Abstract
Background and objectives: Abnormalities in mineral metabolism [calcium, phosphate, and immunoreactive parathyroid hormone (PTH)] and vitamin D have been linked to increases in central arterial stiffness. Central arterial stiffness can be measured using noninvasive technologies, including augmentation index (AIx), a composite measure of arterial stiffness.
Design, setting, participants, and measurements: In 131 outpatients identified from individual cardiac or kidney disease clinics, we examined conventional demographic and laboratory risk factors, vitamin D levels (1,25-OH2D3 and 25-OHD3), and markers of inflammation or endothelial function [C-reactive peptide (hsCRP), matrix metalloproteinase 2 (MMP-2), matrix metalloproteinase 9 (MMP-9), and IL-6] in relationship to AIx.
Results: The median eGFR was significantly different between clinics (range 25–81 ml/min). Subjects with higher phosphate or MMP-9 levels were found to have a higher AIx (P = 0.02 and 0.07, respectively). Lower 1,25-OH2D3 levels or reduced eGFR were associated with higher AIx (P = 0.002 and 0.005, respectively). The associations between 1,25-OH2D3 and phosphate levels and AIx were observed for values within the normal range. No association was noted for calcium, iPTH, 25-OHD3, or hsCRP and AIx. Adjusting for potential confounders [eGFR, calcium, phosphate, and (log) iPTH] the association of lower 1,25-OH2D3 with AIx remained statistically significant.
Conclusion: This exploratory study demonstrates a significant association between AIx and 1,25-OH2D3 in a diverse group with cardiac, kidney disease, or both. These increasing understanding of the role of vitamin D in vascular health lends a context to these findings and raises questions as to additional modifiable risk factors in complex patients. Further studies are required.
Despite significant advances in the prevention and treatment of cardiovascular disease (CVD) the incidence, prevalence, morbidity, and mortality remain high. While traditional risk factors such as hypertension, diabetes, dyslipidemia, and tobacco use offer targets for therapeutic intervention, they do not account for the entire spectrum of disease. In recent years the focus has turned to emerging nontraditional risk factors as well as the concept of central (aortic) arterial stiffness (CAS). Estimates of CAS using radial tonometry and subsequent pulse wave analysis (PWA) have been validated and shown to predict outcomes as diverse as global CV risk, ischemic threshold, and CAD disease burden, as well as CV and all-cause mortality in the general population as well as the elderly and those with diabetes, hypertension, or chronic kidney disease (CKD) (1–5).
CAS has been shown to vary with various physiologic and pathologic states. Aging has long been associated with a hardening of the conduit arteries. Recently it has been shown that a significant independent association exists between inflammation, as measured by hsCRP, IL-6, or soluble intercellular adhesion molecule-1, and CAS (3,6,7). Likewise an association exists between CAS and the gelatinases [matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9)] that mediate the degradation of collagen and elastin and CAS (8).
Abnormalities of mineral metabolism [calcium (Ca2+), phosphate (PO4), and intact parathyroid hormone (iPTH)] and vitamin D have been implicated as risk factors for CVD in both the CKD and non-CKD populations, being associated with increased rates of myocardial infarction, stroke, and heart failure (HF), as well as an increased all-cause mortality (OR 1.71 for men and 1.85 for women) (9–12). Part of this increased risk stems from the recognition that hyperparathyroidism and vitamin D deficiency result in multiple dysregulatory abnormalities including malignant myocardial and valvular calcification, disturbances in the renin–angiotensin system, and vascular endothelial cell dysfunction leading to increased arterial stiffness and resultant hypertension, left ventricular hypertrophy, and diastolic dysfunction (11,13–16). To complicate matters further, vitamin D deficiency is highly prevalent in unscreened populations and is associated with increased inflammation, as well as vascular and myocardial dysfunction (17,18).
Purpose
The purpose of this exploratory observational cross-sectional study was to describe the relationship between nontraditional cardiovascular risk factors in outpatient populations identified predominantly as having either cardiac disease or kidney disease.
Materials and Methods
Subjects
Between the months of March 2004 and April 2006 131 consecutive consenting patients over the age of 18 yr from two cardiac clinics and one renal clinic at a tertiary care institution in Vancouver, BC, Canada, were enrolled. Forty-one patients with predominantly coronary artery disease (CAD) were recruited from the Healthy Heart Program (HHP), 41 with predominantly NYHA class 1–3 HF from the Heart Function Clinic (HFC), and 49 with predominantly CKD from the Kidney Function Clinic (KFC). At the time of enrollment all of these patients were receiving best evidence standardized care. Recruitment did not vary by season. The study protocol was approved by the institutional ethics board, and all patients provided informed written consent.
