Abstract
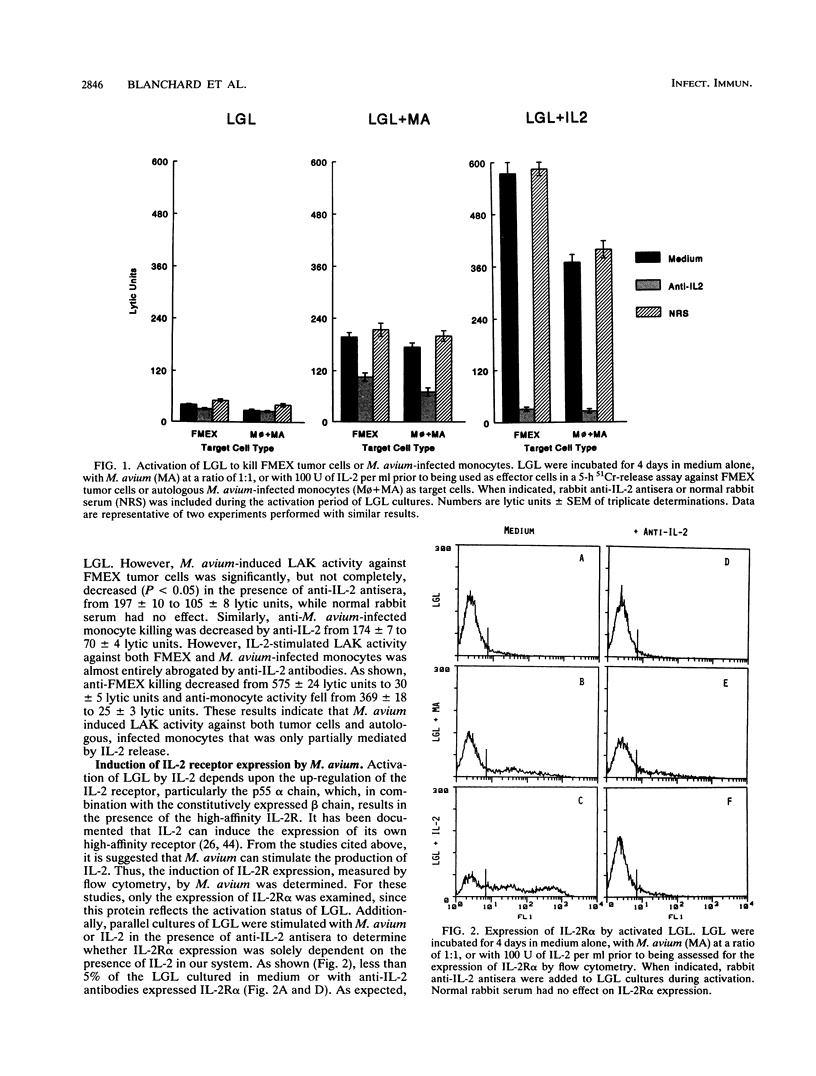
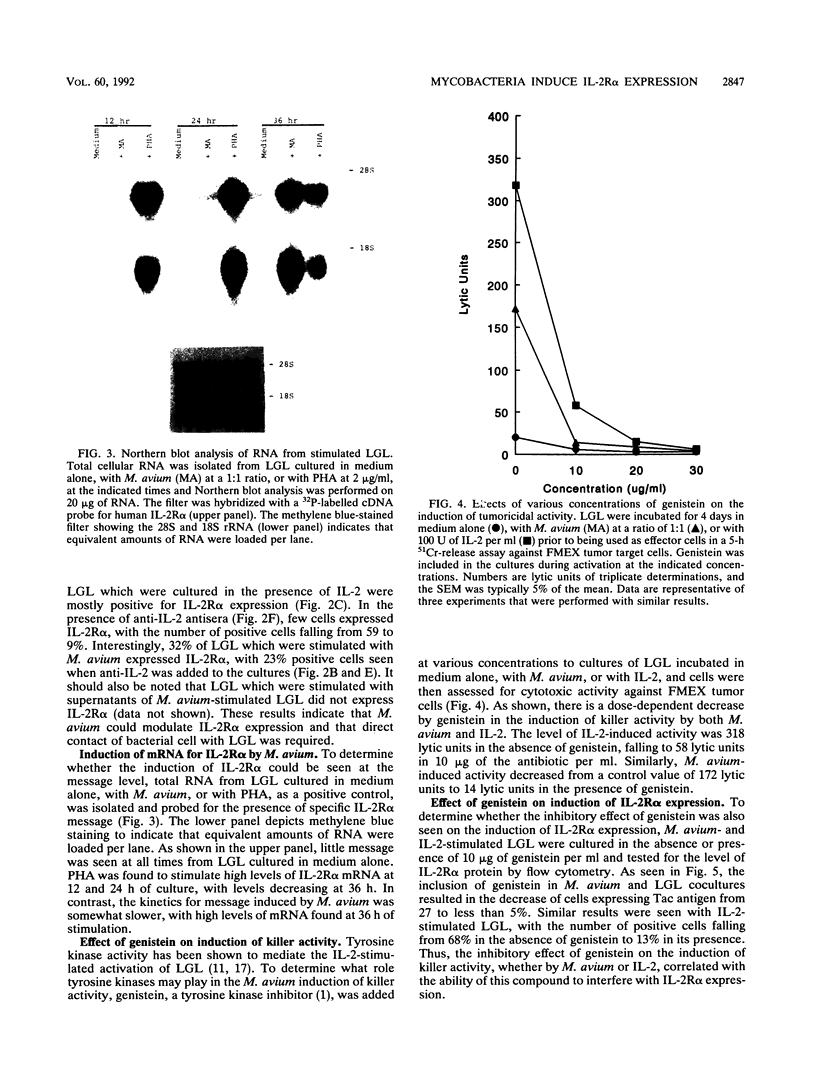
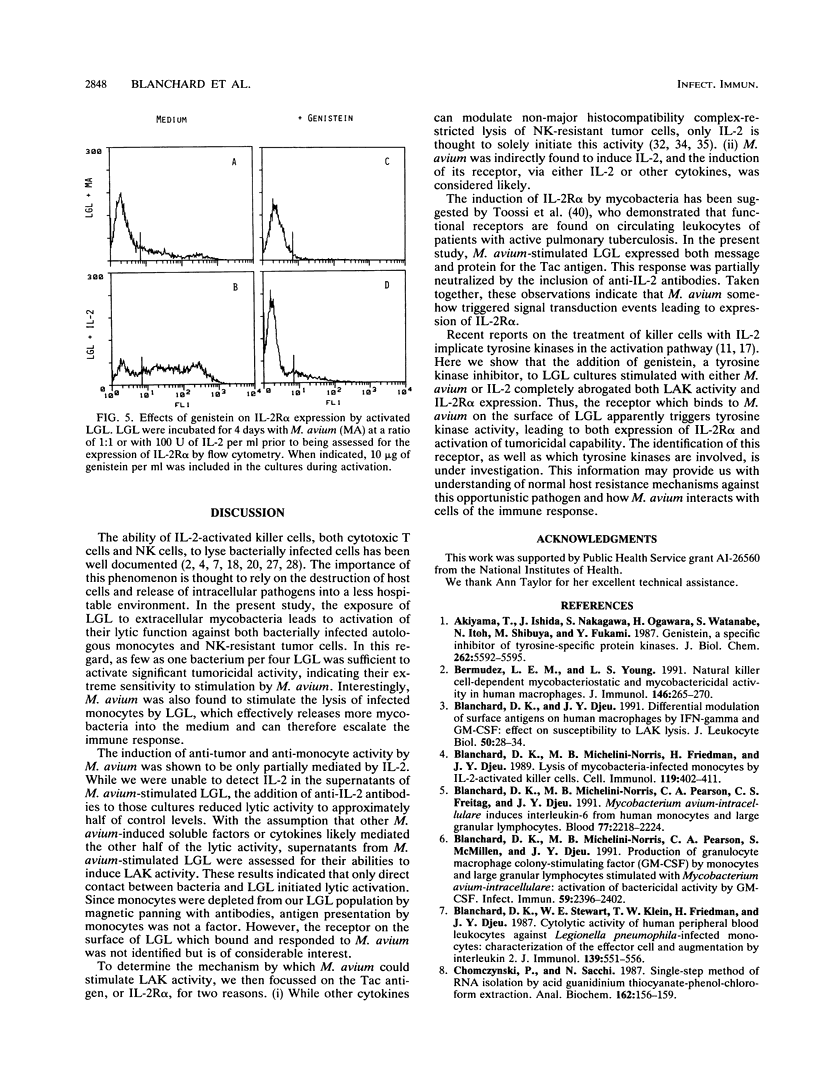
Mycobacterium avium is an intracellular opportunistic pathogen commonly seen in AIDS patients. M. avium-infected monocytes have been recently shown to be lysed by interleukin-2 (IL-2)-activated killer cells. Since some bacterial products can directly augment natural killer activity, we examined the ability of these microorganisms to induce killer cell activity. Coculture of M. avium with large granular lymphocytes (LGL) was found to augment the ability of LGL to lyse both tumor cells and bacterially infected autologous monocytes. The induction of tumoricidal activity by M. avium was only partially neutralized by the presence of anti-IL-2 antibodies, indicating that both IL-2-dependent and IL-2-independent mechanisms are responsible for activation of killer cells. Furthermore, only the direct interaction between bacterium and LGL could induce the expression of both IL-2 receptor alpha protein and mRNA, an effect which was abrogated by the presence of genistein, a tyrosine kinase inhibitor. Thus, M. avium was seen to induce killer cells, an activity that is concomitant with the up-regulation of IL-2 receptor alpha, or Tac antigen, expression and which involves signal transduction mechanisms mediated by tyrosine kinase activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Natural killer cell-dependent mycobacteriostatic and mycobactericidal activity in human macrophages. J Immunol. 1991 Jan 1;146(1):265–270. [PubMed] [Google Scholar]
- Blanchard D. K., Djeu J. Y. Differential modulation of surface antigens on human macrophages by IFN-gamma and GM-CSF: effect on susceptibility to LAK lysis. J Leukoc Biol. 1991 Jul;50(1):28–34. doi: 10.1002/jlb.50.1.28. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Friedman H., Djeu J. Y. Lysis of mycobacteria-infected monocytes by IL-2-activated killer cells: role of LFA-1. Cell Immunol. 1989 Apr 1;119(2):402–411. doi: 10.1016/0008-8749(89)90254-2. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Pearson C. A., Freitag C. S., Djeu J. Y. Mycobacterium avium-intracellulare induces interleukin-6 from human monocytes and large granular lymphocytes. Blood. 1991 May 15;77(10):2218–2224. [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Pearson C. A., McMillen S., Djeu J. Y. Production of granulocyte-macrophage colony-stimulating factor (GM-CSF) by monocytes and large granular lymphocytes stimulated with Mycobacterium avium-M. intracellulare: activation of bactericidal activity by GM-CSF. Infect Immun. 