Abstract
NURR1 is a transcription factor essential for the development, survival, and functional maintenance of midbrain dopaminergic (DAergic) neurons and NURR1 is a potential susceptibility gene for Parkinson’s disease (PD). To determine whether NURR1 gene expression is altered in patients with PD we measured its expression in human peripheral blood lymphocytes (PBL) in 278 patients with PD, 166 healthy controls (HC), and 256 neurological disease controls (NDC) by quantitative real-time PCR. NURR1 gene expression was significantly decreased in patients with PD (particularly those with family history of PD) as compared with HC (p < 0.01) and also as compared with NDC (p < 0.05). There was no significant difference in NURR1 gene expression among PD patients with or without anti-PD medications. When adjusted for gender, age, and ethnicity, lower levels of NURR1 gene expression were associated with significantly increased risk for PD in women, in patients 60 years old or older, and in patients of Caucasian origin. The observed reduction in PBL NURR1 gene expression indicates possible systemic involvement in PD, and the finding may help identify individuals with PD and other disorders associated with impaired central DAergic system.
Keywords: Parkinson’s disease, dopaminergic neurons, transcription factor, biomarkers, peripheral blood lymphocytes
1. Introduction
Parkinson's disease (PD) is a common neurodegenerative disease in adults, characterized by the presence of rigidity, tremor, bradykinesia, and postural instability. The loss of dopaminergic (DAergic) neurons in the substantia nigra pars compacta (SNc), the pathological hallmark of PD, leads to marked depletion of striatal dopamine (DA) [1]. The clinical features are often subtle at onset, and it often takes several years before the disease becomes fully manifested [2]. Because of the inherent difficulty in studying PD pathogenic mechanisms in the central nervous system (CNS) in vivo, research interests have, therefore, focused on other means of early diagnosis, including quantitative assays of cerebrospinal fluid proteomics, serum or plasma metabolomics, and gene expression profile in peripheral blood lymphocytes (PBL) in an attempt to identify potential peripheral biomarkers of the disease [3–12]. For example, α-synuclein levels in cerebrospinal fluid were found to decrease with aging and are significantly reduced in patients with PD [8]. Uric acid levels in serum were negatively correlated with the risk of PD and the progression of the illness [7, 9, 11]. Using high performance liquid chromatography coupled with electrochemical coulometric array detection to search for plasma biomarkers from metabolomic profiling, Bogdanov et al [12] found a complete separation of 25 controls from 66 PD and a significant reduction of uric acid and elevation of glutathione in the plasma of PD patients. Validation of these findings, however, is needed with assays to run in a “blinded” fashion, using a larger number of PD and healthy controls (HC), and comparing PD patients with various neurological disease controls (NDC).
Several studies have used PBL to measure specific changes in DA components, enzyme activities, DA receptors and DA transporters in patients with PD [3, 4, 5, 13, 14]. In addition to the abnormalities in these peripheral markers, a significant increase in caspase-3 activity [15] and a significant decrease in mitochondrial complex I activity [16] has been found in PBL of patients with PD. Furthermore, a systematic scan of genome-wide expression in PBL of PD patients and HC identified cochaperone protein ST13 as a potential molecular marker of early PD [10]. Based on these and other data, it has been proposed that PBL represents a useful reservoir for detecting changes possibly indicative of disease-related pathogenesis occurring in the brains of patients with PD.
NURR1, a member of the steroid/thyroid nuclear superfamily is essential for the development, survival, and functional maintenance of midbrain DAergic neurons [17–19]. It is highly expressed in midbrain DAergic neurons [20] as well as other tissues, including PBL [21]. We previously showed that NURR1-null mice have selective agenesis of DAergic neurons in the SN and ventral tegmental area [22]. We have also demonstrated that aged heterozygous NURR1-null mice (NURR1−/−) have fewer DAergic neurons in the SN [23] and reduced expression of NURR1 increased the vulnerability of mesencephalic DAergic neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced injury [24]. Furthermore, our genetic studies and other reports in patients with PD have indicated that NURR1 might be a potential susceptibility gene for PD [25–29].
