Abstract
The CDC37 gene is essential for the activity of p60v-src when expressed in yeast cells. Since the activation pathway for p60v-src and steroid hormone receptors is similar, the present study analyzed the hormone-dependent transactivation by androgen receptors and glucocorticoid receptors in yeast cells expressing a mutant version of the CDC37 gene. In this mutant, hormone-dependent transactivation by androgen receptors was defective at both permissive and restrictive temperatures, although transactivation by glucocorticoid receptors was mildly defective only at the restrictive temperature. Cdc37p appears to function via the androgen receptor ligand-binding domain, although it does not influence receptor hormone-binding affinity. Models for Cdc37p regulation of steroid hormone receptors are discussed.
INTRODUCTION
The Hsp90 chaperone machinery is known to function in signal transduction processes. These include the regulation of protein kinase activity and stabilization of the high-affinity ligand-binding conformation of steroid hormone receptors. Several other molecular chaperones including Hsp70 and dnaJ proteins appear to function with Hsp90 (Perdew and Whitelaw, 1991; Kimura et al., 1995). Other proteins known to bind Hsp90 include peptidyl prolyl isomerases, p60, p50, p48, and p23 (for review, see Bohen and Yamamoto, 1994).
The p50 protein has been shown to bind quantitatively to Hsp90 (Whitelaw et al., 1991) and to be present in complexes formed between Hsp90 and the protein tyrosine kinase p60v-src, when isolated from animal cells (for review, see Brugge, 1986), or when reconstituted in rabbit reticulocyte lysates (Hutchison et al., 1992). Recently, mammalian p50 was identified as an orthologue of the yeast cell division cycle gene CDC37 and was shown to be involved in stabilization of cyclin-dependent kinases CDK4 and CDK6 (Dai et al., 1996; Perdew et al., 1997; Stepanova et al., 1996). In yeast, Cdc37p is also important for stabilization of the Cdc28 cyclin-dependent kinase (Gerber et al., 1995), suggesting that p50/Cdc37 function is conserved in lower and higher eukaryotes. Both Hsp90 and Cdc37p were also identified as components of the signal transduction pathway leading from the sevenless receptor involved in Drosophila eye development (Cutforth and Rubin, 1994; for a recent review on Cdc37, see Hunter and Poon, 1997).
Further evidence for p50/Cdc37p conservation was suggested by recent genetic studies in yeast showing that mutation in CDC37 reduced p60v-src activity (Dey et al., 1996b). In wild-type yeast cells, expression of p60v-src results in a lethal phenotype due to abnormal protein phosphorylation on tyrosine residues (Boschelli et al., 1993; Florio et al., 1994). Both CDC37 and the YDJ1 molecular chaperone are important for this activity since mutations in either can suppress the lethal phenotype resulting from p60v-src expression (Dey et al., 1996a,b).
Besides regulating p60v-src activity, Ydj1p is a molecular chaperone known to function in hormone-dependent transactivation by steroid hormone receptors (Caplan et al., 1995; Kimura et al., 1995; also see Bohen et al., 1995). P50/Cdc37, however, has only been shown to function in regulating protein kinase activity. Furthermore, it was not observed to associate with unliganded steroid hormone receptor complexes containing Hsp90 (Whitelaw et al., 1991; Stancato et al., 1993; Nair et al., 1996). Together, these observations suggest that p50/Cdc37 is specific for protein kinase activity and is not involved in steroid receptor regulation. On the other hand, conditions that lead to the formation of Hsp90–p60v-src complexes and to the activation of p60v-src protein are similar to those regulating steroid hormone receptor function, suggesting a common activation pathway (Hutchison et al., 1992; Dey et al., 1996a). To clarify whether p50/Cdc37 is also important for steroid hormone receptor activation, we tested whether a mutation in the yeast CDC37 gene affected hormone-dependent transactivation by androgen receptors (AR) and glucocorticoid receptors (GR).
MATERIALS AND METHODS
Yeast Strains and Growth Conditions
Yeast cells were cultured in selective media (0.67% yeast nitrogen base, 2% glucose plus amino acids) using standard procedures. The temperature-sensitive strain 8A7 (MATα cdc37–34, leu2, lys2, trp1, ura3) was used as genetic background for these studies. Plasmid transformation was performed by the LiAc procedure as described by Geitz et al. (1995). Plasmids used in this study were: pG1hAR (human AR; 14), pABC (AR1–660, TRP1; 14), pGAL-37 (GAL1 promoter, CDC37, 2 μ, URA3) pPGKarelacZC (lacZ reporter gene under control of androgen response elements, URA3; Purvis et al., 1991), pPGKgal-hAR (galactose-inducible AR gene, LEU2; Purvis et al., 1991), and pGN795 (rat GR, TRP1; Schena and Yamamoto, 1988), pRSS2 (CDC37, CEN/ARS, URA3).
The isogenic wild type was prepared from the strain AFY14 (8A7 with pG1hAR and pPGKarelacZC) by transformation of a 6-kb DNA fragment containing the wild-type CDC37 gene [isolated by SalI/HindIII digestion from pRSS2 (Dey et al., 1996b] and a selection of colonies that grew at 37°C. The resulting strain was termed AFY17. Replacement of the mutant cdc37–34 gene was verified by genomic DNA sequencing. Strains containing pABC, pPGKgal-hAR, and pGN795 were prepared after deselection of pG1hAR from AFY14 and AFY17 on nonselective media and subsequent transformation by these plasmids. The resulting strains all contained the pPGKarelacZC reporter plasmid. The strain containing the hsp82 temperature-sensitive mutant was described previously (Nathan and Lindquist, 1995; Fang et al., 1996).