1991 Jul;59(7):2396–2402. doi: 10.1128/iai.59.7.2396-2402.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. K., Stewart W. E., 2nd, Klein T. W., Friedman H., Djeu J. Y. Cytolytic activity of human peripheral blood leukocytes against Legionella pneumophila-infected monocytes: characterization of the effector cell and augmentation by interleukin 2. J Immunol. 1987 Jul 15;139(2):551–556. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K. Lysis of human monocytes by lymphokine-activated killer cells. Cell Immunol. 1988 Jan;111(1):55–65. doi: 10.1016/0008-8749(88)90050-0. [DOI] [PubMed] [Google Scholar]
- Fujii M., Abo T., Kumagai K. Cytokines produced by blood mononuclear cells stimulated with the streptococcal preparation OK-432: effect on production by supplementing the medium with xenogeneic serum. Cancer Immunol Immunother. 1988;27(2):97–102. doi: 10.1007/BF00200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M. R., Scearce R. M., Hoffman J. A., Peffer N. J., Hammes S. R., Hosking J. B., Schmandt R., Kuziel W. A., Haynes B. F., Mills G. B. A tyrosine kinase physically associates with the beta-subunit of the human IL-2 receptor. J Immunol. 1991 Aug 15;147(4):1253–1260. [PubMed] [Google Scholar]
- Gaudernack G., Leivestad T., Ugelstad J., Thorsby E. Isolation of pure functionally active CD8+ T cells. Positive selection with monoclonal antibodies directly conjugated to monosized magnetic microspheres. J Immunol Methods. 1986 Jun 24;90(2):179–187. doi: 10.1016/0022-1759(86)90074-8. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Harshan K. V., Gangadharam P. R. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect Immun. 1991 Aug;59(8):2818–2821. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan N. F., Campbell D. E., Douglas S. D. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986 Dec 24;95(2):273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Tsai V. Acute toxoplasma infection of mice induces spleen NK cells that are cytotoxic for T. gondii in vitro. J Immunol. 1986 Jan;136(1):313–319. [PubMed] [Google Scholar]
