Abstract
Background: The mortality rate of maintenance hemodialysis (MHD) patients remains high. Measures of protein-energy wasting, including hypoalbuminemia, are strongly associated with their high mortality. Growth hormone (GH) may improve lean body mass (LBM) and serum albumin levels, and health-related quality of life (HRQoL), which are significantly and positively associated with survival in MHD patients. The OPPORTUNITY™ Trial will examine whether GH reduces mortality and morbidity and improves overall health in hypoalbuminemic MHD patients.
Hypothesis: The primary hypothesis is that daily recombinant human GH injections, compared with placebo, improve survival in hypoalbuminemic MHD patients. Secondary hypotheses are that GH improves morbidity and health, including number of hospitalized days, time to cardiovascular events, LBM, serum protein and inflammatory marker levels, exercise capacity, and HRQoL, and has a favorable safety profile.
Design/Measurements: This is a prospective, double-blind, multicenter, randomized clinical trial involving 2500 MHD patients, up to 50% with diabetes mellitus, from 22 countries. Patients are randomized in a 1:1 ratio to receive daily injections of GH (20 μg/kg per day) or placebo for 104 weeks. Key inclusion criteria include clinically stable and well-dialyzed (Kt/V ≥1.2) adult MHD patients with serum albumin <4.0 g/dl. Exclusion criteria include active malignancy, active proliferative or severe nonproliferative diabetic retinopathy, uncontrolled hypertension, chronic use of high-dose glucocorticoids, or immunosuppressive agents and pregnancy.
Conclusions: The OPPORTUNITY™ Trial is the first large-scale randomized clinical trial in adult MHD patients evaluating the response to GH of such clinical endpoints as mortality, morbidity, markers of body protein mass, inflammation, exercise capacity, and HRQoL.
Adult end-stage renal disease (ESRD) patients undergoing maintenance hemodialysis (MHD) experience high mortality and morbidity with diminished quality of life (1,2). Death and hospitalization rates in MHD patients correlate strongly with indicators of low protein mass, as indicated by low serum albumin and decreased fat-free, edema-free body mass (i.e., lean body mass [LBM]), as well as chronic inflammation (3,4). This is an important association because protein-energy wasting (PEW) occurs in approximately 40% of MHD patients (5). The possibility that improved measures of PEW may lead to decreased mortality or morbidity was recently underscored by several cross-sectional and longitudinal evaluations of cohorts containing up to 58,000 MHD patients who were followed for up to 2 yr. These studies showed that both higher serum albumin and body weight and an increase in these measures are strongly associated with greater survival (6,7). However, there are no prospective, randomized, clinical trials (RCTs) that have confirmed or even assessed whether a pharmacologic intervention that improves indicators of PEW prolongs survival and/or reduces morbidity, including hospitalization, in MHD patients. Thus, major unmet medical needs and important questions exist concerning whether PEW causes mortality and morbidity in MHD patients and whether a treatment that reduces PEW will reduce their high mortality and morbidity.
Growth hormone (GH) has extensive metabolic and, in particular, protein anabolic effects (8), a number of which have also been demonstrated in MHD or chronic peritoneal dialysis patients (9–23). Most of these studies were performed on small numbers of patients. A recent phase 2 RCT involving 139 MHD patients indicated that the GH-treated patients underwent improvement in LBM, serum transferrin, exercise capacity, and a tendency (P = 0.076) for serum albumin to rise (9). Based on these data, a decision was made to conduct a more definitive prospective RCT in hypoalbuminemic MHD patients.
Materials and Methods
Trial Design
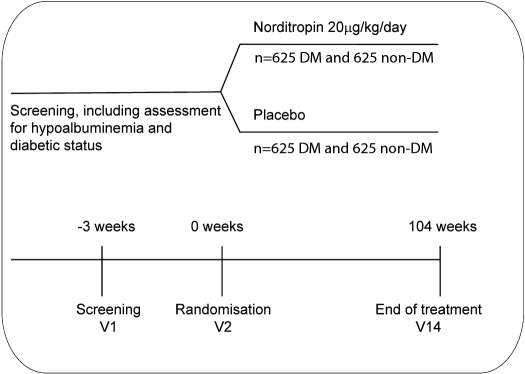
A schematic diagram illustrating the trial design is depicted in Figure 1. This is a 2-yr (104-wk), prospective, randomized, double-blind, parallel-group, placebo-controlled, multicenter international trial investigating the effect of recombinant human GH (Somatotropin, Norditropin, Novo Nordisk A/S.) on survival (time to death) in adult MHD patients (www.Clinicaltrials.gov; NCT00503698). The recruitment period is scheduled to last 28 mo. Patients will be seen at 14 visits over the 2-yr period. All visits will take place in connection with a regular dialysis session. At present, the following countries are scheduled to participate in the trial: Argentina, Australia, Brazil, Canada, China, Czech Republic, Denmark, France, Germany, Hungary, India, Israel, Italy, New Zealand, Poland, Portugal, Russia, South Africa, Spain, Sweden, Turkey, United Kingdom, and United States. A total of 2500 subjects (up to 50% diabetic patients per treatment group) will be randomized to treatment with either GH or matching placebo. Randomization will be stratified by the presence or absence of diabetes mellitus. For the purposes of this trial, subjects diagnosed as diabetic must meet the following criteria: 1) diagnosis of diabetes mellitus (either type 1 or type 2) according to current American Diabetes Association criteria; and 2) current treatment with an oral antidiabetic drug and/or insulin, or patients undergoing dietary management with a fasting plasma glucose ≥126 mg/dl (7.0 mmol/L, median of three measurements analyzed by the central laboratory).
Figure 1.
Schema for the experimental design of the OPPORTUNITY™ Trial.
