Abstract
The molecular genetic mechanism of gene conversion in higher eukaryotes remains unknown. We find it of considerable interest to determine when during spermatogenesis gene conversion occurs. We have therefore purified pachytene spermatocytes and haploid spermatocytes from adult mice and analyzed these fractions for the presence of gene conversion products resulting from the transfer between the major histocompatibility complex class II genes Ebd and Abk in a polymerase chain reaction assay. We have further isolated spermatogenic cells from prepubescent mice and analyzed them for the presence of the same gene conversion products. We can detect gene conversion products in testis cells as early as in 8-d-old mice where the only existing spermatogenic cells are spermatogonia. The frequency of gene conversion products remains the same as the cells reach meiosis in 18-d-old mice, and is unchanged after meiosis is completed in haploid spermatocytes. Gene conversion of this specific fragment therefore appears to be a premeiotic event and, consequently, relies on genetic mechanisms other than normal meiotic recombination.
INTRODUCTION
Gene conversion means an unidirectional transfer of genetic information from one DNA molecule to a homologous counterpart. The term was originally defined in meiotic products of fungi where it refers to a non-Mendelian segregation of genes in tetrads of a heterozygous diploid strain (see Radding, 1978). However, evidence of transfer of DNA segments from one gene to a similar gene has been observed in several gene families and also in higher eukaryotes, and it has been proposed that an analogous mechanism is responsible (Edelman and Gally, 1968; Slightom et al., 1980; Baltimore, 1981; Rubnitz and Subramati, 1986).
In particular, the generation of immunoglobulin diversity in animals like birds (for a review, see Weill and Reynaud, 1996) or rabbits (Becker and Knight, 1990) seems to rely heavily on such mechanisms. It has also been suggested for germline mutations between conserved gene pairs such as globin genes (Slightom et al., 1980; Liebhaber et al., 1981), amyloid genes (Lowell et al., 1986), or the mouse urinary protein (Clark et al., 1985), as well as a mechanism for de novo mutations in the polymorphic major histocompatibility complex (MHC) gene system (see inter alia, Kohn et al., 1978; Nairn and Yamaga, 1980; Pease et al., 1983; Weiss et al., 1983,). These suggestions have generally been based on after-the-fact sequence analyses, and the validity of such an approach has been questioned (Klein, 1984).
However, mutations producing a detectable phenotype from two adjacent genes with complementing expression defects have been observed in both transformed cultured cells (Rubnitz et al., 1986; Letsou and Liskay, 1987) and in transgenic mice (Murti et al., 1992). Finally, we have directly observed gene conversion on the DNA level between MHC class II genes (Högstrand and Böhme, 1994) in an unmanipulated mouse system using a polymerase chain reaction (PCR) assay. Gene conversion of MHC class II genes has also been observed directly in human sperm (Huang et al., 1996).
Spermatogenesis in the mouse has a duration of 33.5 to 35.5 days (Oakberg, 1957). The seminiferous cords of the testis of an adult mouse contains cell types in three principle phases of spermatogenesis (for a review, see Hecht, 1986): spermatogonial renewal and proliferation, meiosis, and spermiogenesis. Spermatogenesis is initiated 3 to 7 days after birth and during the prepubescent period a sequential appearance of spermatogenic cells in the testis can be observed (Nebel et al., 1961; Bellvéet al., 1977).
The mechanisms behind gene conversion in higher eukaryotes are entirely unresolved. We find it of considerable interest to determine when during spermatogenesis gene conversion occurs, both to determine to what extent it is concurrent with standard recombination and to find a cell stage from which mRNA coding for gene products mediating this type of mutation could be isolated. In this communication, we have therefore isolated pachytene and haploid spermatocytes from adult mice by centrifugal elutriation, as well as spermatogenic cells from prepubescent mice of different age, and assayed the same mutation that we have examined previously, to determine when during spermatogenesis gene conversion products first appear.
MATERIALS AND METHODS
Mice
BALB/c and C3H/HeJ mice and (BALB/c × C3H/HeJ)F1 hybrid mice were bred and kept at the animal facility of the Stockholm University.