- Horak I. D., Gress R. E., Lucas P. J., Horak E. M., Waldmann T. A., Bolen J. B. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P., Yeager H., Jr, Whalen G., Evans M., Swartz R. P., Roecklein J. Natural killer cell-mediated lysis of Mycobacterium-avium complex-infected monocytes. J Clin Immunol. 1990 Jan;10(1):71–77. doi: 10.1007/BF00917500. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Niesel D. W., Asuncion M., Klimpel K. D. Natural killer cell activation and interferon production by peripheral blood lymphocytes after exposure to bacteria. Infect Immun. 1988 Jun;56(6):1436–1441. doi: 10.1128/iai.56.6.1436-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimpel G. R., Niesel D. W., Klimpel K. D. Natural cytotoxic effector cell activity against Shigella flexneri-infected HeLa cells. J Immunol. 1986 Feb 1;136(3):1081–1086. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Lindemann R. A. Roles of interferon and cellular adhesion molecules in bacterial activation of human natural killer cells. Infect Immun. 1989 Jun;57(6):1702–1706. doi: 10.1128/iai.57.6.1702-1706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmo T., Davies C., Fodstad O., Morgan A. C. Stable quantitative differences of antigen expression in human melanoma cells isolated by flow cytometric cell sorting. Int J Cancer. 1984 Oct 15;34(4):507–512. doi: 10.1002/ijc.2910340413. [DOI] [PubMed] [Google Scholar]
- Michelini-Norris M. B., Blanchard D. K., Friedman H., Djeu J. Y. Involvement of HLA-DR+ large granular lymphocytes in the induction of tumor necrosis factor by Mycobacterium avium-intracellulare complex. J Leukoc Biol. 1991 Dec;50(6):529–538. doi: 10.1002/jlb.50.6.529. [DOI] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Resnick M., Roguel N., Bercovier H., Enk C., Frankenburg S., Kedar E. Lysis of murine macrophages infected with intracellular pathogens by interleukin 2-activated killer (LAK) cells in vitro. Cell Immunol. 1988 Apr 15;113(1):214–219. doi: 10.1016/0008-8749(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Rollwagen F. M., Dasch G. A., Jerrells T. R. Mechanisms of immunity to rickettsial infection: characterization of a cytotoxic effector cell. J Immunol. 1986 Feb 15;136(4):1418–1421. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Sharon M., Siegel J. P., Tosato G., Yodoi J., Gerrard T. L., Leonard W. J. The human interleukin 2 receptor beta chain (p70). Direct identification, partial purification, and patterns of expression on peripheral blood mononuclear cells. J Exp Med. 1988 Mar 1;167(3):1265–1270. doi: 10.1084/jem.167.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. P., Sharon M., Smith P. L., Leonard W. J. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987 Oct 2;238(4823):75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Smith K. A. The interleukin 2 receptor. Adv Immunol. 1988;42:165–179. doi: 10.1016/s0065-2776(08)60844-5. [DOI] [PubMed] [Google Scholar]