To explore the relevance of peripheral NURR1 gene expression in PD, we conducted a pilot study using PBL collected from 113 PD patients and 42 HC and found a significant reduction in NURR1 mRNA levels in PD patients vs HC (p < 0.001) [30]. To validate this finding we designed another study, using quantitative measurements of NURR1 mRNA in a double-blind manner and in a larger population of PD patients, HC, and various NDC. The aims of this study were to determine (1) whether NURR1 gene expression in PBL is specifically reduced in PD as compared with HC and NDC; (2) whether the NURR1 expression can be used to help identify PD, and (3) whether age, gender, anti-PD medications, or disease severity affects the expression of NURR1.
2. Subjects and methods
2.1. Subjects
We collected a total of 700 PBL samples: 278 from patients with PD (sporadic PD 178; familial PD 100), 166 from HC (138 from spouse of patients with PD, 15 from the spouse of patients with other neurological diseases, and rest of them from the people working at the Department of Neurology, BCM), and 256 from various non-PD NDC which consist of 53 non-movement neurological disorders (14 cerebrovascular disease, 11 Alzheimer disease (AD), 9 multiple sclerosis, 8 seizure, 6 migraine, and 5 peripheral neuropathy), and 203 movement disorder controls, 120 essential tremor (ET), 41 restless leg syndrome (RLS), 21 parkinsonism syndromes including progressive supranuclear palsy, multiple system atrophy, and cortical basal degeneration, and 21 primary or secondary dystonia. Blood samples were collected at the Parkinson’s Disease Center and Movement Disorders Clinic (PDCMDC), Baylor College of Medicine (BCM). The diagnosis of PD was made by one of the authors (JJ) or his colleagues, and was based on established diagnostic criteria [2, 31], and their disease severity was assessed by the Unified Parkinson’s Disease Rating Scale (UPDRS) [32]. Patients with non-PD neurological diseases were evaluated by the members of the Department of Neurology, BCM. All subjects or their legally authorized caregivers signed a written consent, approved by the Institutional Review Board for Human Subject Research for BCM and Affiliated Hospitals.
2.2. Materials and Procedures
Human peripheral blood (10 ml) was drawn from cubital vein into a heparinized plastic syringe. The clinic nurse coded the samples in a blind fashion and delivered them to a research technician who performed the PBL separation using Ficoll/Paque (Pharmacia LKB) method no later than 2 hours after drawing. The resulting pellet was frozen immediately at −80°C until RNA preparation.
Total RNA was extracted from PBL by spin or vacuum total RNA isolation system (Promega Corporation, Madison, WI, USA). One microgram of total RNA from PBL was reverse transcribed into first-strand cDNA by using iScript™ cDNA synthesis kit (Bio-Rad). The cDNA was then assigned in a new code and sent to a research associate in a blind fashion to perform the quantitative measurement of NURR1 gene expression.
2.3. Real-time PCR assay of NURR1 gene expression against internal control GAPDH
The fluorescent real-time PCR reaction was carried out in the Bio-Rad iCycler System (Bio-Rad) with a final volume of 25 µl for each reaction containing with the specific primers targeting human NURR1 (forward: 5’-TCCAACGAGGGGCTGTGCG-3’; reverse: 5’-CACTGTGCGCT TAAAGAAGC-3’) and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH, forward: 5’-GAAGGTGAAGGTCGGAGTC-3’, reverse: 5’-GAAGATGGTGATGGGATTTC-3’). Human GAPDH gene was used as internal control. After 95°C for 3 min, the experimental reaction consists of 40 cycles of 95°C for 15 sec, 59.5°C for 60 sec, the PCR products were detected by the fluorescent probe 5′6-FAM CGGCCTGCCAACACTACGGCGT BHQ-1 3′ (Biosearch Technologies, CA, IDT Inc.) for NURR1 and 5’Texas red CAAGCTTCCCGTTC TCAGCCBHQ-2 3’, (Biosearch Technologies, CA) for GAPDH. The value of threshold cycle (Ct) was generated at every cycle during a run. Fluorescent reading from real-time PCR reaction was quantitatively analyzed by determining the difference of Ct (delta Ct) between Ct of NURR1 and GAPDH, and the NURR1 gene expression was determined by the formation of 2−delta Ct.
2.4. Statistical analysis
After completion of the assays the data was analyzed by an independent statistician (Dr. Maosheng Huang, Department of Epidemiology, M.D. Anderson Cancer Center, Houston, TX). All statistical analysis was performed with the Stata 8.0 Statistical Software Package (Stata Corporation, College Station, TX). The X2 test was used to test for differences between the PD patients and the control subjects in the distributions of gender and ethnicity. A student’s t test or a Wilcoxon Rank-Sum (Mann-Whitney test) was used to test for differences between the two groups in the distribution of age and gene expression. Odds ratios (OR) and 95% confidence intervals (CIs) were calculated as estimates of relative risk by dichotomizing the levels of NURR1 gene expression. One- or two-way ANOVA was performed to evaluate the differences in the mean value of the relative NURR1 gene expression. Unconditional multivariable logistic regression was performed to control for possible confounding by age, gender, and ethnicity, where appropriate. All statistical tests were two-sided. Spearman correlation coefficients were computed to evaluate the correlations among age, duration (year), or the UPDRS scores of PD, and the levels of NURR1 gene expression. P values lower than 0.05 were considered significant.
3. Results
3.1. Characteristics of study population
The demographic characteristics of each group are summarized in Table 1. Among all subjects, 53.0% female; 82.6% were Caucasian, 9.8% Hispanic, 5.6% African-American, and 2.5% represented other races.
Table 1.
Characters of demographic distribution of study population
| Groups | Number (%) |
Gender Male: Female |
P value |
P value |
Ethnic Caucasian: others |
P value |
P value |
Age (years) (Mean±SD) |
P value |
P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy Control | 166 (23.5) | 53:113 | Ref | 142 :24 | Ref | 56.3 ± 12.8 | Ref | |||
| PD | 278 (39.5) | 171:107 | <0.01 | Ref | 240:38 | NS | Reference | 60.1 ± 10.6 | <0.01 | Ref |
| sPD | 178 (25.4) | 110:68 | NA | NA | 155:23 | NA | NA | 58.6 ± 11.2 | NA | NA |
| fPD | 100 (14.2) | 61:39 | NA | NA | 85:15 | NA | NA | 60.8 ± 9.6 | NA | NA |
| NDC | 256 (36.5) | 111:145 | NA | <0.01 | 234:22 | <0.05 | NS | 59.8 ± 11.6 | <0.05 | NS |
| Non-MDC | 53 (7.6) | 28:25 | <0.01 | NS | 47:6 | NS | NS | 60.3 ± 13.6 | NS | NS |
| MDC | 203 (29) | 83:120 | NS | <0.001 | 187:16 | <0.05 | <0.05 | 59.2 ± 11.7 | <0.05 | NS |
X2 and Student-t test. Ref=reference; NA= not analyzed; NS = not significant; PD = Parkinson’s disease sPD = sporadic PD; fPD = familial PD; Non-MDC = non movement disease control; MDC = movement disease control.
3.2. NURR1 gene expression on PBL of all study groups
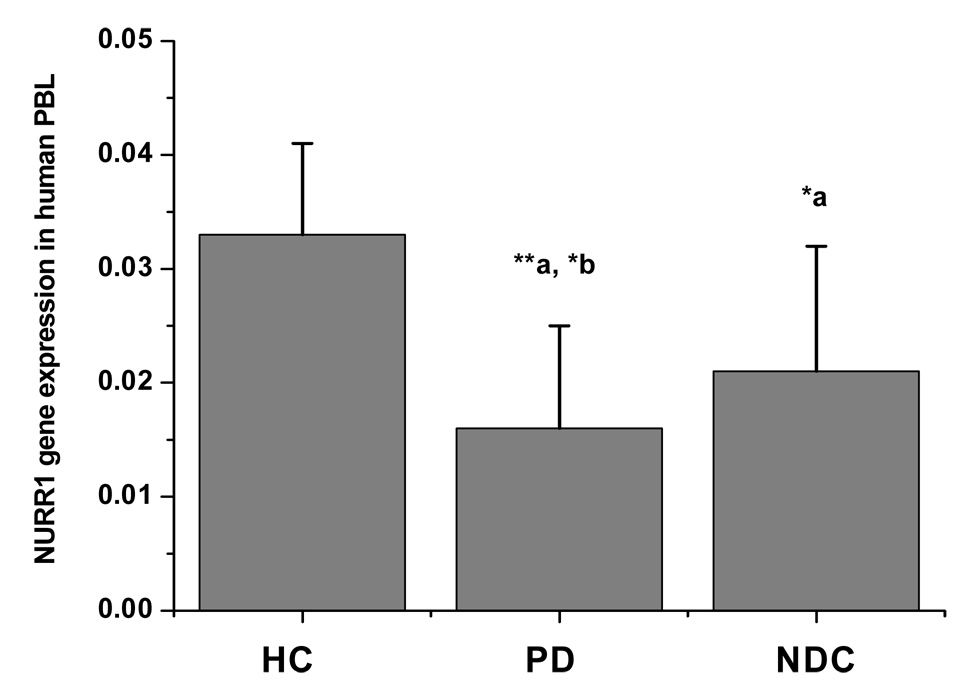
The mean level of NURR1 gene expression in PBL in patients with PD was 52% lower than healthy controls (p < 0.01), and 25% lower than all NDC (p < 0.05; Figure 1; Table 2). The decreased expression in NURR1 gene in PD was more robust in patients with familial history of PD and the difference between sPD and fPD was statistically significant (p < 0.05; Table 2). There was also a significant difference of NURR1 expression when HC and NDC were compared (p < 0.05; Figure 1; Table 2) and when PD and other movement disorders were compared (p < 0.05; Table 2).
Figure 1.
NURR1 gene expression on PBL in different study groups. Fluorescent reading from real-time PCR reaction was quantitatively analyzed by determining the difference of Ct (delta Ct) between Ct of NURR1 and GAPDH, and the NURR1 gene expression was determined by the formation of 2−delta Ct. The level of NURR1 gene expression was markedly low in patients with PD (n = 278) and moderately low in various neurological disease controls (NDC, n = 256) as compared with healthy controls (HC, n = 166). Data presented as mean ± SEM from a total of 700 subjects in duplication assay measured in blind fashion. * p < 0.05 and ** p < 0.01 vs. HC (a), vs. NDC (b).
Table 2.
NURR1 gene expression vs GAPDH as internal control in all groups
| Group | Number | NURR1 mRNA mean ± SEM |
P value | P value | P value |
|---|---|---|---|---|---|
| Healthy control | 166 | 0.033 ± 0.008 | Reference | ||
| PD | 278 | 0.016 ± 0.009 | <0.01 | Reference | |
| sPD | 178 | 0.018 ± 0.008 | <0.05 | NA | Reference |
| fPD | 100 | 0.013 ± 0.010 | <0.01 | NA | <0.05 |
| NDC | 256 | 0.021 ± 0.011 | <0.05 | <0.05 | NA |
| Non-MDC | 53 | 0.019 ± 0.014 | NS | NS | NA |
| MDC | 203 | 0.022 ± 0.012 | <0.05 | <0.05 | NA |
| ET | 120 | 0.027 ± 0.018 | NS | <0.05 | NA |
| RLS | 41 | 0.009 ± 0.007 | <0.05 | NS | NA |
| Parkinsonism | 21 | 0.022 ± 0.016 | NS | NS | NA |
| Dystonia | 21 | 0.013 ± 0.010 | NS | NS | NA |
Data are the means ± SEM from 700 subjects in duplication assay and mRNA levels were determined in blind fashion. Fluorescent reading from real-time PCR reaction was quantitatively analyzed by determining the difference of Ct (delta Ct) between Ct of NURR1 and GAPDH, and the NURR1 gene expression was determined by the formation of 2−delta Ct. PD = Parkinson’s disease; sPD = sporadic PD; fPD = familial PD; NDC = neurological disease control; Non-MDC = non-movement disease control; MDC = movement disease control; ET = essential tremor; RLS = rest leg syndrome; NA= Not analyzed; NS = not significant. *Wilcoxon Rank-Sum (Mann-Whiney Test)
3.3. PD risk estimate after adjusting age, gender and ethnicity
Since the demographic distribution of gender, age and ethnicity was significantly different among all study groups, we determined the odds ratio (OR) values to estimate the relative risk of PD after adjusting for age, gender and ethnicity where appropriate. Using HC as reference, we found that the OR was significantly increased in the overall PD population (p < 0.001). The estimated relative risk for patients with PD was significantly increased in both male (p < 0.05) and female patients (p < 0.01), but was greater in females (Table 2 and Table 3). The risk effect of the level of NURR1 gene expression appeared to be greater in older subjects (p < 0.01, age ≥60 years, and p < 0.05 age <60 years), and in subjects of Caucasian origin (p < 0.001). No difference in risk of was found in non-movement disorder controls or other movement disorder controls when adjusted for age and gender (Table 3).
Table 3.
Relative risk estimates of PD for the levels of PBL NURR1 gene expression
| PD-OR | Non-MDC-OR | MDC-OR | ||||||
|---|---|---|---|---|---|---|---|---|
| Risk estimate in HC and PD groups | Risk estimate in HC and NDC groups | Risk estimate in HC and NDC groups | ||||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||||
| Overall: | Overall: | Overall: | ||||||
| 1.66 (1.02–2.10) | <0.001 | NS | NS | |||||
| Gender | Gender | Gender | ||||||
| Male | 1.44 (0.99–1.89) | <0.05 | Male | NS | Male | NS | ||
| Female | 1.78 (1.01–2.16) | <0.01 | Female | NS | Female | NS | ||
| Age | Age | Age | ||||||
| <60y | 1.69 (1.00–2.21) | <0.05 | <60y | NS | <60y | NS | ||
| >=60y | 1.85 (1.00–2.29) | <0.01 | >=60y | NS | >=60y | NS | ||
| Race | Race | Race | ||||||
| Caucasian | 1.56 (1.02–2.10) | <0.001 | Caucasian | NS | Caucasian | NS | ||
| Other | NS | Other | NS | Other | NS | |||
OR = odds ratio; NS = not significant. The relative risks for patients with PD, NDC, non-movement disease controls (Non-MDC), and movement disease controls (MDC) were estimated by OR value in overall sample after adjusted by age, gender and ethnicity.
3.4. The influence of medication
Among 278 PD patients, 65 were of recent-onset and not yet treated with anti-PD medications (“de novo” PD), the remaining 213 patients were treated with anti-PD medications, including 58 treated with DA receptor agonists, usually pramipexole or ropinirole, 66 were treated with l-dopa, and other 89 patients were treated with the combination of DA agonists and l-dopa. There was no significant influence of medication on NURR1 expression (Table 4).
Table 4.
The influence of medications on NURR1 expression
| Medication history | Number | Male:Female | Age |
NURR1 mRNA mean ± SD |
P value |
|---|---|---|---|---|---|
| de novo | 65 | 38:27 | 57.4 ± 9.8 | 0.018 ± 0.012 | Reference |
| Treated | 213 | 133:80 | 61.6 ± 11.2 | 0.015 ± 0.011 | NS |
| DA agonist | 58 | 38:20 | 60.9 ± 12.3 | 0.013 ± 0.012 | 0.068 |
| l-dopa | 66 | 35:31 | 60.1 ± 10.9 | 0.017 ± 0.014 | NS |
| DA agonist + l-dopa | 89 | 60:29 | 61.8 ± 11.8 | 0.015 ± 0.012 | 0.072 |
NS = not significant.
p values were derived from Student's t-test for gene expression.
3.5. Correlation of disease duration and severity with NURR1 expression in PD
We performed a correlation analysis between disease duration (years after onset of disease symptoms) and severity (total UPDRS score) in 278 patients with PD. There was no significant correlation between the duration of PD and the level of NURR1 gene expression (r=−0.03, p > 0.05), and there was no correlation between the NURR1 gene expression and UPDRS scores (r=−0.02, p > 0.05).
4. Discussion
Although PD is typically considered an age-related neurodegenerative disorder, with symptoms usually starting in the sixth decade of life, the biology of the disease may start decades prior to clinical manifestation [2,32]. Therefore, disease-specific and sensitive biomarkers would be very useful in identifying individuals at risk for the disease at the pre-clinical stage and to serve as potential surrogate markers of disease progression.
Mutations or variations in genes coding for transcription factors involved in regulation of neuronal development and maintenance of nigrostriatal system function may be risk factors for PD [19,33]. NURR1 is of great interest since this gene is highly expressed in the SN and down-regulation of its expression has been shown to cause relatively specific nigro-striatal DAergic dysfunction in animal model [18–20,22–24,33,34]. Furthermore, at least three point mutations in NURR1 gene have been found in association with PD [26,28,29]. We initially reported that mutations in exon (−291Tdel and −245T→G sequence variation in 5’ untranslated region) in familial PD cases [26]. A third novel mutation (heterozygous C-to-G transversion resulting in serine-to-cysteine substitution) in the translated region of exon 3 was found in a non-familial PD resulting in a change of p.Ser125Cys adjacent to ERK1/2 phosphorylation site and subsequently leading to a marked reduction of Nurr1-induced transcriptional activation [28,29]. A polymorphism in NURR1 intron 6 (NI6P) was found to confer a greater risk for PD and diffuse Lewy body disease [25,27]. Moreover, Nurr1 has been found to be down-regulated in the brains of aged individuals and in patients with PD [35,36], and such down regulation is highly correlated with the presence of α-synuclein containing inclusion bodies in SN [36].
In the present study, we determined (in a blinded fashion) levels of NURR1 mRNA in PBL of a large population of patients with PD, various NDC, and HC. We showed that the NURR1 gene expression in PBL in patients with PD is significantly decreased independent of medication, disease duration, or severity. This finding suggests that the decreased NURR1 gene expression in PBL reflects pre-existing disease-related changes rather than a surrogate marker for disease severity or progression. To further determine the association of age, gender and ethnicity of PD with the changes of NURR1 expression, we performed adjusted analyses of OR and found that the NURR1 gene expression was more significantly decreased in older and female patients and in those of Caucasian origin. The results should, however, be interpreted cautiously as the observed changes in PBL may not necessarily represent corresponding abnormalities in NURR1 gene expression in the brains of patients with PD. Despite these limitations we believe that our findings provide support for the notion that PBL can be used as a readily accessible tissue to study DAergic dysfunction relevant to PD pathogenesis.
In order to determine the specificity of reduced PBL NURR1 gene expression in PD, we also measured NURR1 gene expression in PBL in various NDC. The subgroup of movement disorder controls included ET, RLS, various non-PD parkinsonism syndromes, and dystonia. While not specific for PD, as the reduction of NURR1 gene expression may also occur in other disorders involving the DAergic system, the reduction of NURR1 gene expression in PBL is much less robust in other movement disorders and in non-movement disorders than in PD.
The analysis of our findings leads us to the following conclusions: (1) although NURR1 expression is significantly decreased in our PD patients as compared with HC and with a subgroup of disease controls this abnormality may not be specific for PD as it may be also found, although to a lesser degree, in other movement disorders, and it is not known whether the changes in NURR1 expression in PBL reflect any abnormalities in the gene expression in the brain; (2) the changes in NURR1 expression do not seem to correlate with disease progression; (3) patients with familial history seem to correlate with greater reduction in PBL NURR1 expression; (4) levels of NURR1 gene expression seem to be lower in women, in patients of 60 years old or older, and in subjects of Caucasian origin; and. (5) anti-PD medications have limited or no impact on NURR1 expression.
Acknowledgments
This study was supported by a grant from the American Parkinson Disease Association of Post-doc fellow (2003), grants from NINSD-40370, NINSD-043567, and a research grant from M J Fox Foundation for Parkinson’s Research. We thank patients and Christine Hunter, Kevin Nguyen-Vuong, Karen Flores and other staff members of the Parkinson’s Disease Center and Movement Disorder Clinic of Baylor College of Medicine for their assistance with this project. We also thank Drs David Chiu, Rachell Doody, Dennis Mosier, William Ondo, Larry Schneider, and Jinwu Zhang (Department of Neurology, Baylor College of Medicine) for some of the blood samples and information on patients as disease controls.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barzilai A, Melamed E. Molecular mechanisms of selective dopaminergic neuronal death in Parkinson’s disease. Trends Mol Med. 2003;9:126–132. doi: 10.1016/s1471-4914(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 3.Nagai Y, Ueno S, Saeki Y, et al. Decrease of the D3 dopamine receptor mRNA expression in lymphocytes from patients with Parkinson's disease. Neurology. 1996;46:791–795. doi: 10.1212/wnl.46.3.791. [DOI] [PubMed] [Google Scholar]
- 4.Barbanti P, Fabbrini G, Ricci A, et al. Increased expression of dopamine receptors on lymphocytes in Parkinson's disease. Mov Disord. 1999;14:764–771. doi: 10.1002/1531-8257(199909)14:5<764::aid-mds1008>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Caronti B, Tanda G, Colosimo C, et al. Reduced dopamine in peripheral blood lymphocytes in Parkinson’s disease. Neuro Report. 1999;10:2907–2910. doi: 10.1097/00001756-199909290-00006. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Li XJ, Martin DB, Aebersold R. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat Biotechnol. 2003;21:660–666. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- 7.De Lau LM, Koudtall PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 8.Tokuda T, Salem SA, Allsop D, et al. Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson's disease. Biochem Biophys Res Commun. 2006;349(1):162–166. doi: 10.1016/j.bbrc.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Annanmaki T, Muuronen A, Murros K. Low plasma uric acid level in Parkinson's disease. Mov Disord. 2007;22:1133–1137. doi: 10.1002/mds.21502. [DOI] [PubMed] [Google Scholar]
- 10.Scherzer CR, Eklund AC, Morse LJ, et al. Molecular markers of early Parkinson's disease based on gene expression in blood. Proc Natl Acad Sci U S A. 2007;104(3):955–960. doi: 10.1073/pnas.0610204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisskopf M, O'reilly E, Chen H, Schwarzschild M, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol. 2007 doi: 10.1093/aje/kwm127. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogdanov M, Matson WR, Wang Lei, Matson T, Sauders-Pullman R, Bressman SS, Beal MF. Metabolomic profiling to develop blood biomarkers for Parkinson’s disease. Brain. 2008;131:389–396. doi: 10.1093/brain/awm304. [DOI] [PubMed] [Google Scholar]
- 13.Caronti B, Antonini G, Calderaro C, et al. Dopamine transporter immunoreactivity in peripheral blood lymphocytes in Parkinson’s disease. J Neural Transm. 2001;108:803–807. doi: 10.1007/s007020170030. [DOI] [PubMed] [Google Scholar]
- 14.Naoi M, Maruyama W, Naka N, et al. (R)salsolinol N-methyltransferase activity increases in parkinsonian lymphocytes. Ann Neurol. 1999;43:212–216. doi: 10.1002/ana.410430211. [DOI] [PubMed] [Google Scholar]
- 15.Blandini F, Mangiagalli A, Cosentino M, et al. Peripheral markers of apoptosis in Parkinson's disease. The effect of dopaminergic drugs. Ann NY Acad Sci. 2003;1010:675–678. doi: 10.1196/annals.1299.123. [DOI] [PubMed] [Google Scholar]
- 16.Müftüoglu M, Elibol B, Dalmizrak Ö, et al. Mitochondrial complex I and IV activities in leukocytes from patients with parkin mutations. Mov Disord. 2004;19:544–548. doi: 10.1002/mds.10695. [DOI] [PubMed] [Google Scholar]