Recovery of the cdc37–34 Gene
The original genomic clone containing the CDC37 gene in YCp50 was digested with XbaI to remove a 1.79-kb restriction fragment containing 1.14 kb of the open reading frame and 649 bp of upstream sequence. The linearized DNA was isolated from low melting point agarose and transformed into the cdc37–34 strain A34 (Dey et al., 1996b). Plasmids recovered from the resulting temperature-sensitive transformants were transformed into A34 and the cdc37–1 strain 14A (Dey et al., 1996b). Both strains remained temperature-sensitive when harboring this plasmid. The mutation in the cdc37–34 gene was characterized by DNA sequencing.
β-Galactosidase Activity Assay
Yeast cells were grown to early log phase (A600 = 0.2) and preincubated at 25°C or 37°C for 1 h before addition of dihydrotestosterone (DHT) for AR or deoxycorticosterone (DOC) for GR. The cells were incubated for another hour prior to preparation of extracts as described by Caplan et al. (1995). β-Galactosidase activity assays have been described previously by Caplan et al. (1995).
Hormone-binding Assays
Yeast cells were grown in selective media containing 2% raffinose to A600 = 0.2 and incubated at 25°C or 37°C in 1-ml aliquots for 30 min. AR was inducibly expressed from the GAL1 promoter (by addition of galactose to 2% vol/vol) for 30 min. The expression was terminated by addition of glucose to 2% (vol/vol), and the cultures were incubated for another hour at 25°C or 37°C. [3H]R1881 (diluted 1:50 with cold R1881) plus or minus 10 μM DHT was titrated into the cells, which were incubated with shaking for another 1.5 h, washed three times with 1 ml of water, and counted in 5 ml of scintillation fluid. Nonspecific cpm (typically 7% of total counts) were calculated from the samples containing 10 μM DHT and subtracted from the cpm for samples incubated in the absence of excess DHT.
Ligand competition assays were performed by growing yeast cells to A600 = 0.2, incubating at 25°C or 37°C for 30 min, and adding 100 nM R1881 (2 nM [3H]R1881 and 98 nM unlabeled R1881) in the presence or absence of 25 μM hydroxyflutamide (Scherring-Plough, Kenilworth, NJ; stored in ethanol). The cells were incubated for 1.5 h at 25°C or 37°C before being washed three times with water and counted in 5 ml of scintillant in a scintillation counter.
Preparation of Monoclonal Antibody to Cdc37p
The CDC37 gene was fused to Escherichia coli maltose-binding protein by ligating a 2.2-kbp EcoRV-SalI restriction fragment containing the CDC37 gene to the StuI site in the pMALc plasmid supplied by New England Biolabs (Beverly, MA). This construction results in a fusion of residues 49–499 of Cdc37p with the 43-kDa maltose-binding protein to yield a ∼95-kDa protein. A mixture of soluble Cdc37p-maltose-binding protein fusion protein isolated on an amylose-affinity resin (New England Biolabs) and fusion protein isolated from polyacrylamide gels emulsified with Freund’s complete adjuvant was injected s.c. into 6-wk-old BALB/c mice. A second injection of fusion protein in incomplete Freund’s adjuvant was performed 1 month later. Mice with high titer sera were killed, and spleen cells were fused to NS-1 human myeloma cells to produce hybridomas. A producer line was injected i.p. into pristine-treated BALB/c mice. Ascites fluid was recovered and antibody was isolated on protein G agaraose according to the specifications of the manufacturer (Life Technologies, Gaithersburg, MD).
Miscellaneous
Western Blot analysis was performed by standard procedures using a chemiluminescent detection kit (Pierce, Rockford, IL). Polyclonal antisera to AR was described previously (Fang et al., 1996).
RESULTS
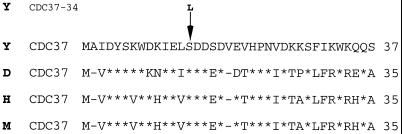
The yeast CDC37 gene was recently identified as a component of the cellular apparatus that controls p60v-src activity (Dey et al., 1996b). To determine whether Cdc37p also functions in steroid hormone receptor activation, a temperature-sensitive cdc37 mutant strain was used to study hormone-dependent transactivation by AR and GR. This mutant contains the same cdc37–34 gene originally isolated as a suppressor of p60v-src lethality (Dey et al., 1996b). The mutant gene was sequenced after plasmid rescue and was found to contain a single base change (C to T) at nucleotide 41 of the open reading frame. This change results in a predicted amino acid substitution at position 14 from serine to leucine. This residue is one of two serines that are phosphorylated in vivo by casein kinase II (the other is serine 17), and replacement of both serines with alanine reduces Cdc37p activity (McCann and Glover, unpublished results). Comparison of p50/Cdc37 sequences from human, mouse, Drosophila, and yeast reveals serine 14 to be part of the most conserved region of these p50/Cdc37 sequences, from amino acids 1 to 38, which are over 50% identical and 70% conserved (Figure 1).
Figure 1.
Cdc37 protein sequences. Comparison of the N-terminal region of p50/Cdc37 proteins in single letter amino acid code from S. cerevisiae (Y), Drosophila melanogaster (D), human (H), and mouse (M). Conserved residues are denoted by a star. The position of the S14L mutation in cdc37–34 is shown by an arrow.
