Abstract
Background and objectives: Evidence exists that variability in hemoglobin may be an independent risk factor for mortality among hemodialysis patients. These observations were based on a 1996 cohort, a time when anemia management differed greatly from present.
Design, settings, participants and measurements: A retrospective cohort study of patients incident to Fresenius Medical Care units between 2004 and 2005 (n = 6644). Hemoglobin variability (Hgb-Var) was defined for each subject as the residual SD of a linear regression model of time on hemoglobin.
Results: The mean (SD) of Hgb-Var was 1.13 (0.55) g/dl. In the primary analysis, each g/dl increase of Hgb-Var was associated with an adjusted hazard ratio (95% confidence interval) for all-cause mortality of 1.11 (0.92 to 1.33). No significant interaction with Hgb-Var and mortality was found on the basis of age (P = 0.22), arterial disease (P = 0.45), Hgb slope (P = 0.68), or mean Hgb (P = 0.78). When Hgb-Var was defined by a regression model that included a quadratic term for time (enabling descriptions of curvilinear hemoglobin trajectories), model fit was greatly improved (P for difference <0.001). The corresponding adjusted hazard ratio (95% confidence interval) for all-cause mortality was 1.17 (0.93 to 1.49).
Conclusions: Hgb-Var was not found to be associated with all-cause mortality when examined in a contemporary incident hemodialysis population. More research is needed to determine whether differences in these findings compared with prior analyses relate to temporal trends in anemia management or from differences in the relationship between Hgb-Var and outcomes among incident versus prevalent hemodialysis patients.
Kidney failure requiring chronic maintenance dialysis is common in the United States, with more than 300,000 people receiving renal replacement therapy in 2005 (1). More than 90% of these patients are treated with erythropoiesis stimulating agents (ESAs) and/or intravenous iron for management of anemia (2). Higher hemoglobin (Hgb) levels in this population result in improved quality of life (3–5) and survival (6–10).
Despite more than a decade of research, the optimal anemia management strategy for dialysis patients has yet to be delineated, and guidelines continue to evolve (11). Presently, the National Kidney Foundation Kidney Disease Outcome Quality Initiative recommends targeting Hgb between 11.0 and 12.0 g/dl, but evidence suggests that only 30% of patients fall within this range at any point in time (12), as fluctuations in Hgb levels result in frequent under and overshooting of targets (13–17).
Evidence suggests that not only does hemoglobin variability (Hgb-Var) complicate maintenance of Hgb within the target range but that it is also independently associated with mortality (18,19). Previously, we introduced a novel metric (18) (the residual SD about a linear regression of time on observed Hgb values) that characterized Hgb-Var independent of absolute Hgb level and demonstrated an independent association between degree of variability and all-cause mortality among prevalent hemodialysis (HD) patients. However, these findings were based on data from a 1996 cohort whose anemia management was substantially different from more current-day cohorts of patients with end-stage renal disease. Given the dramatic changes in anemia management over the past decade, the applicability of our previous findings needs to be investigated among contemporary dialysis patients.
Thus, we conducted this retrospective cohort study to examine Hgb-Var and its relation to all-cause mortality using a contemporary cohort of adult HD patients.
Materials and Methods
Patient Selection
We conducted a nonconcurrent cohort study of incident HD patients who enrolled at any Fresenius Medical Care (FMC) unit between June 2, 2004 and August 25, 2005, using the ArMORR cohort (20). To be eligible for inclusion in the primary cohort, patients must have been 18 yr of age or older as of dialysis initiation and have been on HD for a period of less than one month before enrollment. To enable description of Hgb-Var, subjects were required to maintain continuous enrollment in a study unit for at least 180 d. Dialysis sessions missed because of hospitalization, travel, or nonadherence did not constitute violation of continued enrollment; patients recovering renal function, transferring care, changing dialytic modalities, receiving renal transplants, or dying before day 181 were excluded.
Ascertainment of Exposures, Outcomes, and Covariates
The study data were collected and entered into a centralized database prospectively in a manner previously described (20). No further information was gathered retrospectively. Upon enrollment, patients’ demographic information and comorbidities were recorded. All laboratory tests were performed in a centralized laboratory (Spectra Laboratories, Rockleigh, NJ), and results were recorded in a central database. At each HD session, detailed records of laboratory tests, medications administered during dialysis (including intravenous iron and erythropoietin), and mortality were collected.