Variables of Interest and Data Collection
Detailed baseline characteristics, medications, and comorbidities were ascertained by clinical and chart review. All patients had brachial BP measurement, height and weight determination, and a pulse wave analysis (see below) at the time of enrollment. Venous blood samples were obtained for complete blood count (CBC), electrolytes, lipids, creatinine, and parameters of mineral metabolism (Ca2+, PO4, and iPTH) after an overnight fast. Kidney function was estimated by using the four-variable MDRD formula and standardized creatinine values. For the purposes of data analysis, patients with GFR >60 ml/min/1.73 m2 are assumed to have normal kidney function.
At the time of initial blood draw, samples for vitamin D levels (1,25-OH2D3 and 25-OHD3) as well as inflammatory and endothelial markers (hsCRP, MMP-2, MMP-9, and IL-6) and NT-proBNP were frozen, stored at −70°C, and later run as a separate batch analysis in a central laboratory. 25-OHD3 levels were determined by Sorin RIA (Stillwater, MN), and 1,25-OH2D3 levels were determined by using a competitive binding assay (13). MMP-9 and MMP-2 were measured by using a quantitative sandwich enzyme immunoassay technique. (Quantikine; R&D Systems). IL-6 was measured by using a multiplex Luminex assay (Human Cardiovascular Disease Panel 3 Linco plex kit). High-sensitivity CRP was measured by using a solid-phase chemiluminescent immunometric assay (Immulite 2000; Siemens Healthcare). All analyses were run in the same laboratory under the same conditions using standard protocols.
Measures of Central Stiffness: Pulse Wave Analysis
Pulse wave analysis (PWA) is a validated simple, reproducible, noninvasive, tonometric technique for assessing CAS (2,19). Using a peripheral site, in this case the radial artery, one is able to capture and analyze a composite pulse waveform that consists of a forward traveling pressure wave due to ventricular contraction and a peripherally reflected wave (which normally results in diastolic augmentation). As the central arteries stiffen there is increased conduction with faster translation of the outgoing wave. Eventually the timing of the reflected wave encroaches earlier into systole resulting in increased cardiac afterload (augmented systolic pressure) as well as a fall in coronary perfusion pressure and diastolic BP (3,20).
Data derived from PWA can be used to calculate a validated surrogate of central arterial compliance known as the augmentation index of the central aortic pressure waveform (AIx) (2,19). AIx is a parameter that reflects the degree to which central arterial pressure is enhanced by wave reflection and is defined as the augmentation pressure divided by the pulse pressure. Higher AIx values suggest increased peripheral wave reflection and/or earlier reflected wave return secondary to increased pulse pressure propagation velocity (PWV) (due to increased CAS) (3,20).
Using previously described techniques a trained research assistant performed pulse wave analysis using applanation tonometry of the radial artery. Aortic pulse waveform, central aortic pressure, and augmentation index at an HR of 75 were assessed with the commercially available noninvasive SphygmoCor Aortic BP Waveform Analysis System (AtCor Medical, Sydney, Australia), a validated assessment of CAS (2,19–21). This technique is simple to apply in an outpatient setting given the use of the radial artery, which is easily exposed.
Statistical Analyses
The study cohort's demographics at baseline, as well as the AIx, measures of mineral metabolism, and inflammatory markers of interest were summarized by the clinic of origin where patients were recruited (see Table 1). We then examined these parameters of mineral metabolism and inflammatory markers by the tertile of AIx. Descriptive statistics such as mean with SD or median with interquartile range, depending on the underlying distribution, are reported for continuous variables. Percentages are used to summarize categorical variables. χ2, one-way ANOVA, and Kruskal–Wallis tests were employed, where appropriate, to examine whether the distribution of the aforementioned variables is homogeneous across clinics and across tertile of AIx. All tests were two-sided with P < 0.05 considered to be statistically significant.
Table 1.