Cdc37p Functions in AR Activation
Previous studies with the yeast system have demonstrated that constitutively expressed full-length AR mediates transcription of the E. coli lacZ gene in a DHT-dependent manner (Purvis et al., 1991; Caplan et al., 1995; Fang et al., 1996). Mutations in genes regulating receptor activation result in decreased hormone-dependent transactivation by AR. Two genes which function in this capacity encode the well-characterized molecular chaperones Ydj1p and Hsp90 (Caplan, 1997; Caplan et al., 1995; Fang et al., 1996).
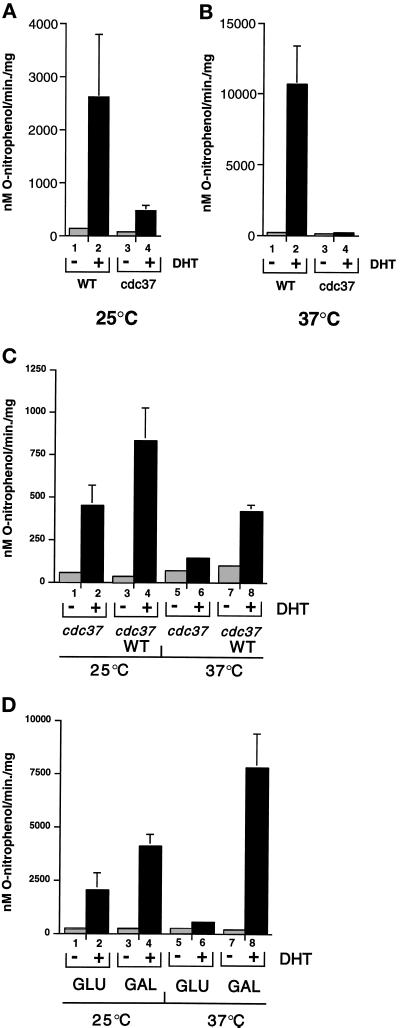
For the present study, the cdc37–34 mutant and an isogenic wild-type strain were assayed for DHT-dependent lacZ gene expression at two different temperatures: 25°C, which is permissive for both strains, and 37°C, which is restrictive for the mutant strain. Incubation of the wild-type strain with 100 nM DHT for 1 h induced β-galactosidase activity to 22-fold above the background at 25°C and 79-fold at 37°C (Figure 2A and B, respectively). β-Galactosidase levels in the uninduced and induced states were similar to those previously observed in other wild-type yeast strains (Caplan et al., 1995; Fang et al., 1996) and were approximately fourfold higher at 37°C than at 25°C (compare Figure 2 A with B). In the cdc37–34 mutant strain, however, β-galactosidase induction was 5-fold lower than the wild type at 25°C, and at 37°C it was almost 80-fold lower than the wild type (Figure 2, A and B). Similar results were obtained using the synthetic androgen R1881. Transactivation by AR is therefore defective at both permissive and restrictive temperatures in the cdc37–34 mutant, although the defect is more severe at 37°C as was previously found with p60v-src activity (Dey et al., 1996b).
Figure 2.
Transactivation by AR is defective in a cdc37 mutant strain. (A) β-Galactosidase activity in wild type (WT; lanes 1 and 2) and cdc37–34 mutant (cdc37; lanes 3 and 4). Cultures incubated at 25°C were treated with (lanes 2 and 4) or without (lanes 1 and 3) 100 nM DHT for 1 h. (B) As in A except that the cultures were incubated at 37°C for 1 h before hormone administration and for 1 h afterward. (C) β-Galactosidase activity in the cdc37–34 mutant strain containing low copy number vector (pRS316; lanes 1, 2, 5, and 6) or plasmid containing CDC37 (pRSS2; WT in lanes 3, 4, 7, and 8). The cells were incubated with (lanes 2, 4, 6, and 8) or without (1, 3, 5, and 7) 100 nM DHT for 1 h at 25°C or 37°C as indicated. (D) β-Galactosidase activity in the cdc37–34 mutant containing the multicopy 2 μ plasmid containing CDC37 under control of the inducible GAL1 promoter. Cells grown in glucose (GLU; lanes 1, 2, 5, and 6) or galactose (GAL, lanes 3, 4, 7, and 8) were incubated with or without DHT at 25°C or 37°C as indicated. All results are the mean of three independent experiments.
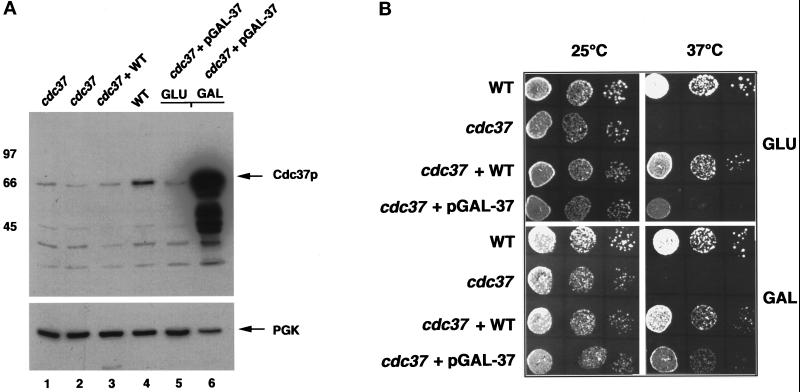
The results show in Figure 2A and B that loss of Cdc37 function negatively affects the activation of human AR expressed in yeast, and that this defect was overcome by replacement of the mutant gene with the wild type. However, the mutant phenotype was barely suppressed in cells coexpressing genomic cdc37–34 and the wild-type gene on a low copy number plasmid (approximately twofold activation above the mutant alone; Figure 2C). Similar results were obtained by crossing the cdc37–34 mutant with wild-type strain W303 1a (Thomas and Rothstein, 1989). The resulting diploid was also defective for DHT-dependent lacZ gene expression, although a similar cross between the isogenic wild-type and W3031a generated a diploid with wild-type DHT-dependent lacZ gene expression. This indicates that the cdc37–34 mutant gene is partially dominant over the wild type. The mutant phenotype could be fully suppressed, however, by overexpression of wild-type CDC37 from the GAL1 promoter on a multicopy plasmid (Figure 2D). In this case, expression of wild-type CDC37 was suppressed by growth in glucose and induced by growth in galactose (Figure 3A). Induction of Cdc37 by growth in galactose restored AR activation to wild-type levels, although even the presence of glucose AR activation was increased in this strain (compare Figure 2D, lane 2 with Figure 2A, lane 4 and Figure 2C, lane 2). This partial suppression may result from leakage of Cdc37p from the GAL promoter in cells grown in glucose, since increased levels of Cdc37p were observed in these cells (Figure 3A and below).
