Abstract
Background and objectives: Patients with chronic kidney disease (CKD) receiving dialysis often develop secondary hyperparathyroidism with disturbed calcium and phosphorus metabolism. The National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (KDOQI) was established to guide treatment practices for these disorders. The ACHIEVE study was designed to test two treatment strategies for achieving KDOQI goals.
Design, setting, participants, measurements: Individuals on hemodialysis treated with vitamin D sterols were enrolled in this 33-week study. Subjects were randomly assigned to treatment with either cinacalcet and low-dose vitamin D (Cinacalcet-D) or flexible vitamin D alone (Flex-D) to achieve KDOQI-recommended bone mineral targets. ACHIEVE included a 6-week screening phase, including vitamin D washout, a 16-week dose-titration phase, and an 11-week assessment phase.
Results: Of 173 subjects enrolled, 83% of Cinacalcet-D and 67% of Flex-D subjects completed the study. A greater proportion of Cinacalcet-D versus Flex-D subjects had a ≥30% reduction in parathyroid hormone (PTH) (68% versus 36%, P < 0.001) as well as PTH ≤300 pg/ml (44% versus 23%, P = 0.006). The proportion of subjects simultaneously achieving targets for intact PTH (150–300 pg/ml) and calcium-phosphorus product (Ca×P) (<55 mg2/dl2) was also greater (21% versus 14%), but this was not statistically significant. This was attributable to 19% of Cinacalcet-D subjects with a PTH value below the KDOQI target range.
Conclusions: Achievement of KDOQI targets was difficult, especially with Flex-D. Maintaining calcium and phosphorus target values precluded the use of vitamin D doses necessary to lower PTH to within the narrow target range and highlighted limitations inherent to the KDOQI treatment algorithm.
Treatment recommendations for patients with secondary hyperparathyroidism (SHPT) due to chronic kidney disease (CKD) have changed considerably in recent years, as has the overall clinical management of those receiving dialysis. Perhaps most notably, the levels of serum calcium and phosphorus considered acceptable are substantially lower than in the past, as stated formally in published guidelines (1). Such recommendations have been provided primarily for reasons of patient safety, in part due to concerns around risk of increasing serum calcium and/or episodes of hypercalcemia when calcium-containing phosphate-binding agents and/or vitamin D sterols are used to manage SHPT (2–17). Vitamin D analogs also raise serum phosphorus despite the ongoing use of phosphate-binding compounds (10–14). Both hypercalcemia and hyperphosphatemia are recognized widely as important risk factors for soft-tissue and vascular calcification among dialysis patients (15–23).
Although vitamin D sterols and phosphate-binding agents can lower parathyroid hormone (PTH) in patients with SHPT, therapeutic strategies that effectively lower PTH while limiting risks of elevated serum calcium and phosphorus are of considerable interest. Previously published clinical trials that have assessed the safety and efficacy of treatments for SHPT have used calcium or phosphorus limits that differ substantially from those now advocated (5–13,24–27). Thus, these results may not accurately reflect the therapeutic efficacy of these approaches for controlling PTH when treatments are adjusted to maintain calcium and phosphorus within ranges now considered appropriate. Additionally, the efficacy of various SHPT treatments has not been evaluated using current guidelines to inform dose modifications with any available therapeutic agent or when a defined range of PTH values is used to measure therapeutic outcomes.
ACHIEVE was the first study designed to directly assess the feasibility of managing SHPT within the context of National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines. This prospective, randomized, controlled trial (RCT) compared the efficacy and safety of two treatment regimens to control PTH among hemodialysis patients with SHPT. One treatment group received the calcimimetic cinacalcet HCl (cinacalcet) together with constant low doses of vitamin D sterols (Cinacalcet-D). This intervention focused on cinacalcet as treatment for SHPT while providing low-dose vitamin D as adjunctive therapy. The second treatment group used higher, but flexible, doses of either paricalcitol or doxercalciferol without cinacalcet (Flex-D). Dosage adjustments for all therapeutic agents were made to maintain mineral and bone target ranges within KDOQI treatment guidelines.
Materials and Methods
Study Population
Patients were considered for the study if they were ≥18 yr of age, had received hemodialysis for ≥3 mo, and were receiving either paricalcitol or doxercalciferol to manage SHPT. Candidates had historical plasma PTH values between 150 and 800 pg/ml, which were confirmed during screening, and albumin-corrected serum total calcium concentrations ≥8.4 mg/dl. Patients with PTH levels 300 to 800 pg/ml were considered without regard to serum calcium-phosphorus product (Ca×P) levels, whereas those with PTH levels 150 to 300 pg/ml were considered only if Ca×P > 55 mg2/dl2. After a 3-wk washout period during which vitamin D therapy was withheld, PTH and calcium levels were measured again on at least two occasions. Patients with mean PTH values >300 pg/ml and mean calcium levels ≥8.4 mg/dl qualified for study.