Isolation of Testis Cells
Young mice (8, 10, and 18 d) were sacrificed by decapitation. The testes were extracted and placed in medium (RPMI 1640 + 1.0 mM sodium pyruvate + 0.06% sodium lactate + 105 IU/l penicillin G and 100 μg/l streptomycin) and kept on ice. The tunica was carefully removed and the testes were incubated in medium with 2 mg/ml collagenase (Sigma C-0103) for 10 to 15 min at 32°C. The spermatogenic tubules were washed three times in medium to remove interstitial cells and were then incubated in medium with 10% trypsin (Sigma T4549) for 10 min at 32°C. A total of 1.25 mg/ml soybean trypsin inhibitor (Sigma T9003) and 10% fetal calf serum was added to the incubation mixture, and the cells were dispersed from the tubule fragments with a Pasteur pipette. The cell aggregates were allowed to settle and the supernatant containing testis cells was transferred to a new tube and the washing procedure was repeated once. A sample was saved for cell staining and the remainder was centrifuged down for DNA preparation.
Isolation of Fractionated Testis Cells
Pachytene and haploid spermatogenic cells were isolated from adult testis by centrifugal elutriation (Meistrich, 1977, Heyting et al., 1987). Twenty male mice between 10 and 15 wk of age were sacrificed using CO2 and their testis were collected. A single testis cell suspension was made with collagenase and trypsin treatment as described above. This cell suspension was loaded into an elutriation rotor (Beckman model J-6M/E) equipped with an elutriation chamber. The cells were first centrifuged at 2500 rpm at a flow rate of 15 ml/min to obtain haploid spermatogenic cells, and subsequently at a speed of 1800 rpm and a flow rate of 35 ml/min to collect pachytene cells. The cell fractions were further purified by an isopychnic centrifugation on a Percoll (Pharmacia 17-0891-01) gradient with a 31% starting concentration of Percoll and spun for 30 min at 10,000 rpm on a Sorvall SS-34 fixed-angle rotor. A sample of the cell suspension was stained and the remainder was used for mutation analysis.
Cell Culture
A primary Sertoli cell culture was made essentially according to Dorrington and Fritz (1974). A single testis cell suspension was made from 8-d-old (BALB/c × C3H/HeJ)F1 mice with collagenase and trypsin treatment as described. The cells were placed in DMEM (Life Technologies 41965-039) supplemented with 8% fetal calf serum, 2 mM l-glutamine, 0,5 mM sodium pyruvate, 0,05 mM 2-mercaptoethanol, 50 IU/ml penicillin, and 50 μg/ml streptomycin in a 37°C cell incubator. The medium, including nonadherent cells, was changed at d 2, 4, and 6 of cell culture. At d 8, the medium was removed and the adherent cells were washed twice in 1× PBS before treatment for 15 min with 10% trypsin in 1× PBS. The cells were washed twice in medium, a sample was taken for cell staining, and the remainder was used for mutation analysis.
Cell Staining and Cell Counting
Fifty microliters of the cell suspension were placed in 200 μl of saline buffer on a glass specimen slide. The cells were fixed with a few drops of methanol:acetic acid (3:1). The cells were stained in Gurr’s solution for 5 min, rinsed in deionized water, and allowed to dry. The cell counts were determined by classifying nuclei present in about 2000 fixed testis cells from three separate cell preparations. Cells with abnormal morphology or unidentified cells (4 to 6 %) have not been included in the data.
DNA Isolation
The cells were digested in a buffer containing 0.3 mg/ml proteinase K, 0.5% SDS, 10 mM Tris-HCl, 5 mM EDTA, and 0.3 M NaAc for 2 to 4 h at 56°C before standard phenol/chloroform extraction and ethanol precipitation.