Figure 3.
Characterization of the cdc37–34 mutant. (A) Western Blot analysis of Cdc37 protein in whole-cell extracts from cdc37–34 cells (cdc37) grown at 25°C (lane 1) or 37°C for 1 h (lane 2), cdc37–34 cells containing pRSS2 (wild-type CDC37 on a low copy number plasmid; lane 3), wild-type cells (lane 4), and cdc37–34 cells containing pGAL-37 grown in glucose (lane 5) or galactose (lane 6). Full-length Cdc37p is arrowed. Bottom panel, reprobing the same filter with antisera against phosphoglycerate kinase (PGK; arrowed). (B) Serial dilutions of yeast cells and growth at 25°C or 37°C. WT, wild-type cells; cdc37, cdc37–34; cdc37/WT, cdc37–34 with pRSS2 (low copy number plasmid with CDC37); cdc37/pGAl-37, cdc37–34 with multicopy plasmid containing CDC37 under galactose promoter control.
To further investigate the cdc37–34 mutant phenotype, the levels of Cdc37 protein were analyzed by Western blot analysis of whole-cell extracts prepared from strains expressing either the mutant gene, the wild-type gene, or a combination of both genes. The results of these experiments (Figure 3A) show that the level of Cdc37 protein in the cdc37–34 mutant was reduced by approximately 20-fold compared with the levels found in the wild-type strain (compare lanes 1 and 4). Furthermore, the mutant appears to have a dominant effect on the level of wild-type Cdc37p, since there appears to be very little increase in the levels of protein in cells expressing both genes in single or low copy, even though these cells can grow at 37°C, albeit with a reduced rate compared with wild-type cells (Figure 3B). Overexpression of Cdc37p from the GAL1 promoter on a multicopy plasmid also resulted in reduced cell growth at 37°C, even though activation of the AR was restored to wild-type levels in these cells. There was only a slight change in the levels of mutant Cdc37 protein in cells grown at 37°C compared with 25°C.
Cdc37p Functions via the AR Ligand-binding Domain But Does Not Affect Hormone-binding Affinity
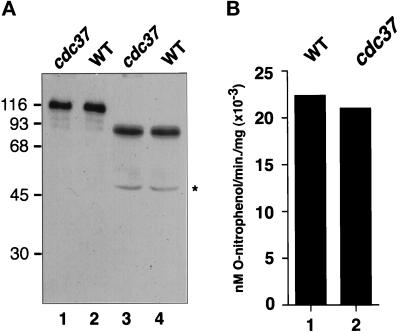
Although there is a marked decrease in the hormone-dependent transactivation by AR in the cdc37–34 mutant, there was no significant difference in receptor protein levels compared with the wild type (Figure 4A, lanes 1 and 2). Similar protein levels were also observed upon expression of a truncated version of the AR gene (AR1–660) which lacks the ligand-binding domain (Figure 4A, lanes 3 and 4).
Figure 4.
Transactivation by AR1–660 in wild-type and cdc37–34 mutant strains. (A) Western blot analysis of AR (lanes 1 and 2) and AR1–660 (lanes 3 and 4) in wild-type (WT) and cdc37–34 (cdc37) mutant cell extracts. Analysis was performed using whole-cell extracts (1 μg in lanes 1 and 2 and 5 μg in lanes 3 and 4) probed with anti-AR polyclonal antisera. Molecular weight size standards are shown in kDa. Star denotes breakdown product from AR1–660. (B) Steady-state β-galactosidase activity in wild-type (WT; lane 1) and cdc37–34 (cdc37; lane 2) mutant strains constitutively expressing AR1–660. Results are the mean of three independent experiments.
Previous studies have shown that deletion of the ligand-binding domain liberates the receptor from hormone dependence and also from factors that function in AR regulation via this region. A mutation in the YDJ1 gene, for example, results in defective transactivation by AR (Caplan et al., 1995). This defect was suppressed by deletion of the ligand-binding domain however, suggesting that Ydj1p functions via this region. To determine whether Cdc37p also functions via the ligand-binding domain, AR1–660 was constitutively expressed in wild-type and cdc37–34 mutant strains. Hormone-independent lacZ reporter gene expression was then assayed by measuring steady-state β-galactosidase activity. β-Galactosidase activity in these strains was observed to be 200-fold higher than the hormone-independent levels normally found in wild-type cells expressing full-length AR (Figure 4B, compare with Figure 2A, lanes 1 and 3). Furthermore, the levels in both wild-type and cdc37–34 mutant strains were very similar, suggesting that Cdc37p loss of function affected neither the folding of AR1–660 nor its ability to induce lacZ gene expression (or the folding and activity of β-galactosidase). This suggests that Cdc37p functions specifically via the ligand-binding domain in hormone-dependent activation of AR.