Patients were excluded from the study if they were pregnant or nursing, had undergone a parathyroidectomy within the previous 3 mo, were involved in any other clinical trial within the past 30 d, or had received cinacalcet previously. The study was conducted in accordance with the principles of the Declaration of Helsinki, and all patients provided written informed consent. The study protocol was reviewed and approved by the institutional review board at each study site.
Study Design and Treatments
This was a multicenter, open-label, RCT conducted at 42 sites. The 33-wk study was comprised of three phases: a 6-wk screening phase (including a 3-wk vitamin D washout period in which vitamin D therapy was withheld), a 16-wk dose titration phase, and an 11-wk efficacy-assessment phase. Subjects with PTH >300 pg/ml and corrected serum calcium ≥8.4 mg/dl were assigned randomly in a 1:1 ratio to receive either oral cinacalcet with low doses of paricalcitol or doxercalciferol (2 μg or 1 μg, respectively, thrice weekly) (Cinacalcet-D), or flexible, escalating doses of paricalcitol or doxercalciferol (Flex-D). During the 16-wk titration phase, adjustments to cinacalcet or vitamin D doses were made at 4-wk intervals during study visits on weeks 4, 8, 12, and 16. All biochemical measurements were determined at Covance Laboratory Services (Indianapolis, IN).
Cinacalcet-D subjects received an initial cinacalcet dosage of 30 mg/d orally. These subjects also received either 2 μg of paricalcitol or 1 μg of doxercalciferol intravenously thrice weekly (with each dialysis session) provided the corrected serum calcium was <10.2 mg/dl and/or serum phosphorus was <6.0 mg/dl. Cinacalcet dosage was raised incrementally at 4-wk intervals to 60, 90, 120, or 180 mg/d to achieve a PTH level between 150 and 300 pg/ml. Cinacalcet was withheld if PTH was <150 pg/ml and the vitamin D dosage could not be reduced, if calcium values were <7.5 mg/dl, or if symptoms of hypocalcaemia persisted. Cinacalcet therapy was restarted at the next lowest dose after these manifestations resolved. Additionally, vitamin D was withheld if PTH levels were <150 pg/ml and if corrected serum calcium was ≥8.4 mg/dl. If PTH was ≥150 pg/ml but corrected serum calcium >9.5 mg/dl or phosphorus >5.5 mg/dl, vitamin D was reduced. Vitamin D was restarted at a 25% to 50% lower dose after these manifestations resolved.
Flex-D subjects received initial doses of either 2 μg of paricalcitol or 1 μg of doxercalciferol with each thrice-weekly hemodialysis session. Dosage could be titrated upward at 4-wk intervals if calcium was <10.2 mg/dl and if phosphorus was <6.0 mg/dl to achieve a PTH of 150 to 300 pg/ml. Vitamin D dosage was adjusted according to KDOQI guidelines, i.e., withheld for PTH <150 pg/ml, for calcium >10.2 mg/dl, for phosphorus >5.5 mg/dl, or for Ca×P > 55 mg2/dl2. Vitamin D therapy was resumed after lowering the dose by 25% to 50% when these biochemical abnormalities resolved.
Investigators were allowed to adjust concomitant medications, such as phosphate binders and dialysate calcium, without constraint throughout the study for both treatment groups. During the 11-wk assessment phase, PTH, calcium, phosphorus, and Ca×P levels were determined monthly at study weeks 19, 23, and 27 but increases to cinacalcet or vitamin D dosage were not permitted during this interval.
Statistical Analysis
Results are presented as medians with interquartile range (Q1, Q3) or as means ± 1 SD. All subjects who received at least one dose of study drug were included in the safety analyses; subjects who had at least one biochemical measurement during the assessment phase were included in the efficacy analyses. The primary endpoint was the proportion of subjects who simultaneously achieved a mean PTH between 150 and 300 pg/ml and a mean Ca×P value <55 mg2/dl2 during the assessment phase. Secondary endpoints included the proportion of subjects who achieved KDOQI targets for PTH, calcium, phosphorus, and Ca×P individually; the absolute and percentage change from baseline in values for PTH, calcium, phosphorus, and Ca×P; and the proportion of subjects with ≥30% reduction in PTH. The Cochran-Mantel-Haenszel test was used to compare proportions (of achievers) between groups, whereas analysis of covariance on ranked data were used to assess continuous variables. All P values <0.05 were considered statistically significant.