Time to death from any cause was the primary outcome. At-risk time began immediately after dialysis day 180 and continued for a maximum of 185 d (at-risk day 185 corresponds to chronic HD day 365). Patients contributed at-risk time until they reached an endpoint (death) or were censored administratively on dialysis day 365 (at-risk day 185), or for change in dialytic modality, transfer of care, recovery of renal function, or receipt of renal transplantation.
Hgb was measured regularly (at least monthly) in the course of ongoing dialytic care; Hgbs measured during hospitalization or office visits were not available. To minimize ascertainment bias (e.g., sicker subjects having Hgb measured more frequently), we limited consideration to the first Hgb measure in each month of the observation period; the impact of this choice was explored in sensitivity analyses. These Hgb measurements were used to define each subject's absolute Hgb level (mean of the six values). Hgb-Var and Hgb trend were defined by creation of within-subject linear regression models of time on Hgb. Hgb-Var was considered as the residual SD; Hgb trend was defined as the slope term from these regression models (18).
Statistical Analysis
The distribution of all continuous variables (including Hgb parameters) was examined graphically and via summary statistics; frequency distributions were examined for all categorical variables. The association between pair-wise combinations of Hgb parameters (e.g., Hgb-Var versus mean Hgb) was examined with scatter plots and by calculating Pearson correlation coefficients.
The unadjusted association between each Hgb parameter (Hgb-Var, mean Hgb level, and Hgb slope) and all-cause mortality was examined using Kaplan-Meier methods. In these analyses, mean Hgb and Hgb slope were categorized as follows: <11, 11 to 12, 12 to 13, and >13 g/dl, and <0, 0 to 0.5, 0.5 to 1, and >1 g/dl per month, respectively. Because no obvious clinical thresholds exist for Hgb-Var, it was categorized according to observed quartile.
In addition, we fit unadjusted and adjusted Cox proportions hazard models to examine the association between Hgb-Var, Hgb slope, and mean Hgb (considered as continuous predictors) and survival. In adjusted models, covariate terms were included for remaining Hgb parameters, demographic, comorbid disease, laboratory, and medication variables. The proportional hazards assumption was tested by inclusion of two-way cross product terms for each Hgb exposure and time.
Potential a priori-defined interactions on the bases of Hgb slope, mean Hgb level, arterial disease, and age were explored using two-way cross-product terms in multivariable proportional hazards models and assessed using likelihood ratio testing.
Multiple sensitivity analyses were conducted to explore the potential for selection and misclassification biases, including analyses, which included all subjects with definable Hgb-Var (sensitivity analysis I), measured Hgb-Var based on all available Hgb measurements (sensitivity analysis II), and defined Hgb-Var using models that allowed for nonlinear Hgb trajectories (sensitivity analysis III). In each instance, Cox proportional hazards models were specified in an analogous manner to those used in the primary analysis.
All analyses were completed using Stata 9.0 (Stata Corporation, College Station, TX).
Results
Patient Characteristics
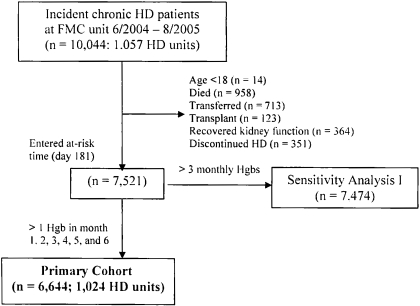
Among the 10,044 incident HD patients who began treatment at any FMC unit between June 2, 2004 and August 25, 2005, 6644 met criteria for inclusion in the primary cohort (Figure 1). The mean ± SD age was 62.0 ± 15.1 yr. Table 1 presents demographic, comorbidity, laboratory, and medication characterization of the cohort. Included subjects were significantly younger, had higher body mass indices, were less likely to be white, and were more likely to have hypertension and diabetes than excluded patients.
Figure 1.
Flow diagram for enrollment.
Table 1.
