Abstract
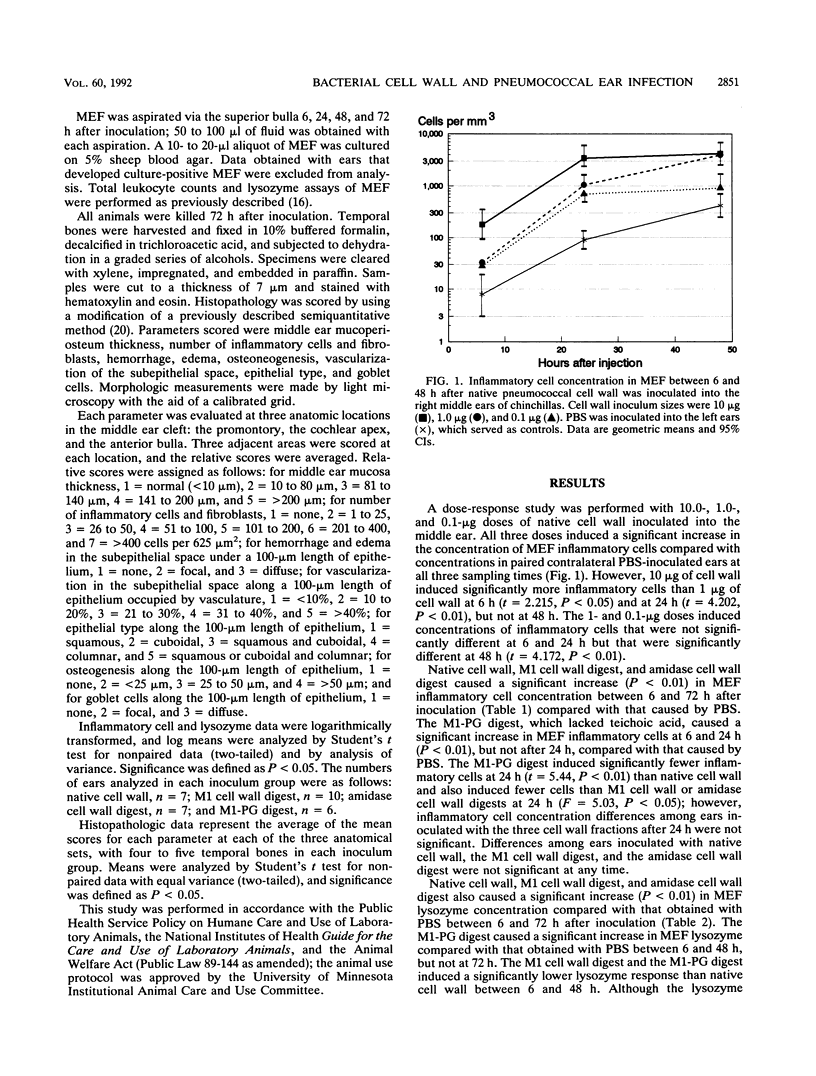
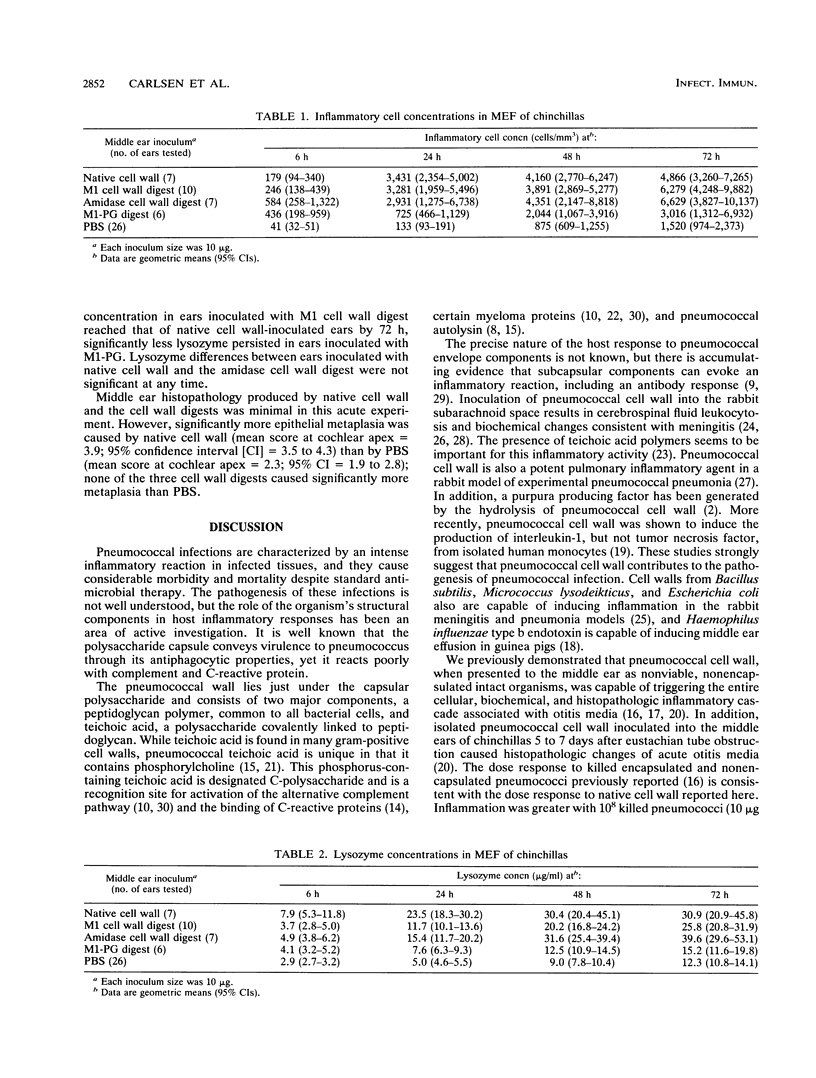
The pathogenesis of middle ear inflammation caused by Streptococcus pneumoniae was explored in the chinchilla model with different pneumococcal cell wall (CW) preparations, including isolated native CW, M1 muramidase CW (M1-CW) digest, amidase CW digest, and M1 peptidoglycan (M1-PG) digest. Inflammatory cell and lysozyme concentrations in middle ear fluid (MEF) were measured between 6 and 72 h after the middle ears were inoculated with one of the preparations or sterile saline. Middle ear histopathology was measured quantitatively at 72 h. Native CW, M1-CW digest, and amidase-CW digest caused significantly more inflammatory cell influx and lysozyme accumulation in MEF than saline did. M1-PG digest also caused more inflammatory cell influx and lysozyme accumulation in MEF than saline did but caused less inflammation than native CW or either CW digest. Epithelial metaplasia was significantly greater in ears inoculated with native CW than in ears inoculated with the CW or PG digest or with saline. Pneumococcal CW is, therefore, the principal factor that initiates middle ear inflammation in acute pneumococcal otitis media, and CW teichoication seems to be important in initiating this response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canafax D. M., Nonomura N., Erdmann G. R., Le C. T., Juhn S. K., Giebink G. S. Experimental animal models for studying antimicrobial pharmacokinetics in otitis media. Pharm Res. 1989 Apr;6(4):279–285. doi: 10.1023/a:1015938205892. [DOI] [PubMed] [Google Scholar]
- Chetty C., Kreger A. Generation of purpura-producing principle from pneumococcal cell walls. J Bacteriol. 1985 Jul;163(1):389–391. doi: 10.1128/jb.163.1.389-391.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria T. F., Prior R. B., Briggs B. R., Lim D. J., Birck H. G. Endotoxin in middle-ear effusions from patients with chronic otitis media with effusion. J Clin Microbiol. 1984 Jul;20(1):15–17. doi: 10.1128/jcm.20.1.15-17.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bustos J. F., Tomasz A. Teichoic acid-containing muropeptides from Streptococcus pneumoniae as substrates for the pneumococcal autolysin. J Bacteriol. 1987 Feb;169(2):447–453. doi: 10.1128/jb.169.2.447-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebink G. S., Juhn S. K., Weber M. L., Le C. T. The bacteriology and cytology of chronic otitis media with effusion. Pediatr Infect Dis. 1982 Mar-Apr;1(2):98–103. doi: 10.1097/00006454-198203000-00007. [DOI] [PubMed] [Google Scholar]
