Abstract
The TOR proteins, originally identified as targets of the immunosuppressant rapamycin, contain an ATM-like “lipid kinase” domain and are required for early G1 progression in eukaryotes. Using a screen to identify Saccharomyces cerevisiae mutants requiring overexpression of Tor1p for viability, we have isolated mutations in a gene we call ROT1 (requires overexpression of Tor1p). This gene is identical to DNA2, encoding a helicase required for DNA replication. As with its role in cell cycle progression, both the N-terminal and C-terminal regions, as well as the kinase domain of Tor1p, are required for rescue of dna2 mutants. Dna2 mutants are also rescued by Tor2p and show synthetic lethality with tor1 deletion mutants under specific conditions. Temperature-sensitive (Ts) dna2 mutants arrest irreversibly at G2/M in a RAD9- and MEC1-dependent manner, suggesting that Dna2p has a role in S phase. Frequencies of mitotic recombination and chromosome loss are elevated in dna2 mutants, also supporting a role for the protein in DNA synthesis. Temperature-shift experiments indicate that Dna2p functions during late S phase, although dna2 mutants are not deficient in bulk DNA synthesis. These data suggest that Dna2p is not required for replication fork progression but may be needed for a later event such as Okazaki fragment maturation.
INTRODUCTION
Helicases are a class of nucleoside triphosphate (NTP)-dependent enzymes that catalyze the unwinding of double-stranded nucleic acids by disrupting the hydrogen bonds between complementary base pairs (bp). DNA helicases that unwind duplex DNA are essential for DNA replication, recombination, and repair, while RNA helicases are required for transcription, pre-mRNA processing, regulation of RNA stability, ribosome assembly, and protein translation. All helicases contain seven characteristic regions of similarity, termed I, Ia, and II–VI (Gorbalenya et al., 1989). Motifs I and II comprise a nucleotide binding/hydrolysis domain, and motif II contains the so-called DEAD, DEAH, or DEXH boxes that are found in a variety of viral and eukaryotic RNA helicases (Schmid and Linder, 1992). The exact function of the other motifs is not clear, although each region is presumed to represent a functional domain having a role in either NTP binding and/or hydrolysis as well as in catalyzing the separation of duplex nucleic acid.
Although originally envisioned as being required for separation of double-stranded template DNA to allow advancement of the replication fork, it is now appreciated that DNA helicases are required for a variety of processes, including that of Okazaki fragment maturation and repair of replicative errors (Matson et al., 1994). In budding yeast, many helicases have been identified genetically or biochemically that are thought to have a role in DNA replication (Li et al., 1992a, 1992b; Shimizu and Sugino, 1993; Schulz and Zakian, 1994; Biswas et al., 1995; Budd and Campbell, 1995). Despite this, definitive roles for these enzymes in either leading or lagging strand synthesis have not yet emerged.
Recently, Campbell and co-workers identified the Dna2p protein as an essential helicase required for DNA synthesis in S. cerevisiae (Budd et al., 1995; Budd and Campbell, 1995). They demonstrate that the enzyme catalyzes separation of DNA:DNA hybrids in vitro and speculate that the 3′→5′ polarity of the enzyme is consistent with a role in leading strand synthesis. Recent evidence suggests that Dna2p interacts functionally and physically with the product of the RAD27/RTH1 gene (Budd and Campbell, 1997). Because the mammalian homolog of this gene product, FEN-1, is required for Okazaki fragment processing in vitro, the authors have suggested that Dna2p (via interaction with the yeast FEN1 gene product) might, in fact, have a role in lagging strand DNA synthesis (Goulian et al., 1990; Turchi et al., 1994). However, there are no clear data to support a role for Dna2p in either leading or lagging strand synthesis.