Inclusion Criteria
Males or females undergoing MHD, age ≥18 yr, serum albumin <4.0 g/dl (determined by the median of three measurements analyzed by the central laboratory), clinically stable and receiving adequate MHD (as defined by a single pool Kt/V ≥1.20 OR no less than 3 dialysis sessions per week with a total dialysis time ≥12 h per week) for at least 3 mo before enrollment. Diabetic patients must be willing to commence insulin therapy if deemed necessary for plasma glucose control.
Exclusion Criteria
Active malignant disease (defined as less than 5 yr since receiving a diagnosis of being malignancy-free), critical illness as defined by the need for respiratory or circulatory support (e.g., in an intensive care unit), active vasculitis, severe congestive heart failure (New York Heart Association class IV), severe chronic systemic infectious or inflammatory disease, liver disease (defined as serum alanine aminotransferase or aspartate aminotransferase levels greater than three times the upper limit of normal), active proliferative or severe nonproliferative diabetic retinopathy, uncontrolled hypertension (predialysis systolic blood pressure >180 mmHg or predialysis diastolic blood pressure >110 mmHg) in two of the last three consecutive dialysis sessions, known or suspected allergy to trial product(s) or related products, females of childbearing potential who are pregnant, breast-feeding, intend to become pregnant, or not using adequate contraception, treatment with corticosteroids in doses >10 mg/d prednisolone (or equivalent), treatment with immunosuppressive agents or receipt of any investigational drug within one month preceding screening, known GH deficiency, mental incapacity, unwillingness or language barrier precluding adequate understanding, any condition considered to interfere with trial participation or evaluation of results or that makes the trial potentially hazardous to the patient, and a scheduled renal transplantation within the trial period.
Primary Endpoint
The primary endpoint, time to event (death), will be evaluated on a 1% significance level in a stratified Cox proportional hazard regression model for right censored survival time. The hypothesis, no treatment effect, will be tested in a one-sided log-likelihood test accounting for gender, age, and time on dialysis at baseline and stratified for diabetic status.
Secondary Endpoints
Several secondary efficacy and safety endpoints will be assessed during the trial (Tables 1 and 2): Two-year mortality rate, frequency and duration of hospitalizations, time from randomization to first hospitalization, time to next cardiovascular event, composite of all-cause mortality, nonfatal myocardial infarction, cardiac insufficiency, stroke and other thromboembolic events, cardiovascular events per year (number of myocardial infarctions, cardiac failure, strokes, and other thromboembolic events), serum albumin, transferrin, homocysteine, and lipids pattern (total cholesterol, low density lipoprotein, high density lipoprotein, and triglycerides), LBM, and fat mass as assessed by bioelectrical impedance analysis, normalized protein equivalent of total nitrogen appearance calculated from predialysis and postdialysis plasma urea levels and dialysis characteristics, appetite assessed by the visual analog scale, serum C-reactive protein, TNF-α, and IL-6, hand grip strength, maximal walking speed, activities of daily living, and HRQoL assessed by EQ-5D and SF-36, version 2. All secondary efficacy endpoints except HRQoL and the 2-yr mortality rate are structured hierarchically and will be analyzed accordingly. Safety endpoints (Tables 1 and 2) will include adverse events assessed from the patient's medical history, physical examination, and laboratory tests, including routine hematology tests and chemical analyses and particularly fasting serum glucose and insulin, self-monitored blood glucose, HbA1C and parathyroid hormone, IGF-I, IGF binding protein-3 (IGFBP-3), and the IGF-I/IGFBP-3 molar ratio.
Table 1.
Trial procedure schedule: subject demographics and clinical characteristics, key outcomes
| Visit | V 1
|
V 2
|
V 3
|
V4
|
V5
|
V6
|
V7
|
V8
|
V9
|
V10
|
V11
|
V12
|
V13
|
V14
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week −3 to 0 | Week 0 | Week 2 | Week 4 | Week 8 | Week 12 | Week 18 | Week 26 | Week 39 | Week 52 | Week 65 | Week 78 | Week 91 | Week 104 | |
| Visit window in relation to date of visit 2 | ± 2 days | ± 2 days | ± 2 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ||
| Subjects | ||||||||||||||
| informed consent obtained | × | |||||||||||||
| allocation of study number to subjecta | × | |||||||||||||
| inclusion/exclusion criteria | × | × | ||||||||||||
| demographics | × | |||||||||||||
| medical history | × | |||||||||||||
| concomitant illnesses | × | |||||||||||||
| concomitant medications | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| dialysis dose (Kt/V) | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| body weight | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| body height | × | × | ||||||||||||
| randomizationb | × | |||||||||||||
| end of trialc | ×g | |||||||||||||
| Efficacy | ||||||||||||||
| mortality | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| morbidity | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| serum albumind | ×f | × | × | × | × | × | × | × | × | × | × | |||
| BIA: lean body mass and fat mass | × | × | × | × | × | × | ||||||||
| hand grip strength | × | × | × | × | × | |||||||||
| walking speed | × | × | × | × | × | |||||||||
| laboratory measurementse | × | × | × | × | × | × | × | × |
BIA, bioelectrical impedance analysis.
Each subject is assigned a sequential six-digit subject number from a range of consecutive subject numbers allocated to each center.
Randomization will take place through IV/WRS.
The End of Trial Form, Affirmation Statement Form, Case Record Form, and Accountability Form are completed for all subjects, including those who prematurely discontinue from the trial.
Blood sampling is done a maximum of 2 hours in advance of the dialysis session. The samples are analyzed centrally.
Laboratory parameters are fasting (a minimum of 6 hours) lipids, insulin, and glucose (visits 1, 10, and 14 only), homocysteine, transferrin, CRP, IL-6 and TNF-α. At visits 1, only fasting lipids, insulin, and plasma glucose will be measured. Blood sampling should be done at a maximum of 2 hours in advance of the dialysis session. The samples will be analyzed centrally.
Serum albumin and fasting (a minimum of 6 hours) plasma glucose are measured three times at three different dialysis sessions before visit 2 within the allowed 3 weeks. The samples are analyzed centrally.