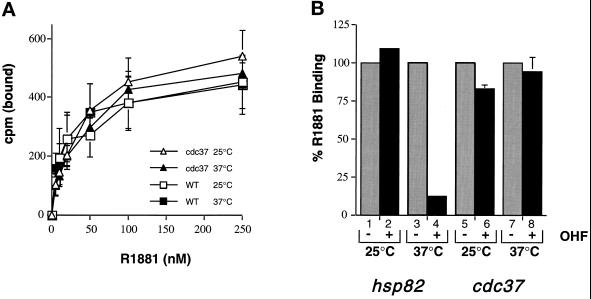
Previous studies have shown that Hsp90 associates with the AR ligand-binding domain (Mariovet et al., 1992) and maintains it in a high-affinity hormone-binding state (Fang et al., 1996). In the absence of functional Hsp90, the AR adopts a low-affinity conformation with a reduced capacity for the agonist R1881 (Fang et al., 1996). To determine whether Cdc37p also affects hormone-binding affinity, direct ligand-binding studies were performed by titrating [3H]R1881 against growing yeast cultures at permissive and restrictive temperatures. The data shown in Figure 5A demonstrate that there was very little difference in the ability of AR to bind [3H]R1881 in wild-type or cdc37–34 mutant cells, at either temperature. This contrasts with the decreased capacity of R1881 to bind to AR upon Hsp90 loss of function (Fang et al., 1996), and indicates that the hormone-binding affinity of AR is not compromised in the cdc37–34 mutant strain.
Figure 5.
Hormone binding to AR in wild-type and cdc37–34 mutant strains. (A) Titration of [3H]R1881 in yeast cells expressing AR. Wild-type (WT; squares) and cdc37–34 mutant cells (triangles) were tested at 25°C (open symbols) and 37°C (closed symbols). (B) Hydroxyflutamide competition assay. hsp82G170D mutant and cdc37–34 mutant cells were incubated at 25°C (lanes 1, 2, 5, and 6, respectively) or at 37°C (lanes 3, 4, 7, and 8, respectively) with (even lanes) or without (odd lanes) 250-fold excess of hydroxyfutamide in the presence of 100 nM [3H]R1881. Results are expressed as a percentage of the [3H]R1881 binding in the presence of hydroxyflutamide (OHF).
This was confirmed using a ligand competition assay with the anti-androgen hydroxyflutamide. This drug is a poor competitor of androgens when the AR is in functional association with Hsp90, but a potent competitive inhibitor upon Hsp90 loss of function (Fang et al., 1996). Ligand competition was performed by incubating yeast cultures with [3H]R1881 in the presence or absence of a 250-fold excess of unlabeled hydroxyflutamide. As a positive control, this experiment was performed with an hsp82 mutant (Nathan and Lindquist, 1995) that displayed conditional competition by hydroxyflutamide (Fang et al., 1996). At the permissive temperature, little competition by hydroxyflutamide occurred in the hsp82 mutant, but at the restrictive temperature a 250-fold excess of hydroxyflutamide reduced specific binding of R1881 by 80% (Figure 5B). In cdc37–34, however, there was negligible competition by hydroxyflutamide at permissive or restrictive temperatures. This lack of competition suggests that the integrity of the high-affinity hormone-binding state is maintained in the cdc37–34 strain.
Transactivation by GR in the cdc37–34 Mutant
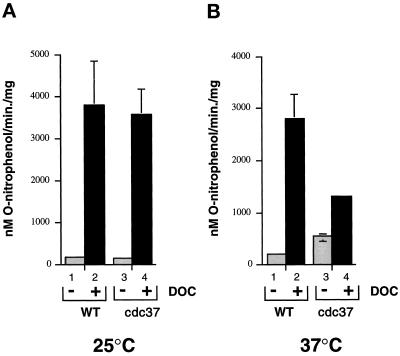
Hormone-dependent transactivation experiments were performed with GR to determine whether the defect in AR activation was general to other steroid hormone receptors. For these studies, a plasmid constitutively expressing full-length rat GR was transformed into wild-type and cdc37–34 strains containing the same reporter plasmid used for the AR studies. The androgen response elements contained in this plasmid also correspond to consensus GR-binding sequences (Ham et al., 1988), indicating that activated GR would induce lacZ gene expression. This was found to be the case, since β-galactosidase activity was induced by DOC to similar levels compared with the wild-type containing AR at 25°C (compare Figure 2A, lanes 1 and 2 with Figure 6, lanes 1 and 2). There was some difference in induction at 37°C, however, since β-galactosidase activity was not enhanced over the levels observed at 25°C, as was found with AR (see Figure 2), but was reduced slightly. The reason for this is unclear since greater induction by GR at 37°C compared with 25°C has been observed in other yeast strains (Nathan and Lindquist, 1995).
Figure 6.
Transactivation by GR in wild-type and cdc37–34 mutant strains. (A) β-Galactosidase activity in wild-type (WT; lanes 1 and 2) and cdc37–34 (lanes 3 and 4) mutant strains containing GR. Cultures were incubated at 25°C (A) or 37°C (B) for 1 h before addition of DOC to 100 nM. Samples in lanes 1 and 3 contained no hormone. Results are the mean of three independent experiments.