PCR and Agarose Gelelectrophoresis
PCR was used to detect gene conversion products essentially as described previously (Högstrand and Böhme, 1994). Briefly, 10 pmol of primers 38 (5′-cgcgcatcctccaggatctc) and 39 (5′-gcaccagttccagccgttctg) were used for 40 cycles in a 50-μl reaction along with 150,000 haploid copies of DNA from testis cells. The copy number was calculated from A260 values by assuming that 1A260 = 50 μg of DNA/ml and that the molecular weight of the haploid mouse genome is 2.15 × 1012. One microliter of the resulting PCR product was removed and subjected to 22 additional cycles with 10 pmol of primers 38 (5′-cgcgcatcctccaggatctc) and 40 (5′-gttccagcccttctgctacttc). All PCRs were performed with a cycle of 94°C for 30 s, annealing at 66°C for 25 s, and extension at 72°C for 1 min on a Corbett FTS-960 Thermal Sequencer. A standard PCR buffer with all four dNTPs (each at 100 μM), 2 mM MgCl2, and 0.2 U Taq DNA polymerase (Life Technologies 18038-026) was used for all reactions. At least five different DNA preparations were used for each different source of DNA. Twenty positive controls containing DNA with gene conversion products, and 10 negative controls containing no DNA, were included in every PCR experiment. A 15-μl sample of the final PCR product was analyzed on a 2.5% agarose gel and screened for the presence of a band of the expected size (186 bp).
Measures to Avoid PCR Contamination
Utmost care was taken to eliminate the possibility of contamination as the cause of the results. Handling of the cells and DNA preparations were carried out in a separate building with protective clothing and a designated set of pipettes and filter tips. For each new batch of reagents, parental cells only were used for DNA preparation to confirm that the reagents were free of previously amplified PCR products. Preparation of the reagents, which were mainly bought ready-to-use, and pipetting the reagents for the first PCR were carried out in a separate building with designated protective clothes, pipettes, and filter tips. Pipetting the ingredients for the second PCR and analysis of the final products were carried out in widely separated rooms with different designated sets of pipettes, filter tips, and protective clothing. In each PCR experiment, 10 negative controls with no added DNA were included. If any smear or distinct band was found in those, the whole experiment was discarded. During the last year, when most of the data here have been collected, this has happened only once.
RESULTS
Gene Conversion Frequency of Pachytene and Haploid Fractions Are Indistinguishable
Testis cells from adult mice were obtained by collagenase treatment and trypsination of the seminiferous tubules (Heyting et al., 1987). The sequential enzymatic dissociation of the testis enables removal of interstitial Leydig cells, cells of the vascular system, and peritubular cells as a source of contamination of the spermatogenic cells. The cells were fractionated into one fraction containing almost exclusively pachytene cells and one fraction containing mainly haploid cells by centrifugal elutriation and further purified in a self-generating Percoll gradient. A sample of the cells was fixed and stained, and the purity of the fractions was assessed under microscope (Table 1). The pachytene fraction was 97% pure, the only contaminating cells being haploid spermatocytes. The haploid fraction was 80% pure, and here the contaminating cells were mainly pachytene cells, causing a considerably lower purity in the DNA preparation.
Table 1.
Spermatogenic cells and DNA contribution of spermatogenic cells in prepubescent mice and fractionated adult testis cells
| Cell type (genome copies)d | Days after birtha
|
Fractionsb
|
|||
|---|---|---|---|---|---|
| 8
|
10
|
18
|
Pach
|
Hapl
|
|
| Cell(DNA) | Cell(DNA) | Cell(DNA) | Cell(DNA) | Cell(DNA)c | |
| Sertoli cells (2n) | 73(73) | 51(39) | 35(23) | −(−) | −(−) |
| Spermatogonia (2n) | 27(27) | 18(14) | 9(6) | −(−) | −(−) |
| Preleptene and leptene (4n) | −(−) | 31(47) | 14(18) | −(−) | −(−) |
| Zygotene (4n) | −(−) | −(−) | 7(9) | −(−) | −(−) |
| Pachytene (4n) | −(−) | −(−) | 34(44) | 97(99) | 20(50) |
| Haploid (n) | −(−) | −(−) | 1(−) | 3(1) | 80(50) |
Testis cells of prepubescent mice sacrificed at 8, 10, and 18 d of age.