Characteristics of study cohort at baselinea
| Variables | Overall | HHP | HFC | KFC | P value |
|---|---|---|---|---|---|
| N | 131 | 41 | 41 | 49 | — |
| Age in years (SD) | 62 (12) | 62 (11) | 67 (11) | 58 (13) | 0.002 |
| Male, % | 67 | 68 | 79 | 57 | 0.08 |
| Caucasian, % | 80 | 80 | 87 | 73 | 0.28 |
| Body mass index in kg/m2 (SD) | 27 (5) | 28 (4) | 28 (5) | 27 (6) | 0.56 |
| eGFR in ml/min/1.73m2 [IQR] | 51 [31-79] | 81 [66-90] | 64 [44-79] | 25 [16-37] | <0.001 |
| Hypertension, % | 66 | 54 | 54 | 86 | 0.001 |
| Diabetes, % | 29 | 29 | 23 | 33 | 0.61 |
| Coronary artery disease, % | 50 | 80 | 74 | 5 | <0.001 |
| Stroke, % | 5 | 2 | 10 | 2 | 0.18 |
| Left ventricular hypertrophy or heart failure, % | 36 | 22 | 87 | 8 | <0.001 |
| Peripheral vascular disease, % | 5 | 5 | 13 | 0 | 0.01 |
HHP, Healthy Heart Program (predominantly CAD); HFC, Heart Function Clinic (predominantly heart failure); KFC, Kidney Function Clinic (predominantly chronic kidney disease); IQR, interquartile range.
If the distribution of any of the mineral metabolism and inflammatory markers was found to be nonhomogeneous among the tertile of AIx, we subsequently examined whether the observed difference was linear in relationship with AIx. To do so, a simple linear regression model was employed. The identified marker was treated as the outcome variable, and the tertile of AIx as a continuous predictor in the model. In the multiple regression model, we further adjusted for eGFR and all other remaining mineral metabolism and/or inflammatory markers.
All analyses were carried out in SAS software, Version 9.1 (SAS Institute, Cary, NC). Graphical presentation of the data was prepared in the statistical software SPLUS, Version 7.0 (Insightful Corporation).
Results
One hundred thirty-one subjects were enrolled for investigation. The demographics of the study population are as stated in Table 1. Overall, the cohort had a mean age of 62 yr and was predominantly men (67%). Sixty-six percent (66%) of the patients enrolled were hypertensive, and 29% had type 1 or type 2 diabetes. The average eGFR for the cohort was 51 ml/min/1.73 m2. With respect to CVD, 50% had underlying CAD, 36% had HF or left ventricular hypertrophy (LVH), and 5% had a history of stroke or peripheral vascular disease (PVD).
When stratified by clinic of origin the demographic results were similar although those originating from KFC were significantly younger with a higher burden of hypertension (P = 0.001) and a lower eGFR (P < 0.001). They had lower prevalence of documented CAD (P < 0.001), PVD (P < 0.01), and LVH or HF (P < 0.001). Not surprisingly, patients from HHP had the highest rate of CAD, whereas patients from HFC had the highest rate of HF or LVH.
Table 2 summarizes the data with respect to the measured variables of interest, including AIx and laboratory data. Overall the AIx was 20.9, but there was a statistically significant difference across the three clinics: those patients with CKD have the highest AIx, and those from the HHP have the lowest (see Table 1). Similar findings were observed in phosphate and iPTH (although note is made that the phosphate levels in all groups are within the normal reference range for our laboratory: 0.65–1.65 mmol/L). As expected, the most abnormal 1,25-OH2D3 level was observed in the CKD cohort; however, a “gradient” is again described through the three clinics (see Figure 1). Note that there were no cases of 25-OHD3 or 1,25-OH2D3 deficiency (defined as <23 nmol/L or <25 pmol/L, respectively). There was no significant difference in serum calcium or 25-OHD3 levels among the clinics.
Table 2.