When induction in the wild-type and cdc37–34 mutant was compared, there were also some surprising differences that contrasted with the AR results shown in Figure 2. The major difference was the finding that GR induction at the permissive temperature (25°C) in the cdc37–34 and wild-type strains was similar (approximately 30-fold above the background in both cases, Figure 6A). At 37°C, however, the levels of induced β-galactosidase were reduced by twofold in the mutant compared with the wild type (Figure 6B). The background β-galactosidase levels were also threefold higher in the mutant incubated at 37°C compared with the same strain incubated at 25°C or the wild type at either temperature. Since the background levels were increased, the induction ratio in the mutant strain was only 2.5-fold compared with a mean 15-fold induction in the wild-type strain. Similar background increases were not observed with AR in this strain.
DISCUSSION
In this report, the yeast CDC37 gene has been shown to have a differential function in the hormone-dependent transactivation by heterologously expressed human AR and rat GR. AR function was reduced severely at both permissive and restrictive temperatures in the cdc37–34 mutant, whereas GR activity was similar to the wild type at the permissive temperature and only mildly reduced at the restrictive temperature. The cdc37–34 allele was also shown to be partially dominant negative for AR activation and also for the levels of wild-type Cdc37 protein (Figures 2 and 3). By correlation, therefore, the AR appears to require at least wild-type levels of Cdc37 protein for its activation, whereas the GR can apparently function quite well even with substantially reduced levels of Cdc37p. This is consistent with previous observations in which the GR was not found to associate with p50/Cdc37 (Stancato et al., 1993).
Similar activation differences between steroid hormone receptors have also been observed in yeast cells expressing mutant forms of Hsp90. The most striking example of this was the hsp82E431K mutant, in which GR activation was reduced to 6% of the wild-type level without affecting hormone-dependent activation of estrogen receptors (ER), mineralcorticoid receptors (MR), or progesterone receptors (PR), whereas the activity of all four receptors was severely reduced in the hsp82G313N mutant (Bohen and Yamamoto, 1993). In other studies, GR activation was severely affected in the hsp82G170D mutant (Nathan and Lindquist, 1995), whereas AR activation was reduced in the same strain by only threefold in the presence of saturating concentrations of hormone (Fang et al., 1996). These examples reflect upon the allele specificity of Hsp90 function in receptor activation, and while the origins of this specificity remain unclear, they demonstrate that individual steroid hormone receptors have distinct properties relating to activation.
Our results provide the first demonstration for Cdc37p function in steroid hormone receptor regulation. In several previous reports, p50/Cdc37p was observed to function only in the regulation of protein kinases (Gerber et al., 1995; Valay et al., 1995; Dey et al., 1996; Stepanova et al., 1996), although other roles have been proposed (Grammatikakis et al., 1995). This raises the possibility that Cdc37p may act indirectly on the AR via a protein kinase. In transfection studies using animal cells, however, deletion of the major phosphorylation sites failed to reduce AR transactivation by more than 30% (Jenster et al., 1994; Zhou et al., 1995). This suggests that phosphorylation does not play a major regulatory role in hormone-dependent gene regulation, although it may be important for hormone-independent mechanisms of activating AR (see Nazareth and Weigel, 1996). Thus, if Cdc37p does not function in AR activation indirectly via a protein kinase, it is probably acting directly on the AR itself, perhaps in association with Hsp90 via the ligand-binding domain. In support of this hypothesis, we have observed Cdc37p to coisolate with His-tagged Hsp90 from yeast cell extracts (our unpublished observations).
Steroid hormone receptors are regulated by the sequential binding of distinct Hsp90 protein subcomplexes. In the case of PR, these bind in a defined order and establish the high affinity hormone-binding state (Smith, 1993; Smith et al., 1995). Hsp90 dissociates in the presence of hormone, which is followed by receptor dimerization and DNA binding. How Cdc37p might fit into this pathway is unclear, since its loss of function did not significantly affect hormone binding to AR (Figure 5), and it does not appear to associate with unliganded steroid hormone receptors (Whitelaw et al., 1991; Stancato et al., 1993; Nair et al., 1996). This leaves open the possibility, however, that Cdc37p functions in the activation pathway downstream of hormone binding. If Cdc37p functions at such a late stage in receptor activation, its action may be related to the conversion of a ligand-bound but inactive receptor to one that is active. Little is known of the allosteric changes which mediate receptor activation. However, since Cdc37p functions via the hormone-binding domain, it is possible that it facilitates some of the structural changes that result in receptor activation.
Previous reports have demonstrated a role for Cdc37 in regulation of protein kinase activity. In the cdc37–1 mutant for example, Cdc28p levels are reduced and complexation with cyclins is impaired (Gerber et al., 1995); furthermore, the activity of the Mps1 kinase is also reduced in the same mutant (Schutz et al., 1997). In the cdc37–34 mutant, p60v-src levels are reduced and the protein aggregates at the restrictive temperature. Also, Kimura et al. (1997) have recently demonstrated that Cdc37p acts as a molecular chaperone by stabilizing partially folded proteins. Perhaps, therefore, Cdc37 acts in a similar manner on the AR, although it appears not to be as important for GR activation.