Testis cells in purified fractions from elutriation centrifugation of testes from adult mice; Pach, pachytene fraction; Hapl, haploid fraction.
Data are presented as percentage of total cells in a testis cell suspension (cell) and as a percentage of total DNA prepared from the same testis cell suspension (DNA). −, no cells found among 2000 assessed testis cells.
The DNA contribution of the different cell types was calculated with consideration taken for the different number of haploid genome copies (n).
We analyzed DNA from the purified fraction for detection of gene conversion events with PCR as described in Figure 1 and in MATERIALS AND METHODS. Table 2 shows the frequency of detected gene conversion events from Ebd to Abk. Detections are measured as number of positive signals/total PCR reactions, and the SDs were calculated regarding the gene conversion events as binomially distributed. The probability per DNA molecule [p(molecule)] of a detected event was calculated according to Rothman (1986). The frequency of gene conversion given here is not an absolute frequency but rather a comparative frequency since the PCR background frequency has not been subtracted in this experiment. Therefore, these results cannot be directly compared to our previous results where a background frequency of 8.4 × 10−8 was subtracted. However, the frequencies can be compared among the different cell populations in this experiment because the background frequencies should be the same in all groups. The haploid spermatid fraction has a frequency of gene conversion events of 1.96 ± 0.29 × 10−6 per DNA molecule. In the pachytene fraction, the gene conversion frequency was indistinguishable from the haploid fraction. Therefore, the gene conversion events that we assay appear to be completed by pachytene.
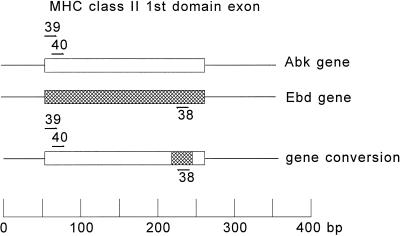
Figure 1.
Location of primers used for detection of gene conversion between Ebd and Abk genes. No product will be amplified unless the Ebd and Abk sequences have been juxtaposed onto one another since both Abk primers are sense directed on one gene and the Ebd primer is antisense directed on another gene 30 kb away. Furthermore, the Abk and Ebd genes are located on two different chromosomes; the Abk on the paternal chromosome 17, and the Ebd on the maternal chromosome 17.
Table 2.
Frequency of detected gene conversion events from Ebd to Abk in the testis of prepubescent mice and in purified testis cells of adult mice
| Micea | Sourceb | Copiesc | Signalsd | p(molecule) alle | p(molecule) germf |
|---|---|---|---|---|---|
| F1 | 8 d | 39,000/150,000 | 57/480 | 0.84 ± 0.15 × 10−6 | 2.66 ± 0.58 × 10−6 |
| F1 | 10 d | 91,500/150,000 | 42/210 | 1.49 ± 0.25 × 10−6 | 2.31 ± 0.41 × 10−6 |
| F1 | 18 d | 115,500/150,000 | 50/260 | 1.42 ± 0.22 × 10−6 | 1.79 ± 0.29 × 10−6 |
| F1 | Pach | 150,000 | 48/200 | 1.83 ± 0.28 × 10−6 | 1.83 ± 0.28 × 10−6 |
| F1 | Hapl | 150,000 | 51/200 | 1.96 ± 0.29 × 10−6 | 1.96 ± 0.29 × 10−6 |
| F1 | Sertoli | 150,000 | 9/300 | 2.03 ± 0.68 × 10−7 | |
| — | No DNA | 0/0 | 0/360 | — |
F1 = (BALB/c × C3H/HeJ)F1 mice.
Cell source for DNA isolation: 8 10, and 18 d, age of mice in days; Pach, fraction enriched in pachytene spermatocytes; Hapl, fraction enriched in haploid spermatocytes; Sertoli, testis cells from 8-d-old mice after 8 d of cell culture; No DNA, no DNA added.