Characterization of the primary cohort (n = 6644)
| Distribution | n | |
|---|---|---|
| Age, years (mean ± SD) | 62.0 ± 15.1 (25th percentile, 51.7; median, 63.0; 75th percentile, 74.2) | 6644 |
| Gender | ||
| male, % | 54.2 | 3603 |
| female, % | 45.8 | 3041 |
| Race | ||
| white, % | 57.7 | 3830 |
| nonwhite, % | 42.4 | 2814 |
| BMI, kg/m2 (mean ± SD) | 27.9 ± 7.6 | 6643 |
| ≤20, % | 9.7 | 642 |
| 20-25, % | 30.8 | 2044 |
| 25-30, % | 28.9 | 1919 |
| 30-35, % | 15.9 | 1053 |
| >35, % | 14.8 | 985 |
| Comorbidities, % present | ||
| diabetes mellitus | 24.8 | 1648 |
| hypertension | 40.4 | 2682 |
| arterial diseasea | 14.3 | 951 |
| coronary artery disease | 9.8 | 654 |
| peripheral vascular disease | 5.1 | 337 |
| cerebrovascular disease | 3.1 | 207 |
| congestive heart failure | 12 | 797 |
| cancer | 2.9 | 193 |
| Laboratory measures (3-month mean ± SD)b | ||
| urea reduction ratio, % | 71.3 ± 6.8 | 6514 |
| Kt/V | 1.33 ± 0.27 | 6438 |
| albumin, g/dl | 3.7 ± 0.4 | 6644 |
| bicarbonate, mEq/L | 23.8 ± 2.9 | 6609 |
| calcium, mg/dl | 9.0 ± 0.6 | 6637 |
| phosphate, mg/dl | 5.4 ± 1.3 | 6644 |
| creatinine, mg/dl | 7.4 ± 2.9 | 6559 |
| intact PTH, pg/ml | 176 ± 158 | 6390 |
| Laboratory measures (6-month mean ± SD)c | ||
| ferritin, ng/ml | 380 ± 414 | 6631 |
| transferrin saturation, % | 24.9 ± 6.7 | 6596 |
| Treatment measuresc | ||
| cumulative 6-month intravenous iron dose, mg | 943 ± 798 | 6452 |
| cumulative 6-month erythropoietin dose, U | 559,700 ± 351,000 | 6613 |
| average no. of doses per subject | 60.5 ± 13.3 | 6613 |
| average dose per treatment | 9540 ± 7580 | 6613 |
Arterial disease is not mutually exclusive.
Averaged over dialysis days 91 to 180.
Considered over dialysis days 0 to 180.
At-risk time began immediately after dialysis day 180. Thus, each subject could contribute a maximum of 185 at-risk days. The cohort contributed an aggregate of 3082 patient-years of at-risk time, during which 447 deaths were observed; median follow-up time was 185 d.
Hemoglobin Variability Parameters
Hemoglobin parameters were defined using monthly Hgb levels over the first 180 study days. Within-subject linear regression models of time on Hgb were used to define Hgb-Var (residual SD) and Hgb trend (slope); in addition, the mean Hgb level for each subject was determined over the same period. The distribution of these parameters is presented in Table 2.
Table 2.
Distribution of hemoglobin variability parameters across the primary cohort (n = 6644)
| Mean | SD | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|---|---|
| Mean Hgb (g/dl) | 12.0 | 0.93 | 6.7 to 11.5 | 11.6 to 12.1 | 12.2 to 12.6 | 12.7 to 15.8 |
| Hgb slope (g/dl per month) | 0.37 | 0.42 | −1.30 to 0.09 | 0.10 to 0.36 | 0.37 to 0.65 | 0.66 to 2.11 |
| Hgb-Var (g/dl) | 1.13 | 0.55 | 0.05 to 0.72 | 0.73 to 1.04 | 1.05 to 1.47 | 1.48 to 4.39 |
Independence of the three Hgb parameters (mean, slope, and variability) was investigated by scatter plot analysis and by calculating Pearson's correlation coefficient for each pair-wise combination. The correlation coefficient between Hgb-Var and mean Hgb and Hgb slope were 0.17 and −0.08, respectively; the correlation coefficient between mean Hgb and Hgb slope was 0.10. In each instance, the low absolute value of the correlation coefficients confirms that these parameters represent independent descriptors of longitudinal Hgb experience.
Survival and Hemoglobin Variability
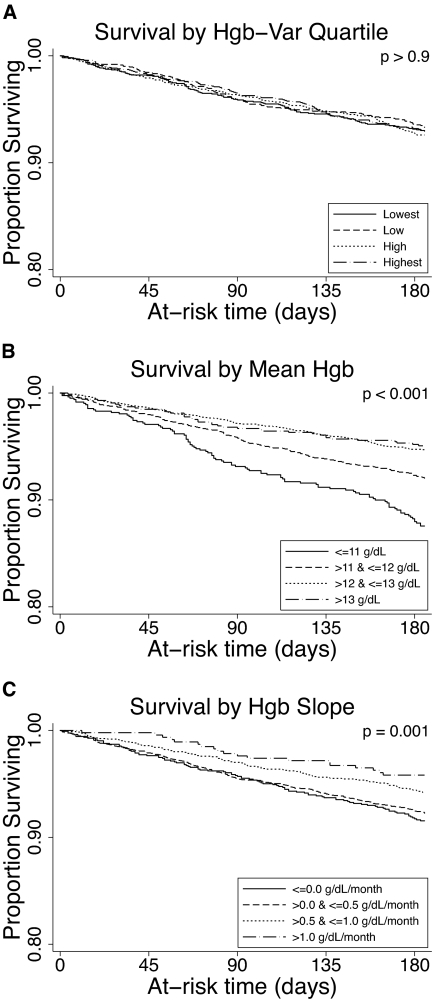
Using unadjusted Kaplan Meier analysis, Hgb-Var (considered in quartiles) was not associated with mortality (Figure 2A; P > 0.9). Unadjusted and adjusted estimates of association between Hgb-Var and mortality were assessed using Cox proportional hazards models. Considered as a linear term, each g/dl increase in Hgb-Var was associated with an unadjusted and adjusted hazard ratio (HR) (95% confidence interval [CI]) for all-cause mortality of 0.96 (0.81 to 1.14) and 1.11 (0.92 to 1.33), respectively (Table 3).