Several aspects of p60v-src and steroid hormone receptor regulation appear to be very similar. Activation of both requires almost identical conditions and protein components, including the molecular chaperones Hsp90, Hsp70, and dnaJ homologues (Smith et al., 1992, Hutchison et al., 1994, Kimura et al., 1995; Dey et al., 1996a). Also, complexes formed between Hsp90 and protein kinases or steroid hormone receptors are stabilized by molybdate ions and both are sensitive to the action of geldanamycin (Hutchison et al., 1992; Stancato et al., 1993; Johnson and Toft, 1995; Smith et al., 1995; Whitesell et al., 1994; Schulte et al., 1995). These similarities suggest that a single pathway exists for the regulation of different types of signal transduction molecules by the Hsp90 chaperone machine. The experimental detection of specific Hsp90 co-chaperones may therefore depend on the relative kinetics of their action, or their stability in multi-chaperone complexes.
Further studies will be required to distinguish between these possible modes of Cdc37p action in AR and GR activation. The studies described in this report suggest, however, that hormone binding to the AR does not by itself lead to activation. Instead, there appear to be distinct events which involve the action of Cdc37p. What these steps are and which other components of the Hsp90 chaperone machinery are involved remain to be determined.
ACKNOWLEDGMENTS
The authors thank Dr. Robert Donnelly (New Jersey Medical School) for DNA sequencing and Dr. Claiborne Glover for discussions and communication of results prior to publication. We also thank Drs. Susan Lindquist, Elizabeth Wilson, and Keith Yamamoto for plasmids and yeast strains. This work was supported by grants from the National Institutes of Health (R01 DK-49065) and the Sinsheimer Foundation to A.J.C.
REFERENCES
- Bohen S P, Yamamoto K R. Modulation of steroid receptor signal transduction by heat shock proteins. In: Morimoto R, Tissieres A, Georgopoulos C, editors. The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Press; 1994. pp. 313–334. [Google Scholar]
- Bohen SP, Kralli A, Yamamoto KR. Hold ‘em and fold ‘em: chaperones and signal transduction. Science. 1995;268:1303–1304. doi: 10.1126/science.7761850. [DOI] [PubMed] [Google Scholar]
- Boschelli F, Uptain SM, Lightbody JL. The lethality of p60v-src in Saccharomyces cerevisiae and the activation of p34CDC28 kinase are dependent on the integrity of the SH2 domain. J Cell Sci. 1993;105:519–528. doi: 10.1242/jcs.105.2.519. [DOI] [PubMed] [Google Scholar]
- Brugge JS. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–21. doi: 10.1007/978-3-642-70810-7_1. [DOI] [PubMed] [Google Scholar]
- Caplan AJ. Yeast molecular chaperones and the mechanism of steroid hormone action. Trends Endocrinol Metab. 1997;8:271–276. doi: 10.1016/s1043-2760(97)00079-9. [DOI] [PubMed] [Google Scholar]
- Caplan AJ, Langley E, Wilson EM, Vidal J. Hormone dependent transactivation by the human androgen receptor is regulated by a dnaJ protein. J Biol Chem. 1995;270:5251–5257. doi: 10.1074/jbc.270.10.5251. [DOI] [PubMed] [Google Scholar]
- Cutforth T, Rubin GM. Mutations in Hsp83 and cdc37 impair signalling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994;77:1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Dai, Kobayashi K, R, Beach D. Physical interaction of mammalian Cdc37 with CDK4. J Biol Chem. 1996;271:22030–22034. doi: 10.1074/jbc.271.36.22030. [DOI] [PubMed] [Google Scholar]
- Dey B, Caplan AJ, Boschelli F. The YDJ1 molecular chaperone facilitates formation of active p60v-src in yeast. Mol Biol Cell. 1996a;7:91–100. doi: 10.1091/mbc.7.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B, Lightbody JJ, Boschelli F. CDC37 is required for p60v-src activity in yeast. Mol Biol Cell. 1996b;7:1405–1417. doi: 10.1091/mbc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Fliss AE, Robins DM, Caplan AJ. Hsp90 regulates androgen receptor hormone binding affinity in vivo. J Biol Chem. 1996;271:28697–28702. doi: 10.1074/jbc.271.45.28697. [DOI] [PubMed] [Google Scholar]
- Florio M, Wilson LK, Trager JB, Thorner J, Martin GS. Aberrant protein phosphorylation at tryosine is responsible for the growth-inhibitory action of pp60v-src expressed in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 1994;5:283–296. doi: 10.1091/mbc.5.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geitz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- Gerber M, Farrell A, Deshaies RJ, Herskowitz I, Morgan DO. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc Nat Acad Sci USA. 1995;92:4651–4655. doi: 10.1073/pnas.92.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatikakis K, Grammatikakis A, Yoneda M, Yu Q, Banerjee SD, Toole BP. A novel glycosaminoglycan-binding protein is the vertebrate homologue of the cell cycle control protein Cdc37. J Biol Chem. 1995;270:16198–16205. doi: 10.1074/jbc.270.27.16198. [DOI] [PubMed] [Google Scholar]
- Ham J, Thomson A, Needham M, Webb P, Parker M. Characterization of response elements for androgens, glucoccorticoids and progesterone in mouse mammary tumour virus. Nucleic Acids Res. 1988;16:5263–5277. doi: 10.1093/nar/16.12.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Poon RY C. Cdc37: a protein kinase chaperone? Trends Cell Biol. 1997;7:157–161. doi: 10.1016/S0962-8924(97)01027-1. [DOI] [PubMed] [Google Scholar]