At the time of the last patient's last visit (approximately 36 months after the first patient's first visit), survival data on all patients who were still alive at visit 14 will be obtained from the medical records or local registries.
Table 2.
Trial procedure schedule: questionnaires, quality of life, safety, compliance, retraining
| Visit | V 1
|
V 2
|
V 3
|
V4
|
V5
|
V6
|
V7
|
V8
|
V9
|
V10
|
V11
|
V12
|
V13
|
V14
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week −3 to 0 | Week 0 | Week 2 | Week 4 | Week 8 | Week 12 | Week 18 | Week 26 | Week 39 | Week 52 | Week 65 | Week 78 | Week 91 | Week 104 | |
| Visit window in relation to date of visit 2 | ± 2 days | ± 2 days | ± 2 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ± 5 days | ||
| Quality of life and safety | ||||||||||||||
| adverse events (AEs), serious AE s | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| physical examination | × | × | × | × | × | |||||||||
| self-measured blood glucosea | × | × | × | × | × | × | x | × | x | × | x | × | ||
| fasting plasma glucose | ×d | × | × | |||||||||||
| HbA1c | × | × | × | × | × | × | × | × | × | × | ||||
| plasma insulinb | × | × | × | |||||||||||
| IGF-I, IGFBP-3 | × | × | × | × | × | × | ||||||||
| hematology and biochemistry | ×e,f | × | × | × | × | × | × | × | ||||||
| pregnancy testc | × | × | ||||||||||||
| vital signs | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| withdrawal criteria | × | × | × | × | × | × | × | × | × | × | × | × | ||
| Questionnaires | ||||||||||||||
| dispense/collect PRO questionnaires | × | × | × | × | × | |||||||||
| dispense/collect morbidity diary | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Trial products | ||||||||||||||
| treatment with trial product and instruction/practice in the use of the delivery device as needed | × | × | × | × | × | × | × | × | × | × | × | × | ||
| supply of trial product | × | × | × | × | × | × | × | × | × | × | × | |||
| drug accountability | × | × | × | × | × | × | × | × | × | × | × | × |
Self-measured blood glucose is only done by the subjects diagnosed with diabetes.
Insulin is measured only in subjects without diabetes. The samples are analyzed centrally.
Only females of childbearing potential undergo the pregnancy test. This test is carried out at visit 1 and visit 14 or according to the regulations of the relevant government.
At screening, serum fasting (for a minimum of 6 hours) plasma glucose is measured three times at three different dialysis sessions before visit 2 within the allowed 3 weeks. The samples are analyzed centrally.
Only PTH and AST/ALT are measured at visit 1. Blood sampling is done at a maximum of 2 hours in advance of the dialysis session. The samples are analyzed centrally.
The time interval can vary between 0 and 3 weeks before visit 2 as long as three measurements of serum albumin and fasting plasma glucose are obtained within this period of time.
Statistical Analyses
The primary endpoint, i.e., time to event (death), will be evaluated on an ongoing basis by applying an event-driven group sequential design. The method will control the type I error by using a general Rho function, as previously proposed (24), in three incorporated interim analyses executed after a prespecified number of events (567, 851, and 1134 deaths). Each evaluation will take both efficacy and futility into account. The results of the first interim analysis, but not the subsequent two analyses, could lead to an extension of the treatment and follow-up period. Any recommendations for these changes will be made by the independent Data Monitoring Committee. Safety will be assessed unblinded monthly by the Data Monitoring Committee. For the secondary endpoints, the general approach for statistical analyses will be a baseline-adjusted analysis of variance on the observed change from baseline to end of trial. In the matter of frequency, a negative binomial model or a logistic regression model will be applied, if feasible. Primary analyses of outcomes will be by intention to treat.
Sample Size Calculation
The sample size calculation is based on a 2-yr background mortality rate of 40% and an expectation of reducing the 2-yr mortality rate by 20% corresponding to a hazard ratio of 0.802. It is estimated that 95% of the included subjects will contribute to survival data, even though we anticipate that close to 50% probably will drop out of the study protocol for various reasons. To obtain 90% power, 2500 MHD subjects will be included (up to 50% with DM). This number should be sufficient to obtain 1134 events over 3 yr (16 mo of accrual and 2-yr follow-up), which would be needed applying a one-sided log-rank test for two exponential survival curves evaluated on a 1% significance level and accounting for 5% exponential drop-outs in both treatment groups.
Study Organization
Steering Committee: The Steering Committee is responsible for overseeing the conduct of the study, monitoring its progress, reviewing and approving proposed protocol amendments, if any, and analyzing and writing the results. The Steering Committee provides advice to Novo Nordisk on these matters.
Clinical Endpoint Committee: This committee, derived from members of the Harvard Clinical Research Institute, adjudicates study endpoints in an unbiased and consistent manner according to prespecified event definitions and criteria.
Clinical Research Organization (COVANCE): This organization monitors and manages research sites in several participating European countries.
Data Monitoring Committee: This committee continuously monitors the safety and efficacy data and makes recommendations to the Steering Committee and to Novo Nordisk regarding safety and efficacy aspects of the Trial.
Independent Biostatistics Group (Cyncron): This organization supports the Data Monitoring Committee through unblinded safety and efficacy data analyses, specifically evaluating the safety of the Trial and whether the possibility remains of demonstrating statistically significant beneficial effects.
Central Laboratory: Two central laboratories are used.
Advisory Board: The Advisory Board is composed of nephrologists, endocrinologists, other academicians, and Novo Nordisk personnel who consult concerning long-range global issues regarding this trial and related research. Novo Nordisk is the study sponsor and oversees the global execution of the study.