Augmentation index, mineral metabolism, and inflammatory markers overall and by clinic typea
| Variables | Overall | HHP | HFC | KFC | P value |
|---|---|---|---|---|---|
| Augmentation index (SD) | 20.9 (8.9) | 17.6 (9.0) | 20.9 (7.8) | 23.8 (8.8) | 0.005 |
| Calcium in mmol/L (SD) | 2.31 (0.12) | 2.29 (0.09) | 2.33 (0.14) | 2.29 (0.14) | 0.30 |
| Phosphate in mmol/L (SD) | 1.12 (0.22) | 1.04 (0.17) | 1.08 (0.21) | 1.22 (0.24) | <0.001 |
| Intact PTH in pmol/L [IQR] | 6.25 [3.9-9.8] | 4.25 [3.50-6.20] | 6.70 [4.60-9.30] | 9.30 [5.15-15.95] | <0.001 |
| 25-OHD3 in nmol/L [IQR] | 75 [55-93] | 69 [51-83] | 82 [63-96] | 78 [52-108] | 0.13 |
| 1,25-OH2D3 in pmol/L [IQR] | 95 [57-138] | 137 [106-166] | 103 [63-173] | 57 [32-76] | <0.001 |
| CRP in mcg/ml [IQR] | 0.92 [0.39-3.04] | 0.99 [0.37-2.52] | 1.29 [0.64-3.69] | 0.81 [0.37-2.03] | 0.15 |
| MMP-2 in ng/ml [IQR] | 215 [190-256] | 190 [169-210] | 243 [214-281] | 229 [205-252] | <0.001 |
| MMP-9 in mcg/ml [IQR] | 0.47 [0.32-0.79] | 0.56 [0.32-0.83] | 0.42 [0.33-0.91] | 0.49 [0.30-0.66] | 0.75 |
| IL-6 < 0.64 pg/ml, % | 72 | 80 | 59 | 76 | 0.07 |
| NT proBNP in pg/ml [IQR] | 244 [60-658] | 194 [61-308] | 1,021 [443-1,930] | 94 [44-265] | <0.001 |
Significant differences between clinics were observed for AIx, phosphate, iPTH, 1,25OH2D3, MMP-2, and NT-pro BNP. For AIx, phosphate, and iPTH there was a “gradient” of effect through the three clinics with HHP having the lowest values, KFC having the highest, and HFC sitting in the middle. The opposite effect was observed for 1,25OH2D3. HHP, Healthy Heart Program (predominantly CAD); HFC, Heart Function Clinic (predominantly heart failure); KFC, Kidney Function Clinic (predominantly chronic kidney disease); AIx, augmentation index; CRP, C-reactive peptide; MMP-2, matrix metalloproteinase 2; MMP-9,- matrix metalloproteinase 9; IQR, interquartile range.
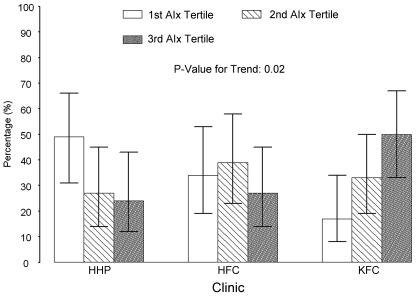
Figure 1.
Patient distribution based on augmentation index tertile by clinic of origin. The KFC group had proportionally more patients in the highest AIx tertile, whereas those in the HHP clinic had more patients in the lowest tertile group. Those in HFC had a relatively even distribution through the tertiles. The whisker in each bar represents the 95% confidence interval of the proportion based on multinomial distribution.
A significant difference was found between clinics for MMP-2 (P < 0.001) but not MMP-9 (P = 0.75). With respect to inflammatory markers, no difference was observed in hsCRP or IL-6 levels when stratified by clinic. Patients from the CKD clinic had the lowest NT-proBNP level whereas patients from the HFC, expectedly, had the highest level.
Augmentation Index: Univariate Analysis
Table 3 describes the results of laboratory testing when subjects were categorized by tertile of AIx. Interesting results emerged with respect to MMP-9, phosphate, and 1,25-OH2D3: those with higher phosphate or MMP-9 levels were found to have a higher AIx (P = 0.02 and 0.07, respectively). Similarly, those with lower 1,25-OH2D3 levels or lower values for renal function (eGFR) were more likely to have higher AIx (P = 0.002 and 0.005, respectively). Strikingly, with respect to 1,25-OH2D3 and phosphate levels, this finding was observed for values that remained in the normal reference range for our laboratory (phosphate normal range 0.65–1.65 mmol/L). No association was noted for calcium, iPTH, 25-OHD3, or hsCRP with respect to the tertile of AIx. These findings remain when AIx was analyzed as a continuous variable (data not shown).
Table 3.