Figure 2.
Survival according to Hgb parameters. (A) Kaplan-Meier survival function for all-cause mortality according to quartile of Hgb-Var. (B) Kaplan-Meier survival function for all-cause mortality according to mean Hgb level (≤11, 11–12, 12–13, >13 g/dl). The number (%) of patients with mean Hgb ≤ 11 was 829 (12.5%), mean Hgb 11 to 12 was 2198 (33.1%), mean Hgb 12 to 13 was 2752 (41.4%), and mean Hgb >13 was 865 (13.0%). (C) Kaplan-Meier survival function for all-cause mortality for subjects with Hgb slope ≤0, 0 to 0.5 g/dl per month, 0.5 to 1 g/dl per month, and >1 g/dl per month. The number (%) of patients with Hgb slope ≤ 0 was 1248 (18.8%), Hgb slope 0 to 0.5 was 2936 (44.2%), Hgb slope 0.5 to 1 was 1984 (29.9%), and Hgb slope >1 was 476 (7.2%).
Table 3.
Unadjusted and multivariable analyses of Hgb parameters
| Unadjusted | Adjusted for other Hgb parametersa | Adjusted for other Hgb parameters and demographicsb | Adjusted for other Hgb parameters, demographics, and comorbiditiesc | Adjusted for other Hgb parameters, demographics, comorbidities, amd labsd | Adjusted for other Hgb parameters, demographics, comorbidities, labs, and medicationse | |
|---|---|---|---|---|---|---|
| Hgb-Var (per g/dl) | 0.96 (0.81–1.14) | 1.03 (0.87–1.23) | 1.04 (0.87–1.24) | 1.06 (0.89–1.27) | 1.07 (0.89–1.30) | 1.11 (0.92–1.33) |
| Mean Hgb (per g/dl) | 0.72 (0.65–0.79) | 0.73 (0.66–0.80) | 0.69 (0.62–0.75) | 0.70 (0.63–0.77) | 0.78 (0.71–0.86) | 0.76 (0.68–0.86) |
| Hgb slope (per g/dl per month) | 0.66 (0.53–0.82) | 0.71 (0.57–0.88) | 0.83 (0.66–1.04) | 0.86 (0.68–1.08) | 0.93 (0.74–1.18) | 0.97 (0.75–1.24) |
Other variables in the table.
Age, sex, race (white, nonwhite, unknown), BMI (≤ 20, 20–25, 25–30, 30–35, >35 kg/m2).
Diabetes, hypertension, arterial disease (coronary and/or cerebrovascular and/or peripheral vascular disease), cancer, and congestive heart failure.
Serum creatinine, phosphate, albumin, and ekT/V averaged over days 90 to 180.
Cumulative 6-month intravenous iron and erythropoietin doses.
Effect modification of association between Hgb-Var and mortality was explored by the introduction of potential effect modifier cross product terms (e.g., Hgb-Var X age, Hgb-Var X, mean Hgb) into the fully adjusted model and assessed using the likelihood ratio test. No significant interaction on the basis of age (P = 0.22), arterial disease (P = 0.45), Hgb slope (P = 0.68), or mean Hgb (P = 0.78) was detected.
Sensitivity Analyses
Exclusion of subjects missing one or more monthly Hgb levels may have imposed survivor bias, as subjects missing Hgb measurements were likely hospitalized. To explore this possibility, sensitivity analysis I was conducted, which considered all subjects with definable Hgb-Var (i.e., with three or more monthly Hgb levels recorded, n = 7474). Unadjusted and adjusted HRs (95% CIs) for all-cause mortality per g/dl increase in Hgb-Var were 0.94 (0.80 to 1.10) and 1.11 (0.92 to 1.33), respectively (Table 4).