Discussion
Effects of GH in Normal Individuals and People with Advanced Chronic Kidney Disease (CKD)
GH stimulates protein synthesis, bone growth, calcium retention, bone mineralization, and lipolysis with decrease in adipose tissue (25). GH reduces hepatic glucose uptake and promotes gluconeogenesis, thereby opposing the glucose-lowering actions of insulin. The effects of GH vary according to whether the patient is fasting or fed. GH exerts its actions, in part, by stimulating the secretion of IGF-I, but GH also has direct, IGF-I-independent effects, including stimulation of gluconeogenesis and lipolysis (8,25–27). Individuals with advanced CKD may be resistant to GH, which may be the result of decreased GH receptors and/or postgrowth hormone receptor defects (28–30) and also to decreased bioavailability and resistance to the actions of IGF-I (31,32).
Because children with CKF commonly have impaired growth and adults with CKF have a high prevalence of PEW, GH treatment has been studied in these individuals. GH stimulates growth in children with CKF, including those undergoing maintenance dialysis therapy and kidney transplant recipients (33,34). Studies in adults with ESRD indicate that GH may stimulate anabolism and improve indicators of body composition known to be associated with increased survival. These effects are summarized in Table 3.
Table 3.
Experience with growth hormone injections in patients undergoing maintenance dialysis therapy or with stage 5 chronic kidney disease not on dialysis
| Reference (study design) | N | Dose and duration | Efficacy | Safety |
|---|---|---|---|---|
| Ziegler et al. 1991 (open label, crossover design) (10) | 5 MHD-GH | 5-10 mg 3 × /week for 2 weeks | ↓ Net urea generation, nPNA, and phosphorus ↑ IGF-1 | No reported safety concerns |
| Ikizler et al. 1994 (open label, crossover design) (12) | 10 CAPD -GH | 5 mg subcutaneously daily for 7 consecutive days (∼ 80 μ g/kg per day) | ↓ SUN, urine nitrogen excretion rate, PNA, amino acid concentrations ↓ serum albumin | No AEs reported |
| Garibotto et al. 1997 (open label, crossover design) (13) | 6 MHD-GH | 5 mg subcutaneously 3 × /week for 6 weeks (∼ 2.1 mg/d) | ↑ muscle protein synthesis and net balance | No AEs reported |
| Sohmiya et al. 1998 (open label study) (17) | 8 CKD-GH (patients not on dialysis) | 2 μ g/kg per hour for 72 hours | ↑ in IGF-1 levels, erythropoietin and reticulocyte counts ↓ SUN | No AEs reported |
| Iglesias et al. 1998 (randomized, controlled, prospective trial) (14) | 4 MHD/4 CAPD-GH7 MHD/2 CAPD-PLBO | 0.2 IU/kg per day for 4 weeks (∼ 67 μ g/kg per day) | ↑ 1.2 kg wt gain in GH group No Δ hand grip strength ↓ albumin, total protein, SUN GH group | Pain at the site of injection, headache, nausea, vomiting, hypotension, paresthesisa, and anxiety in the GH treated group |
| Johannsson et al. 1999 (randomized, controlled, prospective trial) (18) | 7 MHD-GH10 MHD-PLBO | 66.7 μ g/kg for 3× /week | No change in weight | No AEs led to reduction of GH dose; 2 subjects in the GH group died of unspecified causes |
| For 6 months (∼ 28.6 μ g/kg per day) | ↑ FFM (3.9 kg), hand grip strength and normal gait speed in the GH-treated group | |||
| Hansen et al, 2000 (randomized, controlled, prospective trial) (16) | 9 MHD-GH | 4I U/m2 per day (∼ 13.7 mg/m2 per day or ∼ 30 μg/kg per day) | ↑ 3.1 kg LBM, ↓ 3.0 kg FM | No AEs observed |
| 11 MHD-PLBO | ||||
| Kotzmann et al. 2001 (randomized, controlled, prospective trial) (19) | 9 MHD-GH | 0.125 IU/kg (∼ 42 μ g/kg) 3 × /week for 4 weeks followed by 0.25 IU/kg (∼ 83 μ g/kg) 3 × /week for 4 weeks over 3 months | No Δ albumin, SUN, creatinine, and anthropometry | Arthralgia in 5 patients on GH, 1 control, headache in 1 patient on GH |
| Iglesias et al. 2002 (open label study) (20) | 4 MHD-GH4 CAPD-GH | 0.2 IU/kg per day (∼ 66.7 μ g/kg per day) subcutaneously for 4 weeks | Significant correlations between leptin and IGF-1 concentration | No AEs reported |
| Ericsson et al. 2004 (randomized, controlled, prospective trial) (21) | 35 MHD-GH | 0.025 IU/kg per day (∼ 8.3 μg/kg per day)for 1 week, increasing to 0.05 IU/kg per day (∼ 16.7 μ g/kg per day) for 8 weeks | ↑ nPCR No Δ in serum albumin and weight | AEs were equally distributed between two groups |
| Pupim et al. 2005 (open label, crossover study) (22) | 7 MHD-GH | 75 μ g/kg per day for 3 consecutive days | ↑ whole-body net protein balance ↓ Essential amino acid concentrations and muscle breakdown | No AEs reported |
| Kopple et al. 2005 (open label study) (23) | 6 MHD-GH | 50 μ g/kg per day subcutaneously for 8-21 days | ↑ IGF-1; ↑ nitrogen balance and ↓ SUN during GH treatment | 2 subjects who were acutely ill appeared to be GH resistant |
| Feldt-Rasmussen et al. 2007 (randomized, controlled, prospective trial) (9) | 105 MHD-GH | Three dose groups (20, 35, and 50 μ g/kg per day) for 6 months | ↑ LBM and ↑ QoL in all GH groups | No difference of SAE across dose groups; no Δ in LV mass |
| 34 MHD-PLBO | ↑ serum albumin in low dose GH group |
MHD, maintenance hemodialysis; CAPD, continuous ambulatory peritoneal dialysis; PLBO, placebo-treated controls; nPNA, normalized protein equivalent of total nitrogen appearance; GH, growth hormone; SUN, serum urea nitrogen; FFM, fat free mass; LBM, lean body mass; QoL, quality of life; LV, left ventricular; AE, adverse event; SAE, serious adverse event.