Rapamycin is a potent macrolide immunosuppressant that inhibits G1 progression in both mammalian and yeast cells (Schreiber, 1991; Terada et al., 1993; Barbet et al., 1996). The TOR proteins, which include yeast Tor1p and Tor2p and the mammalian homologues FRAP/mTOR/RAFT/RAPT1, were originally identified by both biochemical and genetic methods as the functionally relevant targets of rapamycin (Heitman et al., 1991; Brown et al., 1994; Chiu et al., 1994; Sabatini et al., 1994; Sabers et al., 1995). These proteins contain a lipid kinase domain found in phosphatidyl inositol (PI) kinases, enzymes that are required for the production of PI-derived second messengers (Carpenter and Cantley, 1990). However, the TORs belong to the so-called phosphatidyl inositol kinase (PIK)-related family of kinases, whose members have roles in a variety of processes such as cell cycle progression, meiotic and V(D)J recombination, chromosome maintenance and repair, and DNA checkpoint signal transduction (Keith and Schreiber, 1995). It is postulated that the TORs, like other PIK-related family members, such as DNA-PK, function as protein kinases rather than (or in addition to) lipid kinases (Hunter, 1995; Keith and Schreiber, 1995). While some groups have reported associated PI 4-kinase activity with immunoprecipitated Tor2p and RAFT1 (Cardenas and Heitman, 1995; Sabatini et al., 1995), others have failed to do so, and it is unclear whether this activity is intrinsic to or associated with the TOR proteins.
Multiple studies implicate the TOR/FRAP proteins in the control of protein translation. Rapamycin blocks protein synthesis in yeast by almost 90% (Drebot et al., 1987), while the effect in mammalian cells is cell type-specific, ranging from 10 to 75% (Terada et al., 1994; Giasson and Meloche, 1995). In addition, TOR/FRAP activity is required for activation of p70 S6 kinase, the physiologic regulator of phosphorylation of the S6 ribosomal subunit that is believed to have a role in the control of protein translation in higher eukaryotes (Hershey, 1989; Chung et al., 1992). In addition, rapamycin blocks initiation of translation of transcripts containing 5′ polypyrimidine tracts (Jefferies et al., 1994). More recently, it has been shown that rapamycin inhibits the growth factor-induced up-regulation of eIF-4E activity, a factor required for the initiation of translation of all 5′ capped transcripts (Graves et al., 1995; Lin et al., 1995).
However, it is not clear that the only role for the TOR proteins is to regulate protein translation. That is, the TOR proteins may be involved in the control of a wide variety of events that are required to execute a cellular response to an environmental stimulus, one of these being modulation of the translational apparatus. For example, yeast treated with rapamycin display all of the characteristics of starved cells (Barbet et al., 1996). In addition, the yeast TOR proteins have been implicated in the control of the activity of Sit4p, a type 2A phosphatase required for G1 progression (Di Como and Arndt, 1996). Control of phosphatase function provides a potential mechanism for how the TORs might control a pleiotropic array of responses, since phosphatases are involved in a variety of distinct cellular processes. Recent evidence suggests that Tor2p is localized to the vacuole and plays a role in the fidelity of vacuolar segregation to mother and daughter cells (Cardenas and Heitman, 1995). More recently, Hall and colleagues have shown a role for Tor2p in the regulation of Rho-like guanosine triphosphatases and the actin cytoskeleton (Schmidt et al., 1996, 1997).
Regardless of their cellular function, it is still unknown what mechanisms (if any) regulate the TORs or upon what substrates these putative kinases act. In a screen designed to identify genes that operate in the TOR-signaling pathway, we have isolated mutants that are dependent upon overexpression of Tor1p for viability. One such class of yeast were novel dna2 mutants. In this paper we describe the isolation and characterization of these mutants and present the results of genetic experiments designed to illuminate the nature of the interaction between Tor1p and Dna2p. In addition, we describe the construction of temperature-sensitive (Ts) mutants that demonstrate that Dna2p acts late in S phase to perform an essential function that is consistent with a role in lagging strand DNA synthesis.