PTH was measured by either Nichols Biointact PTH or Bayer Advia Intact PTH (iPTH) assays. Before completion of enrollment, the biointact assay was no longer available. This affected all patients in both treatment groups similarly. A factor of 1.85 was used to convert biointact PTH results to iPTH values (28). A 1-μg dose of doxercalciferol was considered to be equal to 2 μg of paricalcitol, and all results for vitamin D sterol dosages are presented as microgram-equivalents of paricalcitol.
Results
Subject Disposition, Baseline Demographics, and Baseline Biochemical Results
A total of 173 subjects were enrolled: 87 were assigned to the Cinacalcet-D group and 86 to Flex-D. For the Cinacalcet-D group, all subjects received at least one dose of cinacalcet and 80 (92%) also received either paricalcitol or doxercalciferol. For the Flex-D group, 82 (95%) subjects received at least one dose of a vitamin D sterol.
Overall, 43 subjects (25%) discontinued the study before completion: 15 of 87 (17%) received Cinacalcet-D and 28 of 86 (33%) received Flex-D. Twelve of 87 (14%) Cinacalcet-D subjects and 19 of 86 (22%) Flex-D subjects withdrew before reaching the assessment phase. Reasons for withdrawal for Cinacalcet-D versus Flex-D subjects included, respectively: adverse events (6% versus 1%), administrative decision (0% versus 8%), withdrawal of consent (3% versus 5%), death (2% versus 2%), loss to follow-up (0% versus 2%), kidney transplantation (0% versus 2%), parathyroidectomy (0% versus 1%), and other (6% versus 10%). Of the subjects who withdrew from the Cinacalcet-D and Flex-D arms, 9 (60%) and 22 (79%) subjects, respectively, had a final PTH value before early termination of >300 pg/ml.
Baseline demographic features, length of time on dialysis, and prevalence of concurrent major medical conditions before randomization did not differ between treatment groups (Table 1). Additionally, the average prewashout vitamin D dose, as well as values for PTH, calcium, phosphorus, or Ca×P before or after vitamin D washout, were similar between groups.
Table 1.
Demographics, medical history, and key baseline laboratory values
| Characteristic | Cinacalcet-D (n = 87) | Flex-D (n = 86) |
|---|---|---|
| Male/female, n (%) | 52/35 (60/40) | 45/41 (52/48) |
| Age, mean (SD), years | 57.7 (14.9) | 59.0 (12.4) |
| Time on dialysis, mean (SD), months | 46.3 (36.4) | 46.8 (44.1) |
| Medical history, positive, n (%)a | ||
| diabetes | 49 (56) | 61 (71) |
| hypertension | 81 (93) | 86 (100) |
| peripheral vascular disease | 18 (21) | 22 (26) |
| cerebrovascular accident | 11 (13) | 14 (16) |
| myocardial infarction | 10 (11) | 18 (21) |
| coronary artery disease | 29 (33) | 36 (42) |
| congestive heart failure | 26 (30) | 32 (37) |
| iPTH, median (Q1,Q3), pg/ml | ||
| pre-washout | 414 (316, 550) | 428 (328, 553) |
| baseline | 597 (471, 775) | 621 (463, 833) |
| Serum calcium, median (Q1,Q3), mg/dl | ||
| pre-washout | 9.8 (9.5, 10.3) | 10.0 (9.5, 10.5) |
| baseline | 9.6 (9.2, 10.0) | 9.7 (9.4, 10.0) |
| Serum phosphorus, median (Q1,Q3), mg/dl | ||
| pre-washout | 5.9 (4.5, 7.0) | 5.4 (4.8, 6.2) |
| baseline | 5.1 (4.2, 6.4) | 5.2 (4.1, 5.9) |
| Ca×P, median (Q1,Q3), mg2/dl2 | ||
| pre-washout | 56.8 (44.7, 67.6) | 53.6 (46.6, 61.6) |
| baseline | 48.9 (39.4, 59.4) | 49.0 (40.2, 56.7) |
| Vitamin D at pre-washout, μg/wkb | ||
| median (Q1,Q3) | 13.5 (9.0, 21.0) | 15.0 (12.0, 24.0) |
| mean (SD) | 16.7 (11.8) | 18.7 (11.3) |
SD, standard deviation; iPTH, intact parathyroid hormone; Q1, first quartile; Q3, third quartile; Ca×P, calcium-phosphorus product.
Subjects could have multiple comorbidities.
Data not available for 2 subjects in the Cinacalcet-D group and 1 subject in Flex-D group.