Many reports indicate that GH injections into adult ESRD patients decrease net urea production (urea nitrogen appearance) and/or serum urea, and sometimes serum phosphorus and potassium (10,12,14,23). These findings suggest more effective utilization of protein and amino acids. Metabolic studies also indicate increased protein synthesis and more positive protein or nitrogen balance (13,22,23). Mild hyperglycemia occurred in some of these short-term studies (12,14). Longer-term interventional trials with GH lasting up to 6 mo often, but not always, showed an increase in muscle mass or lean body mass, decrease in fat mass, and new bone formation (15,16,18,19). Serum albumin (11,18) or transferrin (14) rose in some trials. GH is reported to increase serum erythropoietin, leptin, IGF-I, and IGF binding protein-3, increase target organ sensitivity to parathyroid hormone and bone mineral density in GH-deficient people, and reduce serum parathyroid hormone levels (10,17,20,23,35–37).
Why Study GH Treatment Again in MHD Patients?
The foregoing studies were usually of short duration and involved small numbers of MHD patients: usually, 10 subjects or less per treatment arm (Table 3). They were not all placebo-controlled. The duration of study, doses of GH, and key outcome measures varied substantially. Nonetheless, these observations indicated that GH has potent anabolic effects in ESRD patients and provided a rationale for the pilot and feasibility study for the OPPORTUNITY™ Trial.
The proof of concept and feasibility study was a prospective randomized, double-blind, placebo-controlled, dose-ranging RCT involving 139 MHD patients with a serum albumin of 4.0 g/dl or less (22). The key outcomes were indicators of nutritional or possibly inflammatory status. Patients were randomly assigned to receive daily subcutaneous injections of low (20 μg/kg per day, n = 34), medium (35 μg/kg per day, n = 34), or large (50 μg/kg per day, n = 37) doses of recombinant human GH (Norditropin Novo Nordisk A/S, Denmark) or placebo (n = 34) for 26 wk. During or by the end of the study, the three groups of GH-treated patients, in contrast to the placebo-treated group, exhibited a significant increase in LBM and serum transferrin and high density lipoprotein-cholesterol, a reduction in serum homocysteine, and improvement in HRQoL for physical well-being. Serum albumin tended to rise in these patients (P = 0.08). Indeed, serum albumin rose close to significance in the patients receiving the lowest dose of GH (0.20 g/dl) versus −0.115 g/dl in the placebo group (P = 0.06). Morbidity and mortality rates were not significantly different in the placebo or GH treatment groups, but the study was not powered nor designed to assess changes in these outcomes.
Why Do We Need a Large-scale RCT to Test the OPPORTUNITY™ Hypotheses?
Epidemiologic studies demonstrate that low or worsening measures of protein mass and low HRQoL indicators are significant predictors of mortality and morbidity in MHD patients. The phase 2 clinical trial of GH demonstrated that GH improves markers associated with better clinical outcome in adult MHD patients, including LBM and serum protein levels (22). However, there are no studies examining whether GH therapy, or any other pharmacologic therapy that increases protein mass, improves clinical outcomes and especially mortality in these individuals. Given the large heterogeneity in the clinical course of hypoalbuminemic MHD patients and projected frequency of fatal events (3,4), a greater sample size becomes necessary to adequately test the primary hypothesis.
Safety Aspects of GH
Studies in children with CKF who were treated with GH to increase stature indicate that GH is quite safe. Nevertheless, despite its evidently beneficial effects, it is uncertain whether long-term use of pharmacologic doses of GH may induce undesirable effects. In one clinical trial, GH treatment of MHD patients was associated with an initial increase in serum glucose, which reached a steady state in 2 wk (14). Another study in MHD patients did not show a significant increase in blood glucose (18). In the doses used in the phase 2 study (9), GH did not induce hyperglycemia, although serum glucose rose transiently. GH reduces insulin sensitivity during conditions of stress or food deprivation (38). The impact of GH on insulin sensitivity may vary depending on the MHD patient's body composition. In GH-deficient adults, GH replacement therapy is reported to decrease insulin sensitivity during the initial months of treatment with a subsequent reversal toward improved insulin sensitivity thereafter (39). An initial decrease in insulin sensitivity with GH therapy followed by increased sensitivity may be related to a rise in their LBM and a decrease in fat mass, as was observed in the phase 2 study in MHD patients (9). Such a biphasic response may explain the initial rise in fasting plasma glucose that occurred in the GH-treated patients in the phase 2 study (weeks 2 to 4) with a subsequent (week 12 onward) return to baseline glucose levels (9). Because MHD patients may have glucose intolerance and diabetic MHD patients will also be studied in this trial, the effects of GH treatment on serum glucose levels will be carefully monitored.
If the GH-treated patients are given more insulin to control their serum glucose levels, the insulin might cause a selective anabolic stimulus to the GH-treated group. If there is a greater increase in the quantity or frequency of insulin administered in the GH group, we will attempt to adjust for this difference in insulin dosage and for any associated increase in anabolic status in our statistical analyses of the effects of GH treatment. However, an improvement in anabolism associated with an increase in insulin dosage could be considered a potential benefit of GH treatment.
Another question is whether GH will increase left ventricular mass (LVM); this was not observed in the phase 2 study (40). Jensen et al. described increased LVM, determined by echocardiography, in nine MHD patients receiving GH as compared with 11 MHD patients given placebo (41). However, their sample size was small, the mean baseline LVM values were substantially lower in the GH- versus the placebo-treated patients (172 g versus 281 g), and it is not clear that patients were all studied at the same time interval after completion of a hemodialysis treatment. On the other hand, several studies report that GH-deficient adults without kidney failure have increased left ventricular hypertrophy after GH treatment (42,43).