Summary statistics for mineral metabolism and inflammatory markers by augmentation indexa
| Variables | Overall | AIx
|
P valueb | ||
|---|---|---|---|---|---|
| First tertile | Second tertile | Third tertile | |||
| Augmentation index (SD) | 20.9 (8.9) | [-11, 17] | [17, 25] | [25, 47] | — |
| Clinic type, % | 0.01 | ||||
| HHP | 31 | 49 | 27 | 24 | |
| HFC | 31 | 34 | 39 | 27 | |
| KFC | 38 | 17 | 33 | 50 | |
| eGFR in ml/min/1.73m2 [IQR] | 51 [31-79] | 73 [45-87] | 50 [32-73] | 40 [21-70] | 0.005 |
| Calcium in mmol/L (SD) | 2.31 (0.12) | 2.27 (0.10) | 2.33 (0.12) | 2.30 (0.15) | 0.09 |
| Phosphate in mmol/L (SD) | 1.12 (0.22) | 1.04 (0.19) | 1.14 (0.26) | 1.18 (0.21) | 0.02 |
| Intact PTH in pmol/L [IQR] | 6.25 [3.90-9.80] | 5.45 [3.80-8.00] | 7.30 [4.45-13.3] | 5.90 [3.70-9.30] | 0.24 |
| 25-OHD3 in nmol/L [IQR] | 75 [55-93] | 73 [57-88] | 71 [48-93] | 79 [52-103] | 0.41 |
| 1,25-OH2D3 in pmol/L [IQR] | 95 [57-138] | 131 [101-165] | 88 [49-148] | 65 [42-136] | 0.002 |
| CRP in mcg/ml [IQR] | 0.92 [0.39-3.04] | 0.84 [0.38-2.59] | 1.08 [0.68-2.42] | 0.69 [0.32-2.90] | 0.27 |
| MMP-2 in ng/ml [IQR] | 215 [190-256] | 210 [177-247] | 224 [206-256] | 215 [187-261] | 0.30 |
| MMP-9 in mcg/ml [IQR] | 0.47 [0.32-0.79] | 0.36 [0.29-0.63] | 0.49 [0.40-0.67] | 0.57 [0.31-0.98] | 0.07 |
| IL-6 < 0.64 pg/ml, % | 72 | 68 | 78 | 68 | 0.52 |
| NT proBNP in pg/ml [IQR] | 244 [60-658] | 226 [58-787] | 265 [63-1,117] | 204 [61-464] | 0.70 |
When categorized by tertile of AIx trends were observed between groups. The most abnormal value of AIx was found to be significantly associated with lower eGFR and 125OH2D3levels as well as higher phosphate levels. There was an association towards higher MMP-9 in the most abnormal AIx group. HHP, Healthy Heart Program (predominantly CAD); HFC, Heart Function Clinic (predominantly heart failure); KFC, Kidney Function Clinic (predominantly chronic kidney disease); AIx, augmentation index; CRP, C-reactive peptide; MMP-2, matrix metalloproteinase 2; MMP-9, matrix metalloproteinase 9; IQR, interquartile range.
Based on one-way ANOVA or Kruskal–Wallis test.
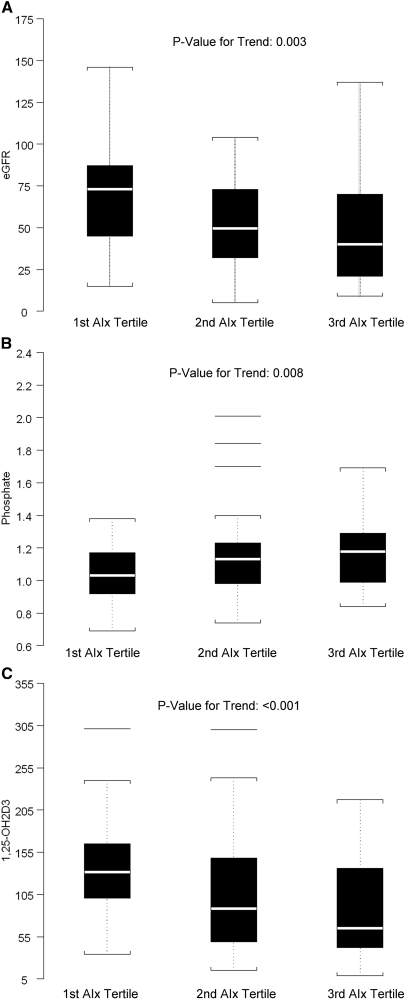
Figure 2 demonstrates linear relationships of eGFR, phosphate, and 1,25-OH2D3 with the tertile of AIx. Phosphate was found to increase with AIx tertile (P = 0.008). An inverse relationship was observed between eGFR and AIx tertile and between 1,25-OH2D3 and AIx tertile where lower values of eGFR were associated with higher AIx tertile (P = 0.003). Likewise, lower values of 1,25-OH2D3 were associated with higher AIx tertile (P < 0.001).
Figure 2.
(A) Box plot of eGFR by the tertile of augmentation index. There was a significant association between lower eGFR and higher AIx tertile (P = 0.003). (B) Box plot of phosphate by the tertile of augmentation index. There was a significant association between higher phosphate levels and higher AIx tertile (P = 0.008) despite phosphate levels within the normal reference range for our laboratory (see text and Table 2 for details). (C) Box plot of 1,25-OH2D3 by the tertile of augmentation index. There was a significant association between lower 1,25-OH2D3 levels and higher AIx tertile (P < 0.001).
