity during those hours may make them particularly vulnerable to cardiovascular events. Future studies of larger groups of patients should confirm these observations and ascertain the prognostic significance of these findings. If hemodynamic reactivity during the course of daily activity is of prognostic importance, then it would pave the way for clinical trials of diet, exercise, and drugs to improve the dismal cardiovascular outcomes in patients with CKD.
Disclosures
None.
Acknowledgments
This study was supported by a grant from Abbott Pharmaceuticals.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Clark LA, Denby L, Pregibon D, Harshfield GA, Pickering TG, Blank S, Laragh JH: A quantitative analysis of the effects of activity and time of day on the diurnal variations of blood pressure. J Chronic Dis 40: 671–681, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Kuwajima I, Hamamatsu A, Suzuki Y, Kuramoto K: The relationship between ambulatory blood pressure and physical activity in young and older shiftworkers: A quantitative assessment of physical activity using a microcomputer with acceleration sensor. Jpn Heart J 34: 279–289, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Van Egeren LF: Monitoring activity and blood pressure. J Hypertens Suppl 9: S25–S27, 1991 [PubMed] [Google Scholar]
- 4.Stewart MJ, Brown H, Padfield PL: Can simultaneous ambulatory blood pressure and activity monitoring improve the definition of blood pressure? Am J Hypertens 6: 174S–178S, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Gretler DD, Carlson GF, Montano AV, Murphy MB: Diurnal blood pressure variability and physical activity measured electronically and by diary. Am J Hypertens 6: 127–133, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Siche JP, Larota C, Charbonnier S, Baguet JP, Diourte B, Bonnet JL, Mallion JM: A quantitative analysis of a predictive model of ambulatory blood pressure monitoring integrating physical activity recording [in French]. Arch Mal Coeur Vaiss 91: 979–984, 1998 [PubMed] [Google Scholar]
- 7.Shapiro D, Goldstein IB: Wrist actigraph measures of physical activity level and ambulatory blood pressure in healthy elderly persons. Psychophysiology 35: 305–312, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Mansoor GA, White WB, McCabe EJ, Giacco S: The relationship of electronically monitored physical activity to blood pressure, heart rate, and the circadian blood pressure profile. Am J Hypertens 13: 262–267, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Kario K, Schwartz JE, Pickering TG: Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension 34: 685–691, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Leary AC, Donnan PT, MacDonald TM, Murphy MB: Physical activity level is an independent predictor of the diurnal variation in blood pressure. J Hypertens 18: 405–410, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE: Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der NP, O'Brien E: Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med 348: 2407–2415, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T, Nishio I: Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension 39: 761–766, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Filipovsky J, Ducimetiere P, Safar ME: Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension 20: 333–339, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Laukkanen JA, Kurl S, Salonen R, Lakka TA, Rauramaa R, Salonen JT: Systolic blood pressure during recovery from exercise and the risk of acute myocardial infarction in middle-aged men. Hypertension 44: 820–825, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O'Brien E: Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: The Dublin outcome study. Hypertension 46: 156–161, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Verdecchia P, Angeli F, Borgioni C, Gattobigio R, Reboldi G: Ambulatory blood pressure and cardiovascular outcome in relation to perceived sleep deprivation. Hypertension 49: 777–783, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Nielsen FS, Rossing P, Bang LE, Svendsen TL, Gall MA, Smidt UM, Parving HH: On the mechanisms of blunted nocturnal decline in arterial blood pressure in NIDDM patients with diabetic nephropathy. Diabetes 44: 783–789, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Portaluppi F, Montanari L, Massari M, Di Chiara V, Capanna M: Loss of nocturnal decline of blood pressure in hypertension due to chronic renal failure. Am J Hypertens 4: 20–26, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Agarwal R, Andersen MJ: Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension 46: 514–520, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, Hirshkowitz M, Kapen S, Kramer M, Loube D, Wise M, Johnson SF: Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: An update for 2002. Sleep 26: 337–341, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Rabe-Hesketh S, Skrondal A: Multilevel and Longitudinal Modeling Using Stata, College Station, TX, Stata Press, 2005
- 25.Lund-Johansen P: Twenty-year follow-up of hemodynamics in essential hypertension during rest and exercise. Hypertension 18: III54–III61, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T, Nishio I, Takeda S: Antihypertensive effects of aerobic exercise in middle-aged normotensive men with exaggerated blood pressure response to exercise. Hypertens Res 25: 507–514, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D: Impaired heart rate response to graded exercise: Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 93: 1520–1526, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Sandvik L, Erikssen J, Ellestad M, Erikssen G, Thaulow E, Mundal R, Rodahl K: Heart rate increase and maximal heart rate during exercise as predictors of cardiovascular mortality: A 16-year follow-up study of 1960 healthy men. Coron Artery Dis 6: 667–679, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Savonen KP, Lakka TA, Laukkanen JA, Halonen PM, Rauramaa TH, Salonen JT, Rauramaa R: Heart rate response during exercise test and cardiovascular mortality in middle-aged men. Eur Heart J 27: 582–588, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Lim PO, MacDonald TM: Step test in hypertension. QJM 93: 703–705, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Pickering TG, Kario K: Nocturnal non-dipping: What does it augur? Curr Opin Nephrol Hypertens 10: 611–616, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Andersen MJ, Agarwal R: Etiology and management of hypertension in chronic kidney disease. Med Clin North Am 89: 525–547, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B: Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med 346: 913–923, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Jones H, Atkinson G, Leary A, George K, Murphy M, Waterhouse J: Reactivity of ambulatory blood pressure to physical activity varies with time of day. Hypertension 47: 778–784, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Muller JE, Tofler GH, Stone PH: Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 79: 733–743, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Sanders PW: Salt intake, endothelial cell signaling, and progression of kidney disease. Hypertension 43: 142–146, 2004 [DOI] [PubMed] [Google Scholar]