GH, in large doses, may cause sodium and water retention in individuals with normal kidney function. This effect is most likely the result of stimulation of sodium reabsorption in the kidney (44). Because MHD patients have little or no renal function, this side effect is not considered likely to occur. GH in high levels may induce acromegaly (8). At the GH dose that will be used in the OPPORTUNITY™ Trial, this side effect is not reported to occur, and it has not been described in previous studies of GH treatment in MHD patients (Table 3).
GH receptor mRNA is present in B lymphocytes (45); GH may stimulate differentiation of B-cell lymphoid precursors (46), and IGF-I may promote cancer growth in vitro (47). Hence, there is concern that GH might induce cancer or promote cancer proliferation. This is particularly relevant because there is increased risk of cancer in ESRD patients (48). Indeed, in people not previously treated with GH, spontaneously high serum IGF-I levels and/or low levels of IGF-binding protein-3 are associated with increased risk of several cancers, including breast, colon, prostate, and lung cancer (49). Not all epidemiologic studies confirm this association. As discussed by Cohen et al., there are explanations for the relationship between higher serum IGF-I and the risk of cancer that do not involve effects of GH (49). Moreover, GH elevates serum IGFBP-3, and this binder of IGF-I may inhibit cancer growth in vitro (50). Mice transgenic for bovine GH, which do not have activated prolactin receptor, do not develop more malignancies (51). However, IGF-I levels in rodents may be inversely associated with the rate of tumor growth, particularly in animals with pre-established malignancies (49). Observations concerning the incidence of cancer in acromegalic patients are controversial. These individuals probably are not at increased risk of cancer (52), although their risk of developing colonic polyps may be increased (53).
A recent retrospective observational study evaluated cancer risk in GH-treated children with advanced CKD who were not receiving renal replacement therapy, who were undergoing maintenance dialysis, or who had received a kidney transplant (54). The findings indicated that children who began to receive GH treatments when they had chronic renal insufficiency and who then received a kidney transplant had a significantly higher incidence of lymphoproliferative disease than children with kidney transplants who had not received GH; however, the odds ratio, adjusted for age and time when manifestations of the lymphoproliferative disease occurred, was 1.88 (95% confidence limits, 1.00 to 3.55, P = 0.05). Moreover, a large proportion of the GH-treated transplant recipients who developed lymphoproliferative disease were Epstein-Barr virus positive, which is a risk factor for this semimalignant disease. Children with CKF who received GH and either did not receive renal replacement therapy or who underwent maintenance dialysis showed no increased risk for lymphoproliferative disease. Moreover, the association between GH treatment and lymphoproliferative disease was only present in the cohort receiving GH in the period 1994 to 1995, but not 1996 to 1998 or 1999 to 2006. Because recombinant human GH has been available since 1986, and since then has only been subjected to formulation changes, other explanations for this association are not unlikely. Thus, this study, although intriguing, can be considered inconclusive. Other studies in children with advanced CKD who were treated with GH to increase stature report GH treatment to be safe (55). A recent consensus statement of several endocrine societies states, “There is no evidence that GH replacement in adults increases the risk of de novo malignancy or recurrence. GH treatment during childhood of survivors of cancer treatment increases slightly the relative risk of a second neoplasia, but there are no comparable data in adults.” “–current recommendations for cancer prevention and early detection in the general population should be implemented” (56). Thus, the foregoing considerations, taken together, do not support a role for GH in inducing cancer, although GH might stimulate the growth of preexisting cancer. Nonetheless, active malignancy is an exclusionary criterion for this trial. Moreover, the OPPORTUNITY™ Trial will use the lowest dose of GH used in the phase 2 trial, 20 μg/kg per day.
GH treatment is reported to increase mortality in intensive care unit patients (57). Although other studies using similar doses of GH, but with much smaller numbers of individuals treated, demonstrated either beneficial effects or no effects of GH treatment in intensive care unit patients (58,59), the trial protocol stipulates that the injections of GH and placebo will be discontinued in any study patient while he/she is living in an intensive care unit.
Conclusion
The OPPORTUNITY™ Trial will be the largest clinical trial ever carried out with GH in adult MHD patients and one of the largest clinical trials conducted in MHD patients. The key question to be tested is the effect of GH on mortality. Additional clinically relevant secondary hypotheses will also be tested. Patients will be randomized to receive daily injections of GH, 20 μg/kg per day, or an equal volume of placebo for 104 weeks in a double-blind, randomized, placebo-controlled, multicenter clinical trial. Safety issues will be carefully monitored.