- Giudicelli S., Tomasz A. Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J Bacteriol. 1984 Jun;158(3):1188–1190. doi: 10.1128/jb.158.3.1188-1190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummell D. S., Berninger R. W., Tomasz A., Winkelstein J. A. The fixation of C3b to pneumococcal cell wall polymers as a result of activation of the alternative complement pathway. J Immunol. 1981 Oct;127(4):1287–1289. [PubMed] [Google Scholar]
- Johnston H. C., Shigeoka A. O., Hurley D. C., Pysher T. J. Nocardia pneumonia in a neonate with chronic granulomatous disease. Pediatr Infect Dis J. 1989 Aug;8(8):526–528. doi: 10.1097/00006454-198908000-00011. [DOI] [PubMed] [Google Scholar]
- Kawana M., Kawana C., Giebink G. S. Penicillin treatment accelerates middle ear inflammation in experimental pneumococcal otitis media. Infect Immun. 1992 May;60(5):1908–1912. doi: 10.1128/iai.60.5.1908-1912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. S., Lim D. J., Lang R. W., Birck H. G. Chronic middle ear effusions. Immunochemical and bacteriological investigations. Arch Otolaryngol. 1975 May;101(5):278–286. doi: 10.1001/archotol.1975.00780340010003. [DOI] [PubMed] [Google Scholar]
- Luotonen J., Herva E., Karma P., Timonen M., Leinonen M., Mäkelä P. H. The bacteriology of acute otitis media in children with special reference to Streptococcus pneumoniae as studied by bacteriological and antigen detection methods. Scand J Infect Dis. 1981;13(3):177–183. doi: 10.3109/inf.1981.13.issue-3.04. [DOI] [PubMed] [Google Scholar]
- Mold C., Nakayama S., Holzer T. J., Gewurz H., Du Clos T. W. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J Exp Med. 1981 Nov 1;154(5):1703–1708. doi: 10.1084/jem.154.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Nonomura N., Giebink G. S., Juhn S. K., Harada T., Aeppli D. Pathophysiology of Streptococcus pneumoniae otitis media: kinetics of the middle ear biochemical and cytologic host responses. Ann Otol Rhinol Laryngol. 1991 Mar;100(3):236–243. doi: 10.1177/000348949110000313. [DOI] [PubMed] [Google Scholar]
- Nonomura N., Giebink G. S., Zelterman D., Harada T., Juhn S. K. Early biochemical events in pneumococcal otitis media: arachidonic acid metabolites in middle ear fluid. Ann Otol Rhinol Laryngol. 1991 May;100(5 Pt 1):385–388. doi: 10.1177/000348949110000507. [DOI] [PubMed] [Google Scholar]
- Nonomura N., Nakano Y., Satoh Y., Fujioka O., Niijima H., Fujita M. Otitis media with effusion following inoculation of Haemophilus influenzae type b endotoxin. Arch Otorhinolaryngol. 1986;243(1):31–35. doi: 10.1007/BF00457904. [DOI] [PubMed] [Google Scholar]
- Riesenfeld-Orn I., Wolpe S., Garcia-Bustos J. F., Hoffmann M. K., Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989 Jul;57(7):1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley-Petzoldt M. L., Giebink G. S., Juhn S. K., Aeppli D., Tomasz A., Tuomanen E. The contribution of pneumococcal cell wall to the pathogenesis of experimental otitis media. J Infect Dis. 1988 Feb;157(2):245–255. doi: 10.1093/infdis/157.2.245. [DOI] [PubMed] [Google Scholar]
- Szu S. C., Clarke S., Robbins J. B. Protection against pneumococcal infection in mice conferred by phosphocholine-binding antibodies: specificity of the phosphocholine binding and relation to several types. Infect Immun. 1983 Feb;39(2):993–999. doi: 10.1128/iai.39.2.993-999.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen U. B., Henrichsen J. Cross-reactions between pneumococci and other streptococci due to C polysaccharide and F antigen. J Clin Microbiol. 1987 Oct;25(10):1854–1859. doi: 10.1128/jcm.25.10.1854-1859.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Saukkonen K. The nature of cell wall-derived inflammatory components of pneumococci. Pediatr Infect Dis J. 1989 Dec;8(12):902–903. doi: 10.1097/00006454-198912000-00034. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Hengstler B., Rich R., Bray M. A., Zak O., Tomasz A. Nonsteroidal anti-inflammatory agents in the therapy for experimental pneumococcal meningitis. J Infect Dis. 1987 May;155(5):985–990. doi: 10.1093/infdis/155.5.985. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Hengstler B., Zak O., Tomasz A. Induction of meningeal inflammation by diverse bacterial cell walls. Eur J Clin Microbiol. 1986 Dec;5(6):682–684. doi: 10.1007/BF02013304. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985 May;151(5):859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Rich R., Zak O. Induction of pulmonary inflammation by components of the pneumococcal cell surface. Am Rev Respir Dis. 1987 Apr;135(4):869–874. doi: 10.1164/arrd.1987.135.4.869. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Tomasz A., Hengstler B., Zak O. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J Infect Dis. 1985 Mar;151(3):535–540. doi: 10.1093/infdis/151.3.535. [DOI] [PubMed] [Google Scholar]
- Wallick S., Claflin J. L., Briles D. E. Resistance to Streptococcus pneumoniae is induced by a phosphocholine-protein conjugate. J Immunol. 1983 Jun;130(6):2871–2875. [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative complement pathway by pneumococcal cell wall teichoic acid. J Immunol. 1978 Jan;120(1):174–178. [PubMed] [Google Scholar]