Multivariate Analysis
We further examined whether the linear relationship between 1,25-OH2D3 (in logarithmic scale) and tertile of AIx remains after adjusting for potential confounders such as eGFR, calcium, phosphate, and iPTH via logarithmic scale. The observed inverse linear relationship between 1,25-OH2D3 and tertile of AIx remained statistically significant (R2 = 0.31; β for AIx tertile = −0.16, P = 0.03). Conversely, the relationship between eGFR and AIx after adjusting for all mineral metabolism markers was no longer significant (R2 = 0.45; β for AIx tertile = −5.05, P = 0.08). Similarly, the relationship between phosphate and AIx was also NS (R2 = 0.23; β for AIx tertile = 0.03, P = 0.22) after controlling for eGFR, calcium, 1,25-OH2D3, and iPTH via logarithmic scale.
Discussion
This exploratory study demonstrates a significant association between AIx (a composite measure of central arterial stiffness) and 1,25-OH2D3 in a diverse group of individuals with CVD, CKD, or both. To our knowledge this is the first study to describe these associations in subjects with milder CKD (stage 2 to stage 4), a group known to be at highest risk of CVD, and in cardiac patients not followed by nephrologists. Importantly, we believe that this information adds credence to the postulated importance of the role of vitamin D in the maintenance of vascular health.
In addition to other factors, vitamin D is known to exert immunoregulatory properties via the ubiquitous vitamin D receptor, a receptor that is present in almost every organ, as well as the vascular wall and most immune cells. In normal conditions vitamin D has been shown to inhibit antigen-presenting cell maturation, angiogenesis, and smooth muscle cell proliferation, as well as alter the cytokine profile to one that less favors inflammation through interaction with the vitamin D receptor (17). Moreover, on a more phenotypic level, vitamin D deficiency may be associated with obesity, metabolic syndrome, and diabetes, conditions known to be associated with adverse vascular health (22). Given the aforementioned effects of vitamin D on mitigating smooth muscle proliferation and inflammation we postulate that a deficiency of 1,25-OH2D3 would lead to multiple dysregulatory vascular effects, eventually leading to abnormalities in central arterial stiffness, and thus our observed relationship with AIx. It is interesting to note that these data describe a relationship despite the relatively “normal” levels of 1,25-OH2D3. It may be that current definitions of deficiency and insufficiency need to be reexamined.
CAS has been shown to be related to a number of pathologic states. Studies from various populations have shown that AIx and PWV are related to anthropomorphic variables (gender, height, aortic width), smoking status, and BP (systolic, mean arterial, and brachial pulse pressure), as well as various pathologic states. Inflammation, a marker of vascular health, has been linked to abnormalities in conduit artery function. Recent studies have shown a relationship between hsCRP, IL-6, or soluble intercellular adhesion molecule-1 and CAS (1,6,7). Likewise, there is an association between CAS and the gelatinases (MMP-2 and MMP-9) that mediate the degradation of collagen and elastin (8).
In this small exploratory study we were not able to find an association between hsCRP or IL-6 and AIx in our analyses. This is potentially congruent with existing literature, with some studies observing a correlation and others failing to find one (1,6,7). This may be a function of small samples sizes or specific population cohort characteristics. There are some assertions that an inflammatory cytokine profile may have differential effects on the central and peripheral vascular beds resulting in central stiffening and peripheral vasodilatation resulting in a neutral net effect on AIx. If true, this may be an additional reason why we failed to detect a difference in AIx (6).
It is known that MMP-9 is associated with higher CV risk (23). However, previous studies have failed to note an association between AIx and MMP. Despite the small numbers in our study on univariate analysis there was a suggestion of a relationship between AIx and MMP-9 (P = 0.07) but not MMP-2. Like inflammation, this may be explained by the differential effects of MMP-9 and MMP-2 on central and peripheral vascular beds where remodeling of only the large elastic arteries is dependent on MMP (8). Interestingly, this finding may be in keeping with the known interaction between MMP-9 and vitamin D. Previous studies have shown that the matrix metalloproteinase/tissue inhibitor of metalloproteinase (MMP/TIMP) system is regulated by circulating 1,25-OH2D3 and that vitamin D deficiency is associated with higher hsCRP, MMP-2, and MMP-9 levels (8,24). Conversely, it may be that the observed univariate relationship, which disappeared after adjustments, was an epiphenomenon predominantly related to vitamin D or other factors.