Disclosures
J.D.K., A.K.C., J.S.C., M.E.N., B.F.-R., W.E.M., C.W., and T.A.I. are OPPORTUNITY® Trial steering committee members and have received consultant fees from Novo Nordisk A/S. M.L., C.B.D., and J.W. are employees of Novo Nordisk A/S.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Lopes AA, Bragg-Gresham JL, Goodkin DA, Fukuhara S, Mapes DL, Young EW, Gillespie BW, Akizawa T, Greenwood RN, Andreucci VE, Akiba T, Held PJ, Port FK: Factors associated with health-related quality of life among hemodialysis patients in the DOPPS. Qual Life Res 16: 545–557, 2007 [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System: Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Vol. II, 2007, Reference tables page H207
- 3.Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD: Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr 80: 299–307, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Kopple JD, Humphreys MH, Block G: Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transplant 19: 1507–1519, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Mehrotra R, Kopple JD: Causes of protein-energy malnutrition in chronic renal failure. In: Nutritional Management of Renal Disease, edited by Kopple JD, Massry SG, Baltimore, MD, Lippincott Williams & Wilkins, 2004, pp 167–182
- 6.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H Jr, Kopple JD, Greenland S: Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant 20: 1880–1888, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, Greenland S: Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis 46: 489–500, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Mφller N, Copeland KC, Nair KS: Growth hormone effects on protein metabolism. Endocrinol Metab Clin North Am 36: 89–100, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Feldt-Rasmussen B, Lange M, Sulowicz W, Gafter U, Lai KN, Wiedemann J, Christiansen JS, El Nahas M: Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J Am Soc Nephrol 18: 2161–2171, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Ziegler TR, Lazarus JM, Young LS, Hakim R, Wilmore DW: Effects of recombinant human growth hormone in adults receiving maintenance hemodialysis. J Am Soc Nephrol 2: 1130–1135, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Schulman G, Wingard RL, Hutchison RL, Lawrence P, Hakim RM: The effects of recombinant human growth hormone and intradialytic parenteral nutrition in malnourished hemodialysis patients. Am J Kidney Dis 21: 527–534, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Ikizler TA, Wingard RL, Breyer JA, Schulman G, Parker RA, Hakim RM: Short-term effects of recombinant human growth hormone in CAPD patients. Kidney Int 46: 1178–1183, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Garibotto G, Barreca A, Russo R, Sofia A, Araghi P, Cesarone A, Malaspina M, Fiorini F, Minuto F, Tizianello A: Effects of recombinant human growth hormone on muscle protein turnover in malnourished hemodialysis patients. J Clin Invest 99: 97–105, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iglesias P, Diez JJ, Fernandez-Reyes MJ, Aguilera A, Burgues S, Martinez-Ara J, Miguel JL, Gomez-Pan A, Selgas R: Recombinant human growth hormone therapy in malnourished dialysis patients: a randomized controlled study. Am J Kidney Dis 32: 454–463, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Jensen PB, Hansen TB, Frystyk J, Ladefoged SD, Pedersen FB, Christiansen JS: Growth hormone, insulin-like growth factors and their binding proteins in adult hemodialysis patients treated with recombinant human growth hormone. Clin Nephrol 52: 103–109, 1999 [PubMed] [Google Scholar]
- 16.Hansen TB, Gram J, Jensen PB, Kristiansen JH, Ekelund B, Christiansen JS, Pedersen FB: Influence of growth hormone on whole body and regional soft tissue composition in adult patients on hemodialysis: a double-blind, randomized, placebo-controlled study. Clin Nephrol 53: 99–107, 2000 [PubMed] [Google Scholar]
- 17.Sohmiya M, Ishikawa K, Kato Y: Stimulation of erythropoietin secretion by continuous subcutaneous infusion of recombinant human GH in anemic patients with chronic renal failure. Eur J Endocrinol 138: 302–306, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Johannsson G, Bengtsson BA, Ahlmen J: Double-blind, placebo-controlled study of growth hormone treatment in elderly patients undergoing chronic hemodialysis: anabolic effect and functional improvement. Am J Kidney Dis 33: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Kotzmann H, Yilmaz N, Lercher P, Riedl M, Schmidt A, Schuster E, Kreuzer S, Geyer G, Frisch H, Horl WH, Mayer G, Luger A: Differential effects of growth hormone therapy in malnourished hemodialysis patients. Kidney Int 60: 1578–1585, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Iglesias P, Diez JJ, Fernandez-Reyes MJ, Bajo MA, Aguilera A, Mendez J, Codoceo R, Selgas R: Effects of short-term recombinant human growth hormone therapy on plasma leptin concentrations in dialysis patients. Nephrol Dial Transplant 17: 260–264, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ericsson F, Filho JC, Lindgren BF: Growth hormone treatment in hemodialysis patients: a randomized, double-blind, placebo-controlled study. Scand J Urol Nephrol 38: 340–347, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Pupim LB, Flakoll PJ, Yu C, Ikizler TA: Recombinant human growth hormone improves muscle amino acid uptake and whole-body protein metabolism in chronic hemodialysis patients. Am J Clin Nutr 82: 1235–1243, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kopple JD, Brunori G, Leiserowitz M, Fouque D: Growth hormone induces anabolism in malnourished maintenance haemodialysis patients. Nephrol Dial Transplant 20: 952–958, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Kim K, DeMets DL: Confidence intervals following group sequential tests in clinical trials. Biometrics 43: 857–864, 1987 [PubMed] [Google Scholar]
- 25.Kopchick JJ: Growth hormone. In: Endocrinology Part III Basic Physiology, 4th ed, edited by DeGroot LJ, Jameson JL, Philadelphia, PA, Saunders, 2001, pp 389–404