Number of haploid copies of germ cell DNA/number of haploid copies of total testis DNA.
Number of detected events/number of examined events.
Probability per molecule to detect a gene conversion event in all DNA molecules.
Probability per molecule to detect a gene conversion event taking only the germ cell contribution into account.
Gene Conversion Detected Already in Spermatogonia
Since gene conversion appears to be already completed by pachytene, we wanted to go further back in spermatogenesis to investigate whether we could find gene conversion products in earlier cell stages. Since early spermatogenic cell stages only constitute a small fraction of adult testis cells and also are hard to purify, we decided to use prepubescent mice of different ages that only have reached a certain maturation stage. Testis cells in suspension were obtained by collagenase and trypsin treatment as described above, and the distribution of the different meiotic cell stages was assessed by examining the morphology of the cells under a microscope. Table 1 shows the cell proportions we obtained from dispersed prepubescent testis cells. We found essentially the same proportions of cells as those previously determined from intact sections (Bellvéet al., 1977), with a couple of percent higher proportion of Sertoli cells in our samples. As follows from Table 1, 8-d-old mice have no more spermatogenic cell stages than spermatogonia; at 10 days no cells had advanced further than the leptene stage and at 18 days the cells were essentially arrested at the pachytene stage. In Table 1, we also show the contribution of DNA from the various cell types. When tetraploid spermatocytes were present, the DNA contribution of these cells was considerably higher than the percentage of cell by cell.
Detection of gene conversion products was measured with PCR as described above. It is evident from Table 2 that detection of gene conversion products in testis cells of prepubescent mice is possible as early as at 8 d of age, when the testis only consists of spermatogonia and Sertoli cells. However, although the number of haploid DNA copies per PCR reaction is constant in all mice, the contribution to that DNA from different cell sources varies depending on the cell percentages and their genome copy number.
Sertoli Cells Have Background Levels of Gene Conversion
We have previously observed that the frequency of gene conversion in DNA from liver cells taken as a representative of non-germline cells is of at least two orders of magnitude lower than the frequency of a germline cell-like sperm (Högstrand and Böhme, 1994). To investigate whether the same is true for a non-germline cell type like Sertoli cells, we therefore cultured testis cells from 8-d-old mice, when they consist mainly of Sertoli cells and spermatogonia. After 8 days in culture and four changes of medium, the spermatogonia cells are almost depleted and the only visible cells left are adherent Sertoli cells (Spruill et al., 1981). Detection of gene conversion products was measured with PCR as mentioned above, and the results are shown in Table 2. The frequency of gene conversion in the adherent non-germline cells was found to be considerably lower than the frequency found in both purified adult testis cells and prepubescent testis cells, and was also indistinguishable from the background frequency of these gene fragments established previously (Högstrand and Böhme, 1994). To estimate a frequency of gene conversion for germline DNA in prepubescent mice, we thus excluded the expected contribution of Sertoli cells and assigned the remaining signals to the germline cells only. The right-most column of Table 2 shows the gene conversion frequency per germline DNA in prepubescent mice after the contribution of gene conversion products from non-germline cells had been subtracted. These were between 1.8 and 2.7 × 10−6 in the testes of all prepubescent mice used, which is at or above the frequency observed in the purified pachytene and haploid fractions shown above, also when the only germ cells present were spermatogonia. Therefore, at least the vast majority of the gene conversion events we observed must have been already completed by that early stage.
DISCUSSION
When we compared the frequencies of gene conversion resulting from the transfer of Ebd onto Abk in germline cells from prepubescent mice and purified pachytene and haploid spermatocytes from adult mice, none of these frequencies were significantly different from each other. These frequencies were, even without correction for PCR background, also in reasonable agreement with what we have reported previously. They also agree with what has been found in the Bm series for MHC class II mutations (Kohn et al., 1978; Nairn and Yamaga, 1980; McIntyre and Seidman, 1984; Egorov and Egorov, 1988) and of what has been calculated by population genetics theory (Otha, 1982).