The importance of phosphate, a parameter of importance in mineral metabolism for CKD patients, has also been described as important in the altered vascular biology of CKD (25,26). Recently, a study by Tonelli et al. showed a graded independent relation between higher serum phosphate levels, within the normal reference range, and the risk of death and CV events in people with prior myocardial infarction (27). The present study similarly describes in the univariate analysis, again within the normal range, a statistically significant relationship between tertiles of AIx and phosphate levels. However, in keeping with the complexity of the relationship, after adjusting for other parameters, the statistical significance of the association was lost.
Although a decrease in 1,25-OH2D3 and an elevation in iPTH are commonly encountered in the CKD population, there is an increased recognition that these abnormalities are more widespread in the general population. A large population-based study identified a high prevalence of reduced 1,25-OH2D3 in the absence of abnormalities in calcium, phosphate, and 25-OHD3 (18). These changes in 1,25-OH2D3 were noted to precede abnormalities in hyperparathyroidism.
In each of the clinic cohorts in our study the markers of mineral metabolism were well within the normal or accepted ranges. Despite this, we observed relationships between arterial stiffness and the various components of mineral metabolism. This cross-sectional study design does not permit ascertainment of causality. Furthermore, the knowledge that interactions between these various parameters are complex further complicates interpretation. For example, circulating vitamin D will have effects on phosphate, calcium, and iPTH levels through a multiplicity of feedback mechanisms. Despite this interaction and our relatively small numbers of subjects a significant association did emerge in patients traditionally considered to be 125OH2D3-deficient who were undergoing treatment (CKD patients) as well as those who were not (cardiac patients).
The observation that vitamin D is associated with measures of CAS must be interpreted with caution. Although biologic plausibility exists, it is possible that the observed association may be the result of residual confounding and does not necessarily indicate a causal link among 125OH2D3, deficiency, and vascular disease. Likewise, our small numbers and the cross-sectional observational nature of this study limit interpretation; therefore, this study remains only hypothesis-generating and should facilitate further studies that examine the degree of variability in outcomes explained by these newer laboratory tests in addition to CAS measures. More importantly, once the biologic relationships are more thoroughly ascertained, large randomized clinical trials might be undertaken to ascertain whether treatment with vitamin D supplementation might impact these measures of CAS (surrogates) or hard CV outcomes.
Currently the most widely available clinical estimates of CAS are derived from applanation tonometry with the resultant calculation of an augmentation index (AIx) as well as measurements of peripheral pulse-wave velocity. Concerns have recently arisen regarding the ability of AIx to assess CAS because of its being a composite measure that is dependent on multiple factors including PWV, pulse pressure propagation distance, and incident and reflected wave overlap (left ventricular ejection time and time to shoulder relationship), as well as a systemic reflection coefficient. Owing to these concerns, recent investigations into the mechanism of CAS have suggested that wave reflection (i.e., AIx) is not pathogenic but merely an epiphenomenon. A recent study examining the etiology of CAS found that the observed increase in pulse pressure is primarily attributable to increased wall stiffness and reduced aortic diameter rather than premature wave reflection (28). Thus, while being a composite measure of arterial stiffness, given that multiple studies have shown AIx to correlate closely with direct measurements of PWV and to be an independent predictor of adverse cardiovascular events, including mortality, we believe the findings herein to be of interest. Consensus remains regarding the prognostic utility of this test (3–5).
In summary, we have reported an exploratory study that examines the role of mineral metabolism parameters and inflammatory markers in patients with CVD, kidney disease, and the combination thereof. In so doing we have attempted to link an emerging literature on vascular biology, conventional and nonconventional risk factors for vascular health, and new understandings about vascular health and disease in CKD patients. Thus, this simple observational study appears to describe the complex interaction of multiple aspects of vascular health and corroborates known relationships. It may be that nondialysis CKD patients represent a severe subtype of the CVD population. As such, studies need to be conducted examining nontraditional risk factors in those with CKD, CVD, or both. It is imperative that we better understand the complexity of the pathobiology of vascular disease in a spectrum of patients so that better therapeutic approaches can be designed and tested.