Table 4.
Unadjusted and fully adjusted HRs (95% CIs) for all-cause mortality seen in sensitivity analyses I, II, and III
| Sensitivity analysis I | Sensitivity analysis II | Sensitivity analysis IIIa | ||||
|---|---|---|---|---|---|---|
| HgbMs considered | First per month | All measured | First per month | |||
| No. required HgbMs | 3 | 6 | 6 | |||
| N | 7474 | 6644 | 6644 | |||
| HR (95% CI) | Unadjusted | Fully adjustedb | Unadjusted | Fully adjustedb | Unadjusted | Fully adjustedc |
| Hgb residual SD (per g/dl) | 0.94 (0.80 to 1.10) | 1.11 (0.92 to 1.33) | 0.99 (0.81 to 1.21) | 1.16 (0.94 to 1.44) | 1.31 (1.05 to 1.64) | 1.17 (0.93 to 1.49) |
| Mean Hgb (per g/dl) | 0.67 (0.62 to 0.72) | 0.76 (0.68 to 0.86) | 0.70 (0.64 to 0.77) | 0.76 (0.67 to 0.85) | 0.72 (0.65 to 0.79) | 0.75 (0.67 to 0.85) |
| Hgb slope (per g/dl per month) | 0.62 (0.51 to 0.76) | 0.97 (0.77 to 1.24) | 0.70 (0.56 to 0.89) | 0.97 (0.75 to 1.26) | NR | NR |
HgbMs, hemoglobin measurements; NR, not reported.
In sensitivity analysis III, Hgb parameters were calculated using regression models that included an additional, quadratic term for time.
Covariates included other Hgb variables, age, sex, race, BMI, comorbidities (diabetes, hypertension, arterial disease (coronary and/or cerebrovascular and/or peripheral vascular disease), cancer, and congestive heart failure), laboratory results (serum creatinine, phosphate, albumin, and ekT/V averaged over days 90-180), and cumulative 6-month intravenous iron and erythropoietin doses.
Adjusted for covariates listed as well as the quadratic representation of hemoglobin over time.
Because of the possibility that use of only the first Hgb value per month may have introduced bias, sensitivity analysis II was conducted in which Hgb parameters were derived from all available Hgb data for each subject. (The correlation coefficients between these refit Hgb parameters and their counterparts in the primary analysis were 0.96, 0.90, and 0.92 for mean Hgb, Hgb-Var, and Hgb slope, respectively.) Unadjusted and adjusted HRs (95% CIs) for all-cause mortality per g/dl increase in Hgb-Var were 0.99 (0.81 to 1.21) and 1.16 (0.94 to 1.44), respectively (Table 4).
For an individual prevalent dialysis patient (in whom little net change in Hgb over time is expected), the trend in Hgb over time was plausibly described by a linear function (18). However, this may not provide the best fit for the data among incident dialysis patients, many of whom are receiving erythropoiesis stimulating therapy for the first time (1). To explore the possibility that nonlinear specification of models provided a better fit of the data, sensitivity analysis III was conducted. In this analysis, quadratic (i.e., time2) and square root terms for time were added (separately) to the within-subject linear regression models used to estimate Hgb-Var and slope. The mean adjusted R2 was significantly higher for the exposure models that included a quadratic term for time (mean R2 = 0.53) than for those that considered only a linear term (mean R2 = 0.22; P for difference <0.001) or included a square root term (mean R2 = 0.27; P < 0.001) in models. When outcome models were fit using Hgb parameters derived from the models including a quadratic term for time, the unadjusted HR (95% CI) per g/dl increase in Hgb-Var was 1.31 (1.05 to 1.64); in the fully adjusted model, the HR (95% CI) was 1.17 (0.93 to 1.49) (Table 4).
Survival and Other Hemoglobin Parameters
Figure 2B demonstrates that incrementally higher mean Hgb levels were associated with lower all-cause mortality (P < 0.001). Considered as a linear term, each g/dl increase in mean Hgb was associated with an unadjusted and adjusted HR (95% CI) for all-cause mortality of 0.72 (0.65 to 0.79) and 0.76 (0.68 to 0.86), respectively (Table 3). The association between mean Hgb level and mortality was quantitatively and qualitatively similar in sensitivity analyses I, II, and III (Table 4).