Crossing over and recombination between homologous chromatids take place in the pachytene stage (see De Robertis and De Robertis, 1987). We found similar gene conversion frequencies in both purified pachytene and haploid fractions. Moreover, in prepubescent testis cells a similar frequency was also obtained when the only germline cells present were spermatogonia. These results clearly indicate that a vast majority of gene conversion events in the MHC genes take place in the mitotic proliferation of germ cells before they enter meiosis. Consequently, we conclude that the molecular mechanisms of gene conversion is clearly separated from the general recombinatorial events during meiosis. Our discovery that germline conversion might be mitotic makes it likely that the analogous phenomenon observed in immunoglobulin gene segments during somatic mutation of B-cells (Weill and Reynaud, 1996) are indeed also mechanically related to germline gene conversions. The mechanisms that can account for mitotic gene conversion are not known, but our results indicate that there must be a highly specific mechanism since only the cells with a spermatogenetic origin in the mouse testis have undergone mitotic gene conversion whereas the Sertoli cells in the same organ or liver cells (Högstrand and Böhme, 1994) have background levels of gene conversion. The specificity of the gene conversion machinery can involve specific enzymes: “convertases,” present only in certain cell types and/or inducible DNA-binding proteins that bind to specific recognition sequences before the chromatids are cut and the donor sequence are copied.
It has previously been proposed that gene conversion might be a premeiotic event when clusters of converted spermatogenic cells were found in the testis of transgenic mice (Murti et al., 1992). Also, several littermates carrying the same mutation were detected for some of the MHC bm mutations (Melvold et al., 1982; Geliebter et al., 1986) even though we favor an alternative explanation for this particular phenomenon. Although we observe gene conversion already in spermatogonia, the frequency of conversions does not increase in later stages, as “unconverted” spermatogonia contribute as much on a per cell basis to spermatogenesis as the “converted” cells do. Mitotic occurrence of gene conversion therefore does not lead to enrichment of conversions in sperm. Furthermore, there is no known mechanism for sperm with a common spermatogonial ancestor to stay in proximity to one another within the epididymis or during ejaculation. Therefore, we find it unlikely that any gene conversion mutant would appear in several littermates, be it mitotic or not, unless the littermates are in fact identical twins. Also, the suggestion that gene conversion should occur more often in females than in males, caused by the longer duration of meiosis in female germ cells which can be arrested in the dictyate stage sometimes for years (Willison, 1985), appears to be unlikely if the main contribution of gene conversion occurs before meiosis.
The frequency of gene conversion in the bm series for the MHC class I genes, and for the mouse H-2 K gene in particular, is at least two orders of magnitude higher than what we have reported for MHC class II genes in this communication. This is in part due to differences in method; with a skin-grafting assay, all possible gene conversions producing phenotypic differences will be observed, whereas our PCR assay only allows for monitoring the transfer of one specific fragment from a given donor gene to a given acceptor gene. Furthermore, the number of potential donor genes is more than one order of magnitude higher in the class I system. If the actual gene conversion frequency per fragment and gene is still higher in the class I situation, one reason might be that the K region might be more accessible due to larger number of adjacent genes that are expressed in testis cells (Yeom et al., 1992).
In this communication, we have shown that gene conversion in the mouse MHC class II, between the Abk and Ebd genes, is a premeiotic event. We can detect gene conversion products in testis cells in 8-d-old mice where the only existing spermatogenic cells are spermatogonia. The gene conversion frequency remains constant among the spermatogenic cells as the mice grow older and the cells reach meiosis. Purified fractions containing mainly pachytene cells, which are in the middle of meiosis, or haploid cells, which have gone through meiosis, have the same gene conversion frequency as the spermatogonia. It therefore seems evident that the main contribution to gene conversion of this specific fragment precedes meiosis and relies on molecular genetic mechanisms other than normal meiotic recombination. These mechanisms remain to be discovered.
ACKNOWLEDGMENTS
This research was supported by grant B96-13C-10835-03 from the Swedish Medical Research Council. We are deeply indebted to Eva Brundell for invaluable help with the centrifugal elutriation, Lena Israelsson for expert technical assistance, Anders Mattsson for generous statistical advice, Gunnel Jansson and Pia Lundell for animal care, and Christer Höög for general discussions.