Effect of Vitamin D Washout
Withdrawal of vitamin D before the administration of study drugs highlights the impact of vitamin D therapy on several indices of mineral metabolism. Median increases in plasma PTH levels were 50%. In contrast, there were corresponding decreases in serum calcium (2.2%), phosphorus (5.9%), and Ca×P levels (8.7%). Responses were similar across subjects who were later randomly assigned to receive either Cinacalcet-D or Flex-D.
Primary Outcomes
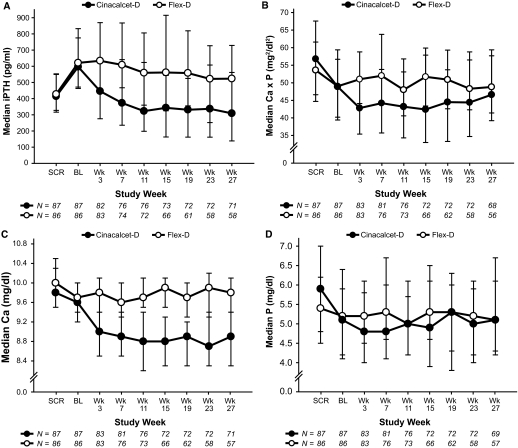
Median (Q1,Q3) PTH levels declined progressively throughout the study in the Cinacalcet-D group, but values changed only modestly in the Flex-D group (Figure 1A). PTH decreased from 597 pg/ml (471, 775 pg/ml) at baseline to 320 pg/ml (211, 589 pg/ml) during the assessment phase for Cinacalcet-D subjects, and from 621 pg/ml (463, 833 pg/ml) to 559 pg/ml (314, 768 pg/ml) over the same interval for Flex-D subjects. Median values for Ca×P were 49 mg2/dl2 (39, 59 mg2/dl2) at baseline and 47 mg2/dl2 (38, 56 mg2/dl2) during the assessment phase for Cinacalcet-D subjects; corresponding results with Flex-D were 49 mg2/dl2 (40, 57 mg2/dl2) and 49 mg2/dl2 (44, 56 mg2/dl2), respectively (Figure 1B). Notably, PTH fell below 150 pg/ml for 14 Cinacalcet-D subjects and one Flex-D subject. Consequently, the difference in the proportion of subjects who achieved the combined primary study endpoint of a PTH between 150 and 300 pg/ml and a Ca×P < 55 mg2/dl2 was not statistically significant (21% versus 14%, P = 0.231).
Figure 1.
Intact parathyroid hormone (iPTH) (A), calcium-phosphorus product (Ca × P) (B), calcium (Ca) (C), and phosphorus (P) (D) values recorded at each scheduled visit for subjects receiving either cinacalcet plus constant low-dose vitamin D (Cinacalcet-D) or flexible vitamin D (Flex-D). Data presented as median ± interquartile range. SCR, screening phase (previtamin D washout); BL, baseline (postvitamin D washout).
Other Biochemical Outcomes
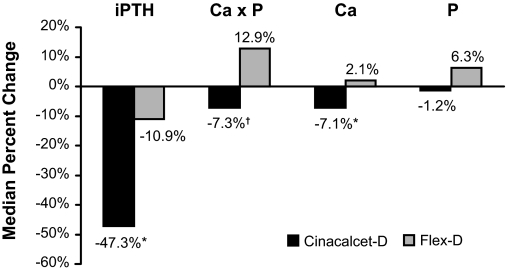
Median (Q1, Q3) calcium declined in Cinacalcet-D subjects, measuring 9.6 mg/dl (9.2, 10.0 mg/dl) at baseline and 8.9 mg/dl (8.4, 9.1 mg/dl) during the assessment phase but remained unchanged in Flex-D subjects, measuring 9.7 mg/dl (9.4, 10.0 mg/dl) at baseline and 9.8 mg/dl (9.5, 10.2 mg/dl) during the assessment phase (Figure 1C). Serum phosphorus did not change appreciably or differ between groups (Figure 1D). Values were 5.1 mg/dl (4.2, 6.4 mg/dl) at baseline and 5.3 mg/dl (4.1, 6.0 mg/dl) during the assessment phase for Cinacalcet-D subjects. Corresponding results were 5.2 mg/dl (4.1, 5.9 mg/dl) and 5.3 mg/dl (4.4, 5.8 mg/dl) for Flex-D subjects. Percent changes between baseline and the assessment phase for PTH, calcium, and Ca×P, but not phosphorus, differed significantly between treatment groups (Figure 2).
Figure 2.