MATERIALS AND METHODS
Strains, Plasmids, and Media
The composition of rich glucose medium (YPD), rich galactose medium (YPGal), synthetic minimal medium (SD), synthetic complete medium (SC), and liquid sporulation medium (Spo) was as described (Sherman, 1991). YPD and YPGal media were adjusted to pH 6.0 with 1 N HCl. Synthetic plates containing 5-fluoroorotic acid (5-FOA) (PCR, Gainesville, FL) were made as described (Sikorski and Boeke, 1991). SD+canavanine plates were prepared by supplementing SD with amino acids and nucleotides dictated by strain auxotrophies as well as 60 g/l canavanine (Sigma Chemical, St. Louis, MO). All solid medium contained 2% agar except the YPGal plates used in the original sectoring screen, which contained 1.8% agar and 100 mg/ml ampicillin (Sigma) to facilitate analysis by spreading colonies and to prevent bacterial contamination. α Factor (Sigma) was added to media from a 1 mg/ml stock in 10 mM HCl (stored at −20°C) to a final concentration of 5 μg/ml. Hydroxyurea (HU) (Sigma) was added to YPD to a final concentration of 0.2 M and sterile filtered before use. Nocodazole (Sigma) was added from a 3 mg/ml stock in dimethylsulfoxide (stored at −20°C) to a final concentration of 15 μg/ml.
Yeast mating, sporulation, and tetrad dissection were performed according to standard methods (Sherman and Hicks, 1991). Yeast were transformed by the lithium acetate method (Ito et al., 1983).
pYDF18 is an N-terminal hemagglutinin (HA)-tag expression vector derived from PG-1 (a 2μ, TRP1 -based plasmid utilizing the G6PD promoter) (Schena et al., 1991). pYDF66, an HA-Tor1p expression vector, was made by cloning the full-length TOR1 gene into pYDF18. The following genomic restriction sites within TOR1 were used to make amino- and carboxyl-terminal deletions of Tor1p using pYDF66 as a template: Spe1 (Δ1–99); NdeI (Δ1–416); XhoI (Δ1–724); SacII (Δ1–1198); NcoI (Δ1–1764); AccI (Δ1–1882); PflM1 (Δ1–2067); BsiW1 (Δ1–2225); AatII (Δ2441–2470); BglII (Δ2260–2470). Kinase-defective mutants of Tor1p (Zheng et al., 1995) were cloned into pYDF66 as C-terminal BsiW1/Sal fragments. pYDF77 is GAL1p→TOR1 on red/white indicator plasmid, made by cloning a NheI/SalI fragment containing the TOR1 gene into pMW29, a URA3-based centromeric plasmid containing the ADE3 gene and the GAL1 promoter with a MCS downstream (Zieler et al., 1995). pYDF88 is a LEU2-based form of pYDF77 without the ADE3 gene and was used for plasmid shuffling as described (Zieler et al., 1995). PYDF125 (a generous gift of S. Schreiber, Harvard University, Boston, MA) is an HA-tagged form of GAL1p→TOR1 in the LEU2-based vector, PRS315 (Sikorski and Hieter, 1989). pYDF117 was created by cloning a ClaI/PstI genomic fragment containing the DNA2 gene into ClaI/PstI-digested PRS304, a TRP1-based integration vector (Sikorski and Hieter, 1989). The TOR1 disruption plasmid (pYDF79) was made by ligating a BamHI/XhoI fragment (nucleotides 988–2172) and a SacII/BamHI fragment (nucleotides 3600–5047) simultaneously into XhoI/SacII-digested PRS304 (Sikorski and Hieter, 1989) to create a disruption fragment that removes amino acids 330–724 of Tor1p and replaces them with the TRP1 gene and pBluescript (Stratagene, La Jolla, CA).
CB018 is a protease-deficient strain constructed by C. Brenner (Brandeis University, Waltham, MA). YDF92-YDF102 are derived from either TW397 (wild-type), TW398 (rad9), or TW308 (mec1–1) (Weinert et al., 1994). Strain YDF20 (tor1-Δ10) was created by transforming YMW1 with BamHI-digested pYDF79 and selecting for TRP+ cells (deletion confirmed by Southern blotting). Strain YDF104 was constructed by mating DBY1034 with DBY1511 (D. Botstein, Stanford University, Stanford, CA). YDF105 was constructed by genomic replacement of the DNA2 gene with the dna2–22 temperature-sensitive (Ts) allele (see below) in strain DBY1034 and mating this strain with DBY1511. YDF106 was constructed by genomic replacement of the DNA2 gene with the dna2–212 Ts allele in both strains DBY1034 and DBY1511 and then mating these two strains. Unless otherwise stated, the dna2–22 Ts mutation was introduced into all strain backgrounds by integrative transformation and never by crossing strains (see below). Thