Abstract
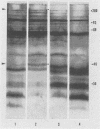
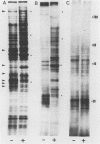
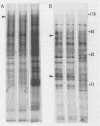
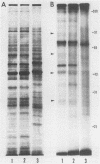
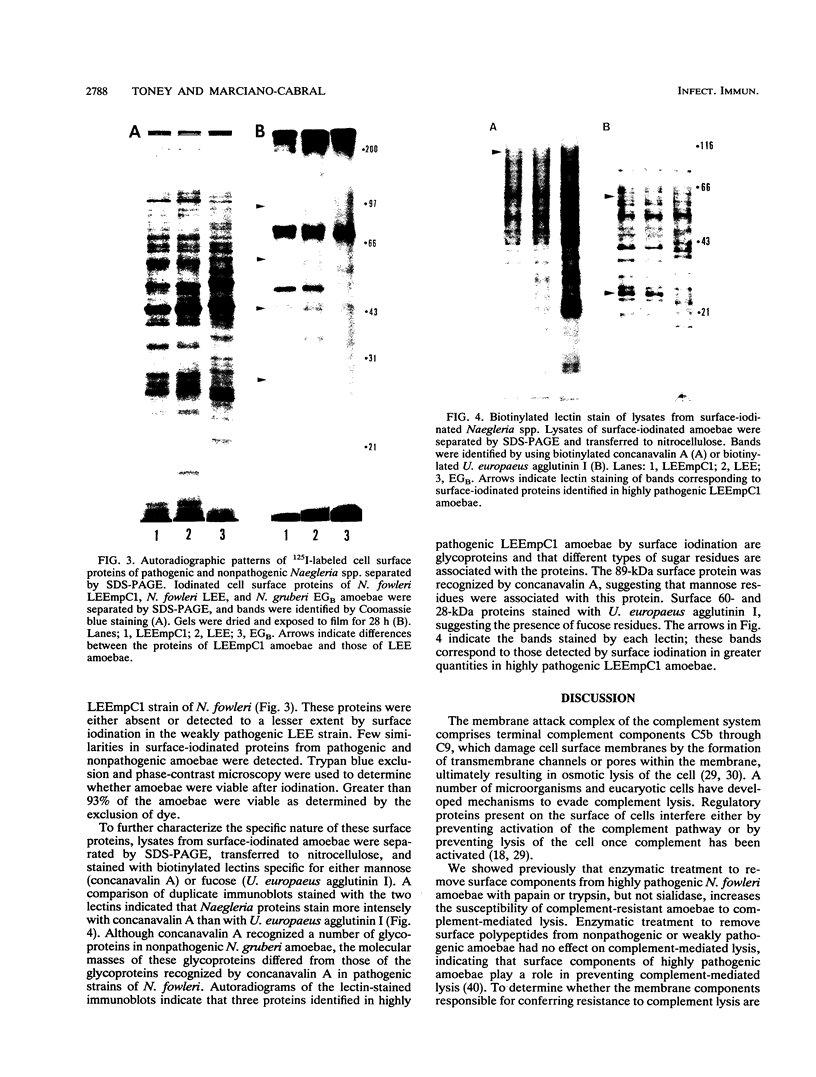
Highly pathogenic strains of Naegleria fowleri activate the alternative complement pathway but are resistant to lysis. In contrast, weakly pathogenic and nonpathogenic Naegleria spp. activate the complement pathway and are readily lysed. The present study was undertaken to determine whether surface components on amoebae accounted for resistance to complement lysis. Enzymatic removal of surface components from highly pathogenic N. fowleri with phosphatidylinositol-specific phospholipase C or with endoglycosidase H increased the susceptibility of these amoebae to complement-mediated lysis. Similar treatment of nonpathogenic amoebae had no effect on susceptibility to complement. Tunicamycin treatment of highly and weakly pathogenic N. fowleri increased susceptibility to lysis by complement in a dose-related manner. Tunicamycin treatment did not alter the susceptibility of nonpathogenic amoebae to complement. Proteins of 234 and 47 kDa were detected in supernatant fluid from phosphatidylinositol-specific phospholipase C-treated highly pathogenic amoebae but not in supernatant fluid from phosphatidylinositol-specific phospholipase C-treated weakly pathogenic amoebae. Electrophoretic analysis of iodinated surface proteins of highly pathogenic N. fowleri revealed species of 89, 60, 44, and 28 kDa. Western immunoblots of lysates from surface-iodinated amoebae were stained with biotinylated concanavalin A or biotinylated Ulex europaeus agglutinin I. Surface proteins, identified in highly pathogenic amoebae by iodination, were shown to be glycoproteins by lectin analysis specific for the detection of mannose and fucose residues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band R. N., Balamuth W. Hemin replaces serum as a growth requirement for Naegleria. Appl Microbiol. 1974 Jul;28(1):64–65. doi: 10.1128/am.28.1.64-65.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carter R. F. Description of a Naegleria sp. isolated from two cases of primary amoebic meningo-encephalitis, and of the experimental pathological changes induced by it. J Pathol. 1970 Apr;100(4):217–244. doi: 10.1002/path.1711000402. [DOI] [PubMed] [Google Scholar]
- Cline M., Marciano-Cabral F., Bradley S. G. Comparison of Naegleria fowleri and Naegleria gruberi cultivated in the same nutrient medium. J Protozool. 1983 May;30(2):387–391. doi: 10.1111/j.1550-7408.1983.tb02936.x. [DOI] [PubMed] [Google Scholar]
- Culbertson C. G. The pathogenicity of soil amebas. Annu Rev Microbiol. 1971;25:231–254. doi: 10.1146/annurev.mi.25.100171.001311. [DOI] [PubMed] [Google Scholar]
- Cursons R. T., Brown T. J., Keys E. A., Moriarty K. M., Till D. Immunity to pathogenic free-living amoebae: role of humoral antibody. Infect Immun. 1980 Aug;29(2):401–407. doi: 10.1128/iai.29.2.401-407.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davitz M. A., Low M. G., Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986 May 1;163(5):1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Haggerty R. M., John D. T. Innate resistance of mice to experimental infection with Naegleria fowleri. Infect Immun. 1978 Apr;20(1):73–77. doi: 10.1128/iai.20.1.73-77.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty R. M., John D. T. Serum agglutination and immunoglobulin levels of mice infected with Naegleria fowleri. J Protozool. 1982 Feb;29(1):117–122. doi: 10.1111/j.1550-7408.1982.tb02892.x. [DOI] [PubMed] [Google Scholar]
- Hickman S., Kulczycki A., Jr, Lynch R. G., Kornfeld S. Studies of the mechanism of tunicamycin in hibition of IgA and IgE secretion by plasma cells. J Biol Chem. 1977 Jun 25;252(12):4402–4408. [PubMed] [Google Scholar]
- Hoffman E. M. Inhibition of complement by a substance isolated from human erythrocytes. I. Extraction from human erythrocyte stromata. Immunochemistry. 1969 May;6(3):391–403. doi: 10.1016/0019-2791(69)90296-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. M. Inhibition of complement by a substance isolated from human erythrocytes. II. Studies on the site and mechanism of action. Immunochemistry. 1969 May;6(3):405–419. doi: 10.1016/0019-2791(69)90297-3. [DOI] [PubMed] [Google Scholar]
- Holbrook T. W., Boackle R. J., Parker B. W., Vesely J. Activation of the alternative complement pathway by Naegleria fowleri. Infect Immun. 1980 Oct;30(1):58–61. doi: 10.1128/iai.30.1.58-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänsch G. M., Weller P. F., Nicholson-Weller A. Release of C8 binding protein (C8bp) from the cell membrane by phosphatidylinositol-specific phospholipase C. Blood. 1988 Sep;72(3):1089–1092. [PubMed] [Google Scholar]
- Joiner K. A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., daSilva W. D., Rimoldi M. T., Hammer C. H., Sher A., Kipnis T. L. Biochemical characterization of a factor produced by trypomastigotes of Trypanosoma cruzi that accelerates the decay of complement C3 convertases. J Biol Chem. 1988 Aug 15;263(23):11327–11335. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Stiernberg J., Waneck G. L., Flavell R. A., Kincade P. W. Cell-specific heterogeneity in sensitivity of phosphatidylinositol-anchored membrane antigens to release by phospholipase C. J Immunol Methods. 1988 Oct 4;113(1):101–111. doi: 10.1016/0022-1759(88)90386-9. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Duksin D. Biological activities of the two major components of tunicamycin. J Biol Chem. 1979 Jul 25;254(14):6572–6576. [PubMed] [Google Scholar]
- Marciano-Cabral F. Biology of Naegleria spp. Microbiol Rev. 1988 Mar;52(1):114–133. doi: 10.1128/mr.52.1.114-133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A. J., Nelson E. C., Jones M. M., Duma R. J., Rosenblum W. I. Experimental Naegleria meningoencephalitis in mice. An electron microscope study. Lab Invest. 1971 Nov;25(5):465–475. [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Morgan B. P. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989 Nov 15;264(1):1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. A novel membrane glycoprotein capable of inhibiting membrane attack by homologous complement. Int Immunol. 1989;1(2):205–208. doi: 10.1093/intimm/1.2.205. [DOI] [PubMed] [Google Scholar]
- Paulson J. C. Glycoproteins: what are the sugar chains for? Trends Biochem Sci. 1989 Jul;14(7):272–276. doi: 10.1016/0968-0004(89)90062-5. [DOI] [PubMed] [Google Scholar]
- Pearce E. J., Hall B. F., Sher A. Host-specific evasion of the alternative complement pathway by schistosomes correlates with the presence of a phospholipase C-sensitive surface molecule resembling human decay accelerating factor. J Immunol. 1990 Apr 1;144(7):2751–2756. [PubMed] [Google Scholar]
- Reilly M. F., White K. L., Jr, Bradley S. G. Host resistance of mice to Naegleria fowleri infections. Infect Immun. 1983 Nov;42(2):645–652. doi: 10.1128/iai.42.2.645-652.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi M. T., Sher A., Heiny S., Lituchy A., Hammer C. H., Joiner K. Developmentally regulated expression by Trypanosoma cruzi of molecules that accelerate the decay of complement C3 convertases. Proc Natl Acad Sci U S A. 1988 Jan;85(1):193–197. doi: 10.1073/pnas.85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster F. L. Intranuclear virus-like bodies in the amoeboflagellate Naegleria gruberi. J Protozool. 1969 Nov;16(4):724–727. doi: 10.1111/j.1550-7408.1969.tb02333.x. [DOI] [PubMed] [Google Scholar]
- Stevens A. R., De Jonckheere J., Willaert E. Naegleria lovaniensis new species: isolation and identification of six thermophilic strains of a new species found in association with Naegleria fowleri. Int J Parasitol. 1980 Feb;10(1):51–64. doi: 10.1016/0020-7519(80)90064-8. [DOI] [PubMed] [Google Scholar]
- Whiteman L. Y., Marciano-Cabral F. Resistance of highly pathogenic Naegleria fowleri amoebae to complement-mediated lysis. Infect Immun. 1989 Dec;57(12):3869–3875. doi: 10.1128/iai.57.12.3869-3875.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman L. Y., Marciano-Cabral F. Susceptibility of pathogenic and nonpathogenic Naegleria spp. to complement-mediated lysis. Infect Immun. 1987 Oct;55(10):2442–2447. doi: 10.1128/iai.55.10.2442-2447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]