- Smyth M. J., Ortaldo J. R. Comparison of the effect of IL-2 and IL-6 on the lytic activity of purified human peripheral blood large granular lymphocytes. J Immunol. 1991 Feb 15;146(4):1380–1384. [PubMed] [Google Scholar]
- Spits H., Yssel H., Paliard X., Kastelein R., Figdor C., de Vries J. E. IL-4 inhibits IL-2-mediated induction of human lymphokine-activated killer cells, but not the generation of antigen-specific cytotoxic T lymphocytes in mixed leukocyte cultures. J Immunol. 1988 Jul 1;141(1):29–36. [PubMed] [Google Scholar]
- Tarkkanen J., Saksela E., Lanier L. L. Bacterial activation of human natural killer cells. Characteristics of the activation process and identification of the effector cell. J Immunol. 1986 Oct 15;137(8):2428–2433. [PubMed] [Google Scholar]
- Tarkkanen J., Saksela E. Potentiation of human natural killer cell cytotoxicity by Salmonella bacteria is an interferon- and interleukin-2-independent process that utilizes CD2 and CD18 structures in the effector phase. Infect Immun. 1991 Aug;59(8):2767–2773. doi: 10.1128/iai.59.8.2767-2773.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkkanen J., Saxén H., Nurminen M., Mäkelä P. H., Saksela E. Bacterial induction of human activated lymphocyte killing and its inhibition by lipopolysaccharide (LPS). J Immunol. 1986 Apr 1;136(7):2662–2669. [PubMed] [Google Scholar]
- Timonen T., Saksela E. Isolation of human NK cells by density gradient centrifugation. J Immunol Methods. 1980;36(3-4):285–291. doi: 10.1016/0022-1759(80)90133-7. [DOI] [PubMed] [Google Scholar]
- Toossi Z., Sedor J. R., Lapurga J. P., Ondash R. J., Ellner J. J. Expression of functional interleukin 2 receptors by peripheral blood monocytes from patients with active pulmonary tuberculosis. J Clin Invest. 1990 Jun;85(6):1777–1784. doi: 10.1172/JCI114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- Welsh R. M. Natural killer cells and interferon. Crit Rev Immunol. 1984;5(1):55–93. [PubMed] [Google Scholar]
- Welte K., Andreeff M., Platzer E., Holloway K., Rubin B. Y., Moore M. A., Mertelsmann R. Interleukin 2 regulates the expression of Tac antigen on peripheral blood T lymphocytes. J Exp Med. 1984 Nov 1;160(5):1390–1403. doi: 10.1084/jem.160.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]