- Hutchison KA, Brott BK, DeLeon JH, Perdew GH, Jove R, Pratt WB. Reconstitution of the multiprotein complex Pp 60src, hsp90 and p50 in a cell free system. J Biol Chem. 1992;267:2902–2908. [PubMed] [Google Scholar]
- Hutchison KA, Dittmar KD, Czar MJ, Pratt WB. Proof that Hsp70 is required for assembly of the glucocorticoid receptor into a heterocomplex with Hsp90. J Biol Chem. 1994;269:5043–5049. [PubMed] [Google Scholar]
- Jenster G, de Ruiter PE, van der Korput HAGM, Kuiper GGJM, Trapman J, Brinkmann AO. Changes in the abundance of the androgen receptor isotypes: effects of ligand treatment, glutamine stretch variation and mutation of putative phosphorylation sites. Biochemistry. 1994;33:14064–14072. doi: 10.1021/bi00251a015. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Toft DO. Binding of p23 and hsp90 during assembly with the progesterone receptor. Mol Endocrinol. 1995;9:670–678. doi: 10.1210/mend.9.6.8592513. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yahara I, Lindquist S. Role of the protein chaperone YDJ1 in establishing Hsp90 mediated signal transduction pathways. Science. 1995;268:1362–1365. doi: 10.1126/science.7761857. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Rutherford SL, Miyata Y, Yahara I, Freeman BC, Yue L, Morimoto RI, Lindquist S. Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 1997;11:1775–1785. doi: 10.1101/gad.11.14.1775. [DOI] [PubMed] [Google Scholar]
- Mariovet S, Van dijck P, Verhoeven G, Heyns W. Interaction of the 90-kDa heat shock protein with native and in vitro translated androgen receptor and receptor fragments. Mol Cell Endocrinol. 1992;88:165–174. doi: 10.1016/0303-7207(92)90021-w. [DOI] [PubMed] [Google Scholar]
- Nair SC, Toran EJ, Rimerman RA, Hjermstad S, Smithgall TE, Smith DF. A pathway of multi-chaperone interactions common to diverse regulatory proteins; estrogen receptor, fes tyrosine kinase, heat shock transcription factor HSF1 and the arylhydrocarbon receptor. Cell Stress Mol Chap. 1996;1:237–250. doi: 10.1379/1466-1268(1996)001<0237:apomci>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DF, Lindquist S. Mutational analysis of Hsp90 function: interactions with a steroid hormone receptor and protein kinase. Mol Cell Biol. 1995;15:3917–3925. doi: 10.1128/mcb.15.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- Perdew GH, Whitelaw ML. Evidence that the 90-kDa heat shock protein (Hsp90) exists in cytosol in heteromeric complexes containing Hsp70 and three other proteins with Mr of 63,000, 56,000 and 50,000. J Biol Chem. 1991;266:6708–6713. [PubMed] [Google Scholar]
- Perdew GH, Wiegand H, Heuval JPV, Mitchell C, Singh SS. A 50 kilodalton protein associated with Raf and pp60v-src protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry. 1997;36:3600–3607. doi: 10.1021/bi9612529. [DOI] [PubMed] [Google Scholar]
- Purvis IJ, Chotai D, Dykes CW, Lubahn DB, French FS, Wilson EM, Hobden AN. An androgen-inducible expression system for Saccharomyces cerevisiae. Gene. 1991;106:35–42. doi: 10.1016/0378-1119(91)90563-q. [DOI] [PubMed] [Google Scholar]
- Schena M, Yamamoto KR. Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science. 1988;241:965–967. doi: 10.1126/science.3043665. [DOI] [PubMed] [Google Scholar]
- Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf1-Hsp90 molecular complex results in destabilization of raf-1 and loss of raf1-ras association. J Biol Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- Schutz AR, Giddings TH, Jr, Steiner E, Winey M. The yeast Cdc37 gene interacts with MPS1 and is required for proper excecution for spindle pole body duplication. J Cell Biol. 1997;136:969–982. doi: 10.1083/jcb.136.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993;7:1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- Smith DF, Stensgard BA, Welch WJ, Tott DO. Assembly of progesterase receptor with heat shock proteins and receptor activation are ATP-mediated events. J Biol Chem. 1992;267:1350–1356. [PubMed] [Google Scholar]
- Smith DF, Whitesell L, Nair SC, Chen S, Prapapanich V, Rimmerman RA. Progesterone receptor structure and function altered by geldanamycin, an hsp90 binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancato LF, Chow Y-H, Hutchison KA, Perdew GH, Jove R, Pratt WB. Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J Biol Chem. 1993;268:21711–21716. [PubMed] [Google Scholar]
- Stepanova L, Leng X, Parker SB, Harper JW. Mammalian p50/Cdc37 is a protein kinase-targeting subunit of hsp90 that binds and stabilizes cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- Thomas BJ, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Valay J-G, Simon M, Dubois M-F, Bensaude O, Facca C, Faye G. The KIN28 gene is required both for RNA polymerase II mediated transcription and phosphorylation of the Rbp1 CTD. J Mol Biol. 1995;249:535–544. doi: 10.1006/jmbi.1995.0316. [DOI] [PubMed] [Google Scholar]
- Whitelaw ML, Hutchison K, Perdew GH. A 50-kDa protein complexed with the 90-kDa heat shock protein (hsp90) is the same protein complexed with Pp 60v-src hsp90 in cells transformed by the rous sarcoma virus. J Biol Chem. 1991;266:16436–16440. [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein Hsp90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z-X, Kemppainen JA, Wilson EM. Identification of the three proline directed phosphorylation sites in the human androgen receptor. Mol Endocrinol. 1995;9:605–615. doi: 10.1210/mend.9.5.7565807. [DOI] [PubMed] [Google Scholar]