Median percent changes from baseline to assessment values for intact parathyroid hormone (iPTH), calcium (Ca), phosphorus (P), and calcium-phosphorus product (Ca × P). Cinacalcet-D, n = 75; Flex-D, n = 64; *P < 0.001; †P = 0.003.
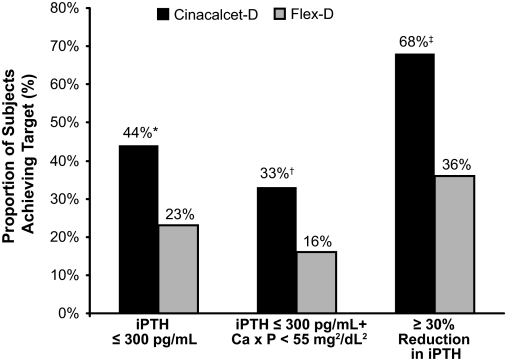
A greater proportion of subjects receiving Cinacalcet-D compared with Flex-D achieved both a mean PTH ≤300 pg/ml during the assessment phase and a combined endpoint of a PTH ≤300 pg/ml and Ca×P < 55 mg2/dl2 (Figure 3). Plasma PTH levels also decreased by ≥30% in a greater percentage of subjects receiving Cinacalcet-D versus Flex-D (Figure 3).
Figure 3.
Proportion of subjects reaching additional intact parathyroid hormone (iPTH) targets by treatment group. Cinacalcet-D, n = 75; Flex-D, n = 64; *P = 0.006, †P = 0.009, ‡P < 0.001; Ca × P, calcium-phosphorus product.
A greater percentage of subjects receiving Cinacalcet-D versus Flex-D achieved the KDOQI target range for calcium of 8.5 to 9.5 mg/dl (63% versus 25%, P < 0.001); 85% of Cinacalcet-D subjects versus only 27% of Flex-D subjects had serum calcium values ≤9.5 mg/dl (P < 0.001). The proportion of subjects who achieved KDOQI target ranges for PTH, phosphorus, and Ca×P did not differ between groups; values for Cinacalcet-D versus Flex-D subjects, respectively, were 25% versus 22% for PTH, 47% versus 63% for phosphorus, and 73% versus 67% for Ca×P. The proportion of subjects in each group who achieved a phosphorus level ≤5.5 mg/dl was 55% versus 66%. The proportion of subjects who achieved simultaneous KDOQI-defined control of all four parameters simultaneously was 8% versus 0% (P = 0.017) for Cinacalcet-D versus Flex-D subjects.
Doses of Study Medications
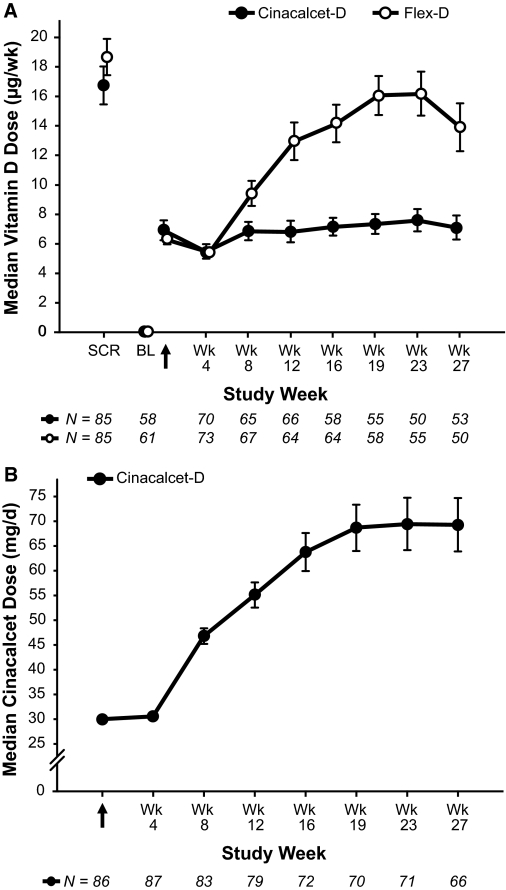
In the Flex-D group, the mean ± SD dose of vitamin D sterols increased gradually after baseline and averaged 13.9 ± 10.4 μg/wk (paricalcitol equivalents, n = 64) during the assessment phase (Figure 4A). Mean doses during the assessment phase, however, were somewhat lower than those that used previtamin D washout because of protocol dosing restrictions.
Figure 4.
Weekly vitamin D dose (A) and daily cinacalcet dose (B) at each measured time point. Data presented as mean ± standard error for subjects that received vitamin D. Zero doses are included. Vitamin D doses are presented as paricalcitol equivalents, where 2 μg paricalcitol equals 1 μg doxercalciferol. SCR, screening phase (previtamin D washout); BL, baseline (postvitamin D washout). Arrow indicates day 1 of treatment.
In the Cinacalcet-D group, the mean ± SD vitamin D dose during the assessment phase was 6.8 ± 5.3 μg/wk (n = 57). The average cinacalcet dose during the assessment phase was 68.5 ± 41.1 mg/d (n = 72), with the average daily dose increasing at each interval of follow-up (Figure 4B).
A smaller percentage of subjects receiving Cinacalcet-D versus Flex-D required a vitamin D dose change during the trial; 51% versus 58% experienced a decrease in vitamin D dose, whereas 44% versus 79% experienced an increase in vitamin D dose, respectively. Doses of vitamin D sterols were withheld less often for Cinacalcet-D versus Flex-D subjects (31% versus 50%) due to fewer episodes of hyperphosphatemia (10% versus 30%) and hypercalcemia (2% versus 17%).
Phosphate Binder Use
Ninety-eight percent of subjects received a phosphate binding agent during the study. The overall use of calcium-free compounds (52% versus 59%), calcium-containing compounds only (20% versus 17%), or combined therapy with both agents (28% versus 21%) did not differ between subjects receiving Cinacalcet-D versus Flex-D, respectively. The most notable change observed was the number of Flex-D subjects receiving calcium-based phosphate binders alone, which dropped from 17 to 8 from the titration phase to the assessment phase. Over the same period, the number of Cinacalcet-D subjects receiving calcium-based phosphate binders alone remained relatively constant, 17 to 18.
Safety
Overall, 71% of subjects in each treatment group reported at least one adverse event. In the Cinacalcet-D group, nausea (10% of subjects), diarrhea, muscle spasms, and abdominal pain (8% each) were observed most often, followed by hypocalcemia (calcium <8.4 mg/dl), dyspnea, and headache (7% each). Pyrexia (6%), vomiting (6%), and hypotension during dialysis procedures (5%) also occurred. For Flex-D subjects, diarrhea (11%), muscle spasms (10%), dyspnea (9%), arthralgia, hyperphosphatemia (phosphorus ≥6.0 mg/dl), hypotension during dialysis, and upper respiratory tract infection (7% each), headache, and pneumonia (6% each) were most often observed. Three deaths were reported in each treatment group, but none was considered treatment-related.
Discussion
The current results highlight the difficulty in achieving adequate PTH control in hemodialysis patients with SHPT while simultaneously maintaining serum calcium and phosphorus levels within the ranges currently considered acceptable, particularly in subjects receiving vitamin D alone (Flex-D). In all studies published previously, reductions in PTH to levels below a predetermined specified value or a defined percentage decrease in PTH during treatment have been used to judge therapeutic efficacy; none was designed to achieve a PTH range of 150 to 300 pg/ml as was done in the current RCT. Here, cinacalcet and vitamin D sterols were used to maintain PTH, as well as calcium and phosphorus, within KDOQI target ranges. Increases to cinacalcet, paricalcitol, and doxercalciferol dosage were constrained if calcium or phosphorus fell above or below recommended guidelines. Earlier published clinical trials using vitamin D sterols to treat SHPT have used substantially higher calcium and phosphorus levels to define episodes of hypercalcemia and hyperphosphatemia, to adjudicate protocol-mandated adjustments to vitamin D dosage, and to determine the need for temporary interruptions to treatment (5–14,24). The current findings thus provide insight into the challenges posed by current management guidelines, their potential impact on the efficacy of treatments for SHPT, and the effect of such recommendations on therapeutic outcomes.
Patients enrolled in the current trial had biochemical evidence of SHPT as documented either by persistent elevations in PTH despite ongoing vitamin D treatment, or by persistent elevations in Ca×P despite adequate PTH control. PTH increased substantially and calcium and phosphorus decreased when paricalcitol or doxercalciferol was withheld during the vitamin D washout period. These findings substantiate widely recognized effects of vitamin D on indices of mineral metabolism (6,9,10,24). Indeed, in a recent meta-analysis of RCTs and the use of vitamin D sterols in CKD, it is these elevations of calcium and phosphorus, along with the paucity of strong data, that require caution when prescribing vitamin D for SHPT (29). Results obtained for the Flex-D group reflected this. Although the average dose of paricalcitol or doxercalciferol increased during the trial, episodes of hypercalcemia and hyperphosphatemia were common, and these limited the doses that could be given to control PTH adequately.
A significantly greater proportion of subjects receiving Cinacalcet-D achieved a PTH ≤300 pg/ml, concurrent values of PTH ≤300 pg/ml and Ca×P < 55 mg2/dl2, concurrent KDOQI-defined control of PTH, calcium, phosphorus, and CaxP, reductions of ≥30% in PTH from baseline, and calcium levels between 8.4 and 9.5 pg/ml as well as ≤9.5 pg/ml. Despite these differences, the proportion of subjects that achieved the primary study endpoint of simultaneous PTH and Ca×P control, as defined by KDOQI target ranges, did not differ between groups. This was attributable to the finding that 14 subjects (19%) receiving Cinacalcet-D, but only one receiving Flex-D had a mean PTH level during the assessment phase <150 pg/ml and, thus, did not reach target (150 to 300 pg/ml) despite evidence of a substantial reduction in PTH. This also accounts for the larger proportion of Cinacalcet-D subjects achieving a PTH level <300 pg/ml at the end of the study and supports the overall efficacy of cinacalcet with low-dose vitamin D as a therapeutic strategy for SHPT. It should be noted, however, that oversuppressed PTH levels may not be beneficial (18) and indeed could be associated with increased risk for low turnover bone disease or other adverse outcomes. Accordingly, a reduction in cinacalcet dose would be appropriate when PTH is <150 pg/ml. Together, these findings underscore the challenges of achieving and maintaining PTH levels within a relatively narrow therapeutic target range.
The study had several limitations, which included the open-label design, the uneven early withdrawal of subjects, and the use of a complex dosing algorithm based upon biochemical criteria. Efficacy was assessed at only three study visits during the assessment phase. Approximately one-third of subjects in each group who would have qualified for upward dosage adjustments based on laboratory values during the assessment phase did not receive increases due to the study protocol disallowance of increases to vitamin D or cinacalcet during the assessment phase. This may have affected the biochemical responses observed, as well as limited the ability to optimally titrate vitamin D or cinacalcet dosage. Finally, efficacy was judged using biochemical measures rather than high level patient-related outcomes, thus limiting the ability to assess fully the risk-benefit balance of each intervention. This was with the intention of testing the ability of the interventions to achieve current KDOQI targets. Studies evaluating patient related outcomes for bone disease interventions should be encouraged.
Conclusion
Despite restrictions to cinacalcet and vitamin D dosing imposed by current guidelines for managing mineral metabolism in CKD patients, cinacalcet and constant low-doses of vitamin D sterols were effective in lowering PTH levels in hemodialysis subjects with SHPT while adequately maintaining acceptable levels of calcium and phosphorus. The results underscore the difficulty of lowering PTH effectively with vitamin D sterols alone while maintaining adequate control of serum calcium and phosphorus, as well as the great difficulty of simultaneously achieving all KDOQI targets.
Disclosures
None.
Acknowledgments
The ACHIEVE study (Study ID Number 20050102) and the preparation of this manuscript were funded by Amgen, Inc. The authors wish to thank Nelson Erlick, DPM, MS, on behalf of Amgen Inc. and Jane Mannion, MS, Amgen Inc. for their assistance in the preparation of this manuscript (Ms Mannion is currently employed by Baxter International, Inc.). Results from the ACHIEVE study were presented in part at the American Society of Nephrology conference, 2007.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl]: S1–S201, 2003 [PubMed] [Google Scholar]
- 2.Dressler R, Laut J, Lynn RI, Ginsberg N: Long-term high dose intravenous calcitriol therapy in end-stage renal disease patients with severe secondary hyperparathyroidism. Clin Nephrol 43: 324–331, 1995 [PubMed] [Google Scholar]
- 3.Gonella M, Calabrese G, Aleo AG, Vagelli G, Deambroglo P: Calcitriol pulse therapy for severe hyperparathyroidism or calcium salts as phosphate binders in renal dialysis patients. Nephron 71: 350–353, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Wallot M, Bonzel KE, Winter A, Geörger B, Lettgen B, Bald M: Calcium acetate versus calcium carbonate as oral phosphate binder in pediatric and adolescent hemodialysis patients. Pediatr Nephrol 10: 625–630, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Kurokawa K, Akizawa T, Suzuki M, Akiba T, Ogata E, Slatopolsky E: Effect of 22-oxacalcitriol on hyperparathyroidism of dialysis patients: results of a preliminary study. Nephrol Dial Transplant 11[Suppl 3]: 121–124, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Tan AU Jr, Levine BS, Mazess RB, Kyllo DM, Bishop CW, Knutson JC, Kleinman KS, Coburn JW: Effective suppression of parathyroid hormone by 1 alpha-hydroxy-vitamin D2 in hemodialysis patients with moderate to severe secondary hyperparathyroidism. Kidney Int 51: 317–323, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Akiba T, Marumo F, Owada A, Kurihara S, Inoue A, Chida Y, Ando R, Shinoda T, Ishida Y, Ohashi Y: Controlled trial of falecalcitriol versus alfacalcidol in suppression of parathyroid hormone in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis 32: 238–246, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D: Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int 63: 1483–1490, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Tsukamoto Y, Hanaoka M, Matsuo T, Saruta T, Nomura M, Takahashi Y: Effect of 22-oxacalcitriol on bone histology of hemodialyzed patients with severe secondary hyperparathyroidism. Am J Kidney Dis 35: 458–464, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Martin KJ, González EA, Gellens M, Hamm LL, Abboud H, Lindberg J: 19-Nor-1-alpha-25-dihydroxyvitamin D2 (paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol 9: 1427–1432, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Frazão JM, Chesney RW, Coburn JW: Intermittent oral 1αhydroxyvitamin D2 is effective and safe for the suppression of secondary hyperparathyroidism in haemodialysis patients. Nephrol Dial Transplant 13[Suppl 3]: 68–72, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Frazão JM, Elangovan L, Maung HM, Chesney RW, Acchiardo SR, Bower JD, Kelley BJ, Rodriguez HJ, Norris KC, Robertson JA, Levine BS, Goodman WG, Gentile D, Mazess RB, Kyllo DM, Douglass LL, Bishop CW, Coburn JW: Intermittent doxercalciferol (1α-hydroxyvitamin D2) therapy for secondary hyperparathyroidism. Am J Kidney Dis 36: 550–561, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Akizawa T, Suzuki M, Akiba T, Nishizawa Y, Ohashi Y, Ogata E, Slatopolsky E, Kurokawa K: Long-term effect of 1,25-dihydroxy-22-oxavitamin D(3) on secondary hyperparathyroidism in haemodialysis patients: one-year administration study. Nephrol Dial Transplant 17[Suppl 10]: 28–36, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Llach F, Yudd M: Paricalcitol in dialysis patients with calcitriol-resistant secondary hyperparathyroidism. Am J Kidney Dis 38[Suppl 5]: S45–S50, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Guerin AP, London GM, Marchais SJ, Metivier F: Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 15: 1014–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Chertow GM, Burke SK, Raggi P: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Ureña P, Malergue MC, Goldfarb B, Prieur P, Guédon-Rapoud C, Pétrover M: Evolutive aortic stenosis in hemodialysis patients: analysis of risk factors. Nephrologie 20: 217–225, 1999 [PubMed] [Google Scholar]
- 19.Marchais SJ, Metivier F, Guerin AP, London GM: Association of hyperphosphataemia with haemodynamic disturbances in end-stage renal disease. Nephrol Dial Transplant 14: 2178–2183, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Block GA, Port FK: Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Dis 35: 1226–1237, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F: Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106: 100–105, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM: Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 39: 695–701, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Moe SM, O'Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K: Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int 61: 638–647, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Maung HM, Elangovan L, Frazao JM, Bower JD, Kelley BJ, Acchiardo SR, Rodriguez HJ, Norris KC, Sigala JF, Rutkowski M, Robertson JA, Goodman WG, Levine BS, Chesney RW, Mazess RB, Kyllo DM, Douglass LL, Bishop CW, Coburn JW: Efficacy and side effects of intermittent intravenous and oral doxercalciferol (1alpha-hydroxyvitamin D(2)) in dialysis patients with secondary hyperparathyroidism: a sequential comparison. Am J Kidney Dis 37: 532–543, 2001 [PubMed] [Google Scholar]
- 25.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446–456, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Qunibi WY, Hootkins RE, McDowell LL, Meyer MS, Simon M, Garza RO, Pelham RW, Cleveland MV, Muenz LR, He DY, Nolan CR: Treatment of hyperphosphatemia in hemodialysis patients: the Calcium Acetate Renagel Evaluation (CARE Study). Kidney Int 65: 1914–1926, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Ross EA, Tian J, Abboud H, Hippensteel R, Melnick JZ, Pradhan RS, Williams LA, Hamm LL, Sprague SM: Oral paricalcitol for the treatment of secondary hyperparathyroidism in patients on hemodialysis or peritoneal dialysis. Am J Nephrol 28: 97–106, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Martin KJ, Akhtar I, Gonzalez EA: Parathyroid hormone: new assays, new receptors. Semin Nephrol 24: 3–9, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GFM: Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med 147: 840–853, 2007 [DOI] [PubMed] [Google Scholar]