- 17.Law SW, Conneely OM, DeMayo FJ, O’Malley BW. Identification of a new brain-specific transcription factor, NURR1. Mol Endocrinol. 1992;6:2129–2135. doi: 10.1210/mend.6.12.1491694. [DOI] [PubMed] [Google Scholar]
- 18.Zetterstrom RH, Solomin L, Jansson L, et al. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 19.Jankovic J, Chen S, Le WD. The role of Nurr1 in the development of dopaminergic neurons and Parkinson's disease. Prog Neurobiol. 2005b;77:128–138. doi: 10.1016/j.pneurobio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Castillo SO, Baffi JS, Palkovits M, et al. Dopamine Biosynthesis Is Selectively Abolished in Substantia Nigra/Ventral Tegmental Area but Not in Hypothalamic Neurons in Mice with Targeted Disruption of the Nurr1 Gene. Mol Cell Neurosci. 1998;11:36–46. doi: 10.1006/mcne.1998.0673. [DOI] [PubMed] [Google Scholar]
- 21.Mages HW, Rilke O, Bravo R, et al. NOT, a human immediate-early response gene closely related to the steroid/thyroid homone receptor NAK1/TR3. Mol Endocrinol. 1994;8:1583–1591. doi: 10.1210/mend.8.11.7877627. [DOI] [PubMed] [Google Scholar]
- 22.Le W, Conneely OM, Zou LL, et al. Selective agenesis of mesencephaolic dopaminergic neurons in Nurr1 deficient mice. Ex Neurol. 1999a;159:451–458. doi: 10.1006/exnr.1999.7191. [DOI] [PubMed] [Google Scholar]
- 23.Jiang C, Wan X, He Y, et al. Age-dependent dopaminergic dysfunction in Nurr1 knockout mice. Exp Neurol. 2005;191:154–162. doi: 10.1016/j.expneurol.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Le W, Conneely OM, He Y, et al. Reduced Nurr1 expression increases the vulnerability of mesencephalic dopamine neurons to MPTP-induced injury. J Neurochem. 1999b;73:2218–2221. [PubMed] [Google Scholar]
- 25.Xu PY, Liang R, Jankovic J, et al. Association of homozygous 7048G7049 variant in the intron six of Nurr1 gene with Parkinson's disease. Neurology. 2002;58:881–884. doi: 10.1212/wnl.58.6.881. [DOI] [PubMed] [Google Scholar]
- 26.Le W, Xu P, Jankovic J, et al. Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet. 2003;33:85–89. doi: 10.1038/ng1066. [DOI] [PubMed] [Google Scholar]
- 27.Zheng K, Heydari B, Simon DK. A common NURR1 polymorphism associated with Parkinson disease and diffuse Lewy body disease. Arch Neurol. 2003;60:722–725. doi: 10.1001/archneur.60.5.722. [DOI] [PubMed] [Google Scholar]
- 28.Grimes DA, Han F, Panisset M, et al. Novel Mutation in the Nurr1 Gene in Parkinson’s Disease. Mov Disor. 2004;19:1120. doi: 10.1002/mds.20820. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen KX, MacDonald H, Lemonde S, et al. A Nurr1 point mutant, implicated in Parkinson's disease, uncouples ERK1/2-dependent regulation of tyrosine hydroxylase transcription. Neurobiol Dis. 2008;29:117–122. doi: 10.1016/j.nbd.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Pan T, Xie W, Jankovic J, Le W. Decreased Nurr1 mRNA in peripheral blood lymphocytes in Parkinson’s disease. Neurol. 2004;62 Suppl 5:A108. [Google Scholar]
- 31.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson’s disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 32.Jankovic J. Progression of Parkinson’s disease: Are we making progress in charting the course? Arch Neurol. 2005b;62:351–352. doi: 10.1001/archneur.62.3.351. [DOI] [PubMed] [Google Scholar]
- 33.Alavian KN, Scholz C, Simon HH. Transcriptional regulation of mesencephalic dopaminergic neurons the full circle of life and death. Mov Disord. 2008;23:319–328. doi: 10.1002/mds.21640. [DOI] [PubMed] [Google Scholar]
- 34.Saucedo-Cardenas O, Quintana-Hua JD, Le WD, et al. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci USA. 1998;95:4013–4018. doi: 10.1073/pnas.95.7.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu Y, Kompoliti K, Cochran EJ, et al. Age-related decreases in Nurr1 immunoreactivity in the human substantia nigra. J Comp Neurol. 2002;450:203–214. doi: 10.1002/cne.10261. [DOI] [PubMed] [Google Scholar]
- 36.Chu Y, Le W, Kompoliti K, et al. Nurr1 in Parkinson's disease and related disorders. J Comp Neurol. 2006;494:495–514. doi: 10.1002/cne.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]