Figure 2C demonstrates that incrementally higher Hgb slopes were associated with lower all-cause mortality (P = 0.001). Unadjusted and adjusted HRs (95% CIs) for all-cause mortality per 1 g/dl per month increment in the Hgb slope were 0.66 (0.53 to 0.82) and 0.97 (0.75 to 1.24), respectively (Table 3). The association between Hgb slope and mortality was quantitatively and qualitatively similar in sensitivity analyses I and II (Table 4).
Comparison of Data from the 2004 Cohort with 1996 Cohort
The differences in findings between those observed in the present cohort and those observed in the 1996 cohort (18) led us to examine differences in the underlying study populations. The 1996 cohort considered both incident and prevalent HD patients, whereas the present cohort included only incident patients.
Observed levels of absolute Hgb level (mean 12.0 versus 10.25 g/dl; P < 0.001), Hgb slope (mean 0.37 versus 0.02 g/dl/mo; P < 0.001), and Hgb-Var (mean 1.13 versus 0.60, P < 0.001) were considerably higher in the present study's cohort than in the full 1996 cohort.
When the 1996 cohort was restricted to incident patients and hemoglobin parameters defined over months 0 through 6, the association between Hgb-Var and mortality was attenuated and no longer reached conventional levels of statistical significance: adjusted HR (95% CI) 1.15 (0.98 to 1.35). This estimate was similar in magnitude to that seen in the present study, suggesting that the relationship between Hgb-Var and mortality may differ between incident and prevalent HD patients. These findings were changed little when follow-up of these patients was censored on at-risk day 185, the adjusted HR (95% CI) was 1.21 (0.99 to 1.49), suggesting that differences in available follow-up time had little effect on estimates.
Recognizing that the 1996 cohort lacked data on many of the covariates included in the present study and incorporated several not used in the present study, we conducted additional analyses to explore the impact of variations in covariate adjustment on our findings. When analyses from the present study were adjusted for only those covariates used in the 1996 study (age, race, sex, diabetes, urea reduction ratio, Kt/V, albumin, bicarbonate, calcium phosphate, parathyroid hormone, ferritin, transferrin saturation, erythropoietin dose and intravenous iron dose, absolute Hgb, Hgb slope), the effect estimate was potentiated and reached conventional levels of statistical significance: adjusted HR (95% CI) was 1.22 (1.01 to 1.48). This suggests that the current analysis may have benefited from more complete adjustment of confounding that was possible for the 1996 analysis.
Discussion
Previously, we reported an independent and potent association between Hgb-Var and all-cause mortality among a 1996 cohort of prevalent and incident HD patients: each 1 g/dl increase in the residual SD was associated with a 33% increase in mortality (18). Given the dramatic changes in anemia management over the past decade, the present study was undertaken to determine whether this association holds for present-day dialysis patients. Using data from a 2004 to 2005 cohort of incident FMC patients, we were unable to demonstrate an association between Hgb-Var and mortality.
At least one other paper has found that Hgb-Var is not associated with mortality (21). That report, as the present study, considered patients from 2004. Unlike the present study, that paper considered both incident and prevalent dialysis patients, and used a stratum-based metric of variability, which may not adequately distinguish among variability, per se, absolute hemoglobin level, and temporal hemoglobin trend. To our knowledge, ours was the first report examining the association between Hgb-Var and mortality using a metric that distinguishes variability from these other descriptors of hemoglobin considered longitudinally.
There are several potential explanations for observed difference in findings between the present and prior studies. One possibility is that the association between Hgb-Var and mortality is fundamentally different among patients with lower and higher absolute Hgb levels. Both our and published data suggest that absolute Hgb levels were >1 g/dl higher in 2004 than in 1996 (1). Although neither study demonstrated significant effect modification of absolute Hgb level on the association between Hgb-Var and mortality, the range of Hgb level in each study was constrained (with little overlap between the studies) and may have failed to detect an interaction.
An additional possibility is that the association between Hgb-Var and mortality results from differences in anemia treatment over time other than those related to the absolute Hgb level. Between 1996 and 2004, mean erythropoietin dose among HD patients rose from approximately 10,500 to 12,000 units weekly (1). In addition, there was a shift toward use of longer-acting erythropoiesis stimulating agents, as well as subcutaneous versus intravenous administration. Use of intravenous iron increased sharply, and there was a trend away from use of iron dextran formulations in favor of iron sucrose or ferric gluconate (1). Any or all of these secular trends may influence the degree of HgbVar seen and may affect the relationship between HgbVar and mortality.
Furthermore, it is possible that the effect of Hgb-Var on mortality among incident HD patients differs from that seen among prevalent patients. Indeed, when the 1996 cohort was restricted to only incident HD patients, effect estimates were attenuated and similar to those observed in the present study, suggesting that results differed because of the incident versus prevalent nature of study cohorts. It is unknown whether this derives from a biologic difference or from inadequacy of the Hgb-Var metric when used in incident patients.