- 26.Djurhuus CB, Gravholt CH, Nielsen S, Moller N, Schmitz O: Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. Am J Physiol Endocrinol Metab 286: E488–E494, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Moller N, Gjedsted J, Gormsen L, Fuglsang J, Djurhuus C: Effects of growth hormone on lipid metabolism in humans. Growth Horm IGF Res 13[Suppl A]: S18–S21, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Chan W, Valerie KC, Chan JC: Expression of insulin-like growth factor-1 in uremic rats: growth hormone resistance and nutritional intake. Kidney Int 43: 790–795, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Villares SM, Goujon L, Maniar S, Delehaye-Zervas MC, Martini JF, Kleincknecht C, Postel-Vinay MC: Reduced food intake is the main cause of low growth hormone receptor expression in uremic rats. Mol Cell Endocrinol 106: 51–56, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Schaefer F, Chen Y, Tsao T, Nouri P, Rabkin R: Impaired JAK-STAT signal transduction contributes to growth hormone resistance in chronic uremia. J Clin Invest 108: 467–475, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding H, Gao XL, Hirschberg R, Vadgama JV, Kopple JD: Impaired actions of insulin-like growth factor 1 on protein synthesis and degradation in skeletal muscle of rats with chronic renal failure: evidence for a postreceptor defect. J Clin Invest 97: 1064–1075, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap J, Tsao T, Fawcett J, Fielder PJ, Keller GA, Rabkin R: Effect of insulin-like growth factor binding proteins on the response of proximal tubular cells to insulin-like growth factor-I. Kidney Int 52: 1216–1223, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Hokken-Koelega AC, Stijnen T, de Muinck Keizer-Schrama SM, Wit JM, Wolff ED, de Jong MC, Donckerwolcke RA, Abbad NC, Bot A, Blum WF: Placebo-controlled, double-blind, cross-over trial of growth hormone treatment in prepubertal children with chronic renal failure. Lancet 338: 585–590, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Fine RN, Kohaut E, Brown D, Kuntze J, Attie KM: Long-term treatment of growth retarded children with chronic renal insufficiency, with recombinant human growth hormone. Kidney Int 49: 781–785, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Joseph F, Ahmad AM, Ul-Haq M, Durham BH, Whittingham P, Fraser WD, Vora JP: Effects of growth hormone administration on bone mineral metabolism, PTH sensitivity and PTH secretory rhythm in postmenopausal women with established osteoporosis. J Bone Miner Res 23: 721–729, 2008 [DOI] [PubMed] [Google Scholar]
- 36.White HD, Ahmad AM, Durham BH, Patwala A, Whittingham P, Fraser WD, Vora JP: Growth hormone replacement is important for the restoration of parathyroid hormone sensitivity and improvement in bone metabolism in older adult growth hormone-deficient patients. J Clin Endocrinol Metab 90: 3371–3380, 2005 [DOI] [PubMed] [Google Scholar]
- 37.White HD, Ahmad AM, Durham BH, Peter R, Prabhakar VK, Corlett P, Vora JP, Fraser WD: PTH circadian rhythm and PTH target-organ sensitivity is altered in patients with adult growth hormone deficiency with low BMD. J Bone Miner Res 22: 1798–1807, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Norrelund H: The metabolic role of growth hormone in humans with particular reference to fasting. Growth Horm IGF Res 15: 95–122, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Fowelin J, Attvall S, Lager I, Bengtsson BA: Effects of treatment with recombinant human growth hormone on insulin sensitivity and glucose metabolism in adults with growth hormone deficiency. Metabolism 42: 1443–1447, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Kober L, Rustom R, Wiedemann J, Kappelgaard A-M, Feldt-Rasmussen B: Evaluation of the effect of growth hormone hormone therapy on left ventricular (LV) mass in patients on maintenance hemodialysis. ERA-EDTA Congress, Stockholm, Sweden, May 10–13, 2008, p 342
- 41.Jensen PB, Ekelund B, Nielsen FT, Baumbach L, Pedersen FB, Oxhoj H: Changes in cardiac muscle mass and function in hemodialysis patients during growth hormone treatment. Clin Nephrol 53: 25–32, 2000 [PubMed] [Google Scholar]
- 42.Amato G, Carella C, Fazio S, La Montagna G, Cittadini A, Sabatini D, Marciano-Mone C, Sacca L, Bellastella A: Body composition, bone metabolism, and heart structure and function in growth hormone (GH)-deficient adults before and after GH replacement therapy at low doses. J Clin Endocrinol Metab 77: 1671–1676, 1993 [DOI] [PubMed] [Google Scholar]
- 43.Cuneo RC, Salomon F, Wilmshurst P, Byrne C, Wiles CM, Hesp R, Sonksen PH: Cardiovascular effects of growth hormone treatment in growth-hormone-deficient adults: stimulation of the renin-aldosterone system. Clin Sci (Lond) 81: 587–592, 1991 [DOI] [PubMed] [Google Scholar]
- 44.Moller J, Nielsen S, Hansen TK: Growth hormone and fluid retention. Horm Res 51[Suppl 3]: 116–120, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C: GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab 86: 4284–4291, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Sumita K, Hattori N, Inagaki C: Effects of growth hormone on the differentiation of mouse B-lymphoid precursors. J Pharmacol Sci 97: 408–416, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Grimberg A, Cohen P: Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol 183: 1–9, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kopple JD, Massry SG: Is there an association between neoplasia and primary or secondary hyperparathyroidism? Am J Nephrol 8: 437–448, 1988 [DOI] [PubMed] [Google Scholar]
- 49.Cohen P, Clemmons DR, Rosenfeld RG: Does the GH-IGF axis play a role in cancer pathogenesis? Growth Horm IGF Res 10: 297–305, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Cohen P, Peehl DM, Rosenfeld RG: The IGF axis in the prostate. Horm Metab Res 26: 81–84, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Wennbo H, Gebre-Medhin M, Gritli-Linde A, Ohlsson C, Isaksson OG, Tornell J: Activation of the prolactin receptor but not the growth hormone receptor is important for induction of mammary tumors in transgenic mice. J Clin Invest 100: 2744–2751, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orme SM, McNally RJ, Cartwright RA, Belchetz PE: Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab 83: 2730–2734, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Colao A, Balzano A, Ferone D, Panza N, Grande G, Marzullo P, Bove A, Iodice G, Merola B, Lombardi G: Increased prevalence of colonic polyps and altered lymphocyte subset pattern in the colonic lamina propria in acromegaly. Clin Endocrinol (Oxf) 47: 23–28, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Dharnidharka VR, Talley LI, Martz KL, Stablein DM, Fine RN: Recombinant growth hormone use pretransplant and risk for post-transplant lymphoproliferative disease: a report of the NAPRTCS. Pediatr Transplant 2007. Dec 2007 [Epub ahead of print] [DOI] [PubMed]
- 55.Banerjee I, Clayton PE: Growth hormone treatment and cancer risk. Endocrinol Metab Clin North Am 36: 247–263, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Ho KK: Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol 157: 695–700, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ: Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 341: 785–792, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Knox J, Demling R, Wilmore D, Sarraf P, Santos A: Increased survival after major thermal injury: the effect of growth hormone therapy in adults. J Trauma 39: 526–530, 1995 [DOI] [PubMed] [Google Scholar]
- 59.Voerman BJ, Strack van Schijndel RJ, Groeneveld AB, de Boer H, Nauta JP, Thijs LG: Effects of human growth hormone in critically ill nonseptic patients: results from a prospective, randomized, placebo-controlled trial. Crit Care Med 23: 665–673, 1995 [DOI] [PubMed] [Google Scholar]