Footnotes
MHC, major histocompatibility complex.
REFERENCES
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981;24:592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Becker RS, Knight KL. Somatic diversification of immunoglobulin heavy chain VDJ genes: evidence for somatic gene conversion in rabbits. Cell. 1990;63:987–997. doi: 10.1016/0092-8674(90)90502-6. [DOI] [PubMed] [Google Scholar]
- Bellvé AR, Cavicchia JC, Milette CF, O’Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepubertal mouse. J Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AJ, Chave-Cox A, Ma X, Bishop JO. Analysis of mouse major urinary protein genes: variation between the exonic sequences of group 1 genes and a comparison with an active gene outwith group 1 both suggest that gene conversion has occurred between MUP genes. EMBO J. 1985;4:3167–3171. doi: 10.1002/j.1460-2075.1985.tb04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EDP, De Robertis EMF., Jr . Meiosis and sexual reproduction. In: De Robertis EDP, De Robertis EMF Jr, editors. Cell and Molecular Biology. Philadelphia: Lea & Febiger; 1987. pp. 439–463. [Google Scholar]
- Dorrington JH, Fritz IB. Effects of gonadotropins on cyclic AMP production by isolated seminiferous tubule and interstitial cell preparations. Endocrinology. 1974;94:395–403. doi: 10.1210/endo-94-2-395. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Gally JA. Antibody structure, diversity and specificity. Brookhaven Symp Biol. 1968;21:328–344. [PubMed] [Google Scholar]
- Egorov IK, Egorov OS. Detection of new MHC mutations by skin grafting, tumor transplantation and monoclonal antibodies: a comparison. Genetics. 1988;118:287–298. doi: 10.1093/genetics/118.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter J, Zeff RA, Melvold RW, Nathenson SG. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA analysis: Kbm9 and Kbm6. Proc Natl Acad Sci USA. 1986;83:3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht NB. Experimental Approaches to Mammalian Embryonic Development. J. Rossant and R.A. Pedersen, New York: Cambridge University Press; 1986. Regulation of gene expression during mammalian spermatogenesis; pp. 151–193. [Google Scholar]
- Heyting C, Moens PB, van Raamsdonk W, Dietrich AJJ, Vink ACG, Redeker EJW. Identification of the two major components of the lateral elements of synaptonemal complexes of the rat. Eur J Cell Biol. 1987;43:148–154. [PubMed] [Google Scholar]
- Huang MM, Erlich HA, Goodman MF, Arnheim N. Analysis of mutational changes at the HLA locus in single human sperm. Hum Mutat. 1996;6:303–310. doi: 10.1002/humu.1380060404. [DOI] [PubMed] [Google Scholar]
- Högstrand K, Böhme J. A determination of the frequency of gene conversion in unmanipulated mouse sperm. Proc Natl Acad Sci USA. 1994;91:9921–9925. doi: 10.1073/pnas.91.21.9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. Gene conversion in MHC genes. Transplantation. 1984;38:327–329. doi: 10.1097/00007890-198410000-00002. [DOI] [PubMed] [Google Scholar]
- Kohn HI, Klein J, Melvold RW, Nathenson SG, Pious D, Shreffler DC. The first H-2 mutant workshop. Immunogenetics. 1978;7:279–294. doi: 10.1007/BF01844019. [DOI] [PubMed] [Google Scholar]
- Letsou A, Liskay RM. Effect of the molecular nature of mutation on the efficiency of intrachromosomal gene conversion in mouse cells. Genetics. 1987;117:759–769. doi: 10.1093/genetics/117.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhaber SA, Goossens M, Kan YW. Homology and concerted evolution at the α1 and α2 loci of human α-globin. Nature. 1981;290:26–29. doi: 10.1038/290026a0. [DOI] [PubMed] [Google Scholar]
- Lowell CA, Potter DA, Stearman RS, Morrow JF. Structure of the murine serum amyloid A gene family-gene conversion. J Biol Chem. 1986;261:8442–8452. [PubMed] [Google Scholar]
- McIntyre KR, Seidman JC. Nucleotide sequence of mutant I-Abm12 gene is evidence for genetic exchange between mouse immune response genes. Nature. 1984;308:551–553. doi: 10.1038/308551a0. [DOI] [PubMed] [Google Scholar]
- Meistrich ML. Methods in Cell Biology. Vol. 15. D.M. Prescott, New York: Academic Press; 1977. Separation of spermatogenic cells and nuclei from rodent testes; pp. 15–54. [DOI] [PubMed] [Google Scholar]
- Melvold RW, Kohn HI, Dunn GR. History and genealogy of the H-2 Kb mutants from the C57BL/6Kh colony. Immunogenetics. 1982;15:177–185. doi: 10.1007/BF00621950. [DOI] [PubMed] [Google Scholar]
- Murti JA, Bumbulis M, Schimenti JC. High-frequency germ line gene conversion in transgenic mice. Mol Cell Biol. 1992;12:2545–2552. doi: 10.1128/mcb.12.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn R, Yamaga K. Biochemistry of the gene products from murine MHC mutants. Ann Rev Genet. 1980;14:241–277. doi: 10.1146/annurev.ge.14.120180.001325. [DOI] [PubMed] [Google Scholar]
- Nebel BR, Amarose AP, Hackett EM. Calendar of gametogenic development in the prepuberal mouse. Science. 1961;134:832–833. doi: 10.1126/science.134.3482.832. [DOI] [PubMed] [Google Scholar]
- Oakberg, E.F. (1957). Duration of the spermatogenesis in the mouse. Nature (4595), 1137–1138. [DOI] [PubMed]
- Ohta T. Allelic and nonallelic homology of a supergene family. Proc Natl Acad Sci USA. 1982;79:3251–3254. doi: 10.1073/pnas.79.10.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease LR, Schulze DH, Pfaffenbach GM, Nathenson SG. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci USA. 1983;80:242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. Meiotic gene conversion. Annu Rev Biochem. 1978;47:847–880. doi: 10.1146/annurev.bi.47.070178.004215. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. Analysis of crude data. In: Rothman KJ, editor. Modern Epidemiology. Boston: Little, Brown and Co.; 1986. pp. 157–173. [Google Scholar]
- Rubnitz J, Subramanti S. Extrachromosomal and chromosomal gene conversion in mammalian cells. Mol Cell Biol. 1986;6:1608–1614. doi: 10.1128/mcb.6.5.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom JL, Blechl AE, Smithies O. Human fetal Gγ- and Aγ-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980;21:627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Spruill WA, White MG, Steiner AL, Tries LL, Kireszenbaum AL. Temporal sequence of cell shape changes in cultured rat sertoli cells after experimental elevation of intracellular cAMP. Exp Cell Res. 1981;131:131–148. doi: 10.1016/0014-4827(81)90414-6. [DOI] [PubMed] [Google Scholar]
- Weill J-C, Reynaud C-A. Rearrangement/hypermutation/gene conversion: when, where and why? Immunol Today. 1996;17:92–97. doi: 10.1016/0167-5699(96)80586-x. [DOI] [PubMed] [Google Scholar]
- Weiss EH, Mellor A, Golden L, Fahrner K, Simpson E, Hurst J, Flavell RA. The structure of a mutant H-2 gene suggests that the generation of polymorphism in the H-2 genes may occur by gene conversion-like events. Nature. 1983;301:671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]
- Willison KR. Sex and frequency of gene conversion in meiosis. Nature. 1985;313:604. doi: 10.1038/313604a0. [DOI] [PubMed] [Google Scholar]
- Yeom YI, Abe K, Bennett D, Artzt K. Testis-/embryo-expressed genes are clustered in the mouse H-2 K region. Proc Natl Acad Sci USA. 1992;89:773–777. doi: 10.1073/pnas.89.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]