A difference in the association between Hgb-Var and mortality between incident and prevalent patients is plausible given the known worse health status among incident patients (1). Thus, their overall high mortality rate may diminish the ability to detect a subtler influence of Hgb-Var on longevity. In addition, the effects of Hgb-Var on mortality may take time to manifest and therefore may not be observable in the short time horizon available for the present cohort. When incident subjects from the 1996 cohort were administratively censored on at-risk day 185 (as in the present cohort), the hazard ratio changed very little (though was slightly potentiated), suggesting that insufficient time to manifest sequelae did not attenuate estimates in the present cohort.
The observed higher levels of Hgb-Var among the present incident cohort and the incident subgroup of the 1996 cohort, as well as the findings of our sensitivity analysis III, suggest that within-person linear regression to derive Hgb-Var metrics may overstate Hgb-Var among incident patients. It is know that upon HD initiation, a majority of patients (>60%) receive erythropoiesis stimulating therapy for the first time. These patients experience a robust rise in Hgb early on, followed by a subsequent flattening, leading to a curvilinear trajectory. Use of linear exposure models therefore would misclassify a curved rise in Hgb as variability and create bias. When quadratic exposure models were used to allow for curvilinear relationships, the observed level of Hgb-Var was reduced to 0.68 g/dl, similar to that seen in the 1996 cohort. In addition, when Hgb-Var was defined by these models, there was potentiation of the association between Hgb-Var and mortality.
Finally, we cannot rule out the possibility that our current findings differ, in part, from those reported in the 1996 cohort because of greater access to potential confounding factors for the analysis. When the Hgb-Var–mortality association was adjusted for those covariates available in the 1996 cohort, the hazard ratio was greater but did not rise to the size observed in the full 1996 cohort (1.22 versus 1.33, respectively). Thus, it is unlikely that residual confounding alone can explain the differences in findings between the two studies.
Ultimately, data from a contemporary cohort of prevalent HD population are needed to distinguish whether difference relate to time period effects or to incident versus prevalent status. Unfortunately, such data were not available for the present study.
Consistent with published reports (7,10,18,20), our data suggest an association between incrementally higher Hgb and decreased mortality up to a level of 12 g/dl. However, the Kaplan-Meier curves for subjects with mean Hgb 12 to 13 g/dl and >13 g/dl were highly similar, suggesting that further increments in mean Hgb may not be associated with improved survival. The cohort did not contain a sufficient number of subjects with higher mean Hgb levels necessary to draw inference in this regard.
In our prior study (18), we demonstrated an association between higher Hgb slope and improved survival. In the present study, this relationship was observed in unadjusted analyses, but not in adjusted analyses, where estimates were greatly attenuated and failed to reach statistical significance. One potential explanation for this discrepancy is that the current study contained data on a greater number of covariates and thus was better able to adjust for the effects of confounding.
There are several limitations to this study that must be noted. As with all observational studies, there is the possibility of residual confounding. Although we attempted to adjust estimates, limitations in the available data did not enable us to adjust on the basis of hospitalization, infection, or other potential confounders. It is noteworthy that, when our 1996 data were analyzed using techniques to address time-dependent confounding, the association between Hgb-Var and mortality was potentiated (22). In addition, nonsignificant study findings raise the possibility of a type II error. Although calculations indicated an 80% power to observe a true association (assuming a magnitude similar to that found in the prior study), we cannot discount the possibility that we failed to observe a true association because of a limited number of subjects and limited follow-up time. Finally, all subjects in this study were patients incident to FMC units. Generalization of findings to subjects to prevalent patients or those dialyzed by other providers should be undertaken cautiously.
Conclusion
The present study was unable to detect an association between Hgb-Var and mortality among incident HD patients. It is unclear whether this lack of association is the result of temporal trends in anemia management or to differences between incident and prevalent dialysis patients. More work is needed to clarify the association between Hgb-Var and mortality before targeting the reduction of Hgb-Var, per se, in clinical practice.
Disclosures
None.
Acknowledgments
S.M.B. was supported by the American Heart Association Fellow-to-Faculty Transition Award 0775021N. Prior work on the 1996 cohort was supported by an unrestricted grant from Hoffman-LaRoche Company.
Published online ahead of print. Publication date available at www.cjasn.org.
Work from this study has been submitted to the American Society of Nephrology Meeting, November 2008; a decision regarding acceptance was pending at the time of submission.