Disclosures
None.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kampus P, Kals J, Ristimae T, Fischer K, Zilmer M, Teesalu R: High-sensitivity C-reactive protein affects central haemodynamics and augmentation index in apparently healthy persons. J Hypertens 22: 1133–1139, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Oparil S, Izzo JL Jr: Pulsology rediscovered: Commentary on the Conduit Artery Function Evaluation (CAFÉ) Study. Circulation 113: 1162–1163, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Hansen TW, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J: Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 113: 664–670, 2006 [DOI] [PubMed] [Google Scholar]
- 4.The CAFE Investigators, for the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Investigators: Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: Principal results of the Conduit Artery Function Evaluation (CAFE) Study. Circulation 113: 1213–1225, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Nürnberger J, Keflioglu-Scheiber A, Opazo Saez AM, Wenzel RR, Philipp T, Schäfers RF: Augmentation index is associated with cardiovascular risk. J Hypertens 20: 2407–2414, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Pietri P, Vyssoulis G, Vlachopoulos C, Zervoudaki A, Gialernios T, Aznaouridis K, Stefanadis C: Relationship between low-grade inflammation and arterial stiffness in patients with essential hypertension. J Hypertens 24: 2231–2238, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Kullo I, Seward J, Bailey K, Bielak L, Grossardt B, Sheedy IIP, Peyser P, Turner S. Am J Hypertens 18: 1123–1129, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Yasmin, Wallace S, McEniery CM, Dakham Z, Pusalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR, Wilkinson IB: Matrix metalloproteinase-9 (MMP-9), MMP-2 and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol 25: 372–378, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Palmer M, Adami HO, Bergstrom R, Akerstrom G, Ljunghall S: Mortality after surgery for primary hyperparathyroidism: A follow-up of 441 patients operated on from 1956 to 1979. Surgery 102: 1–7, 1987 [PubMed] [Google Scholar]
- 10.Lundgren E, Lind L, Palmer M, Jakobsson S, Ljunghall S, Rastad J: Increased cardiovascular mortality and normalized serum calcium in patients with mild hypercalcemia followed up for 25 years. Surgery 130: 978–985, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Hedbäck GM, Odén AS: Cardiovascular disease, hypertension and renal function in primary hyperparathyroidism. J Intern Med 251(6): 476–483, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Slinin Y, Foley RN, Collins AJ: Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS Waves 1, 3, and 4 Study. J Am Soc Nephrol 16: 1788–1793, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Langle F, Abela C, Koller-Strametz J, Mittelbock M, Bergler-Klein J, Stefenelli T, Woloszczuk W, Niederle B: Primary hyperparathyroidism and the heart: Cardiac abnormalities correlated to clinical and biochemical data. World J Surg 18(4): 619–624, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Andersson P, Rydberg E, Willenheimer R: Primary hyperparathyroidism and heart disease—A review. Eur Heart J 25(20): 1776–1787, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Rubin MR, Maurer MS, McMahon DJ, Bilezikian JP, Silverberg SJ: Arterial stiffness in mild primary hyperparathyroidism. J Clin Endocrinol Metab 90(6): 3326–3330, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Kiernan TJ, O'Flynn AM, McDermott JH, Kearney P: Primary hyperparathyroidism and the cardiovascular system. Int J Cardiol 113(3): E89–E92, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Levin A, Li YC: Vitamin D and its analogues: Do they protect against cardiovascular disease in patients with kidney disease? Kidney Int 68: 1973–1981, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Smulyan H, Siddiqui DS, Carlson RJ, London GM, Safar ME: Clinical utility of aortic pulses and pressures calculated from applanated radial-artery pulses. Hypertension 42: 150–155, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Vlachopoulos C, Aznaouridis K, Dima I, Ioakeimidis N, Vasiliadou C, Zervoudaki A, Gialernios T, Stefanadis C: Negative association between serum levels of matrix metalloproteinases-2 and -9 and aortic stiffness in healthy adults. Int J Cardiol 122: 232–238, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Yasmin, Brown MJ: Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. Q J Med 92: 595–600, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Michos ED, Blumenthal RS: Vitamin D supplementation and cardiovascular disease risk. Circulation 115: 827–828, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Tayebjee MH, Nadar SK, Blann AD, Beevers G, MacFadyen RJ, Lip GY: Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in hypertension: Relationship to cardiovascular risk and treatment. Am J Hypertens 17(9): 764–769, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ: Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: Mechanisms for inflammatory damage in chronic disorders? Q J Med 95: 787–796, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Moe SM, O'Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K: Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int 61: 638–647, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM: Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87: e10–e17, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Tonelli M, Sacks F, Pfeffer M, Gao Z Curhan G: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Mitchell GF, Conlin PR, Dunlap ME, Lacourciere Y, Arnold JMO, Ogilvie RI, Neutel J, Izzo JL, Pfeffer MA: Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension 51: 105–111, 2008 [DOI] [PubMed] [Google Scholar]