S.M.B. and K.E.L. contributed equally to this work.
References
- 1.U.S. Renal Data System: USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007; http://www.usrds.org, Accessed October 25, 2007
- 2.Pisoni RL, Bragg-Gresham JL, Young EW, Akizawa T, Asano Y, Locatelli F, Commer J, Cruz JM, Kerr PG, Mendelssohn DC, Held PJ, Port FK: Anemia management and outcomes from 12 countries in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 44: 94–111, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Plantinga LC, Fink NE, Jaar BG, Huang I, Wu AW, Meyer KB, Powe NR: Relation between level of changes of hemoglobin and generic and disease-specific quality of life measures in hemodialysis. Qual Life Res 16: 755–765, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Moreno F, Sanz-Guajardo D, López-Gómez JM, Jofre R, Valderrábano F: Increasing the hematocrit has a beneficial effect on quality of life and is safe in selected hemodialysis patients. J Am Soc Nephrol 11: 335–342, 2000 [DOI] [PubMed] [Google Scholar]
- 5.McMahon LP, McKenna MJ, Sangkabutra T, Mason K, Sostaric S, Skinner SL, Burge C, Murphy B, Crankshaw D: Physical performance and associated electrolyte changes after haemoglobin normalization: a comparative study in haemodialysis patients. Nephrol Dial Transplant 14: 1182–1187, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Robinson BM, Joffe MM, Berns JS, Pisoni RL, Port FK, Feldman HI: Anemia and mortality in hemodialysis patients: accounting for morbidity and treatment variables updated over time. Kidney Int 68: 2323–2330, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Portolés J, López-Gómez JM, Aljama P: A prospective multicenter study of the role of anaemia as a risk factor in haemodialysis patients: the MAR Study. Nephrol Dial Transplant 22: 500–507, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Rao M, Pereira BJ: Optimal anemia management reduces cardiovascular morbidity, mortality, and costs in chronic kidney disease. Kidney Int 68: 1432–1438, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Ma JZ, Ebben J, Xia H, Collins AJ: Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 10: 610–619, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Li S, Collins AJ: Association of hematocrit value with cardiovascular morbidity and mortality in incident hemodialysis patients. Kidney Int 65: 626–633, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Spiegel DM: Anemia management in chronic kidney disease: what have we learned after 17 years? Semin Dial 19: 269–272, 2006 [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Foundation: NKF-K/DOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease: Update 2007, http://www.kidney.org/professionals/kdoqi/index.com, Accessed October 25, 2007
- 13.Fishbane S, Berns JS: Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int 68: 1337–1343, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Fishbane S, Berns JS: Evidence and implications of haemoglobin cycling in anemia management. Nephrol Dial Transplant 22: 2129–2132, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Berns JS, Elzein H, Lynn RI, Fishbane S, Meisels IS, DeOreo PB: Hemoglobin variability in epoetin-treated hemodialysis patients. Kidney Int 64: 1514–1521, 2003 [DOI] [PubMed] [Google Scholar]
- 16.West RM, Harris K, Gilthorpe MS, Tolman C, Will EJ: A description of the variability of patient responses in the control of renal anemia: functional data analysis applied to a randomized controlled clinical trial in hemodialysis patients. J Am Soc Nephrol 18: 2371–2376, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Ebben JP, Gibertson DT, Foley RN, Collins AJ: Hemoglobin level variability: associations with comorbidity, intercurrent events, and hospitalizations. Clin J Am Soc Nephrol 1: 1205–1210, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Yang W, Israni RK, Brunelli SM, Joffe MM, Fishbane S, Feldman HI: Hemoglobin variability and mortality in ESRD. J Am Soc Nephrol 18: 3164–3170, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gibertson DT, Ebben JB, Bradbury B, Dunning SC, Collins AJ: The effect of hemoglobin (Hb) variability and trends on mortality. J Am Soc Nephrol 17: 582A, 2006 [Google Scholar]
- 20.Thadhani R, Tonelli M: Cohort studies: marching forward. Clin J Am Soc Nephrol 1: 1117–1123, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Gilbertson DT, Ebben JP, Foley RN, Weinhardl ED, Bradburry BD, Collins AJ: Hemoglbin level variability: associations with mortality. Clin J Am Soc Nephrol 3: 133–138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunelli SM, Joffe MM, Israni RK, Yang W, Fishbane S, Berns JS, Feldman HI: History-adjusted marginal structural analysis of the association between hemoglobin variability and mortality among chronic hemodialysis patients. Clin J Am Soc Nephrol 3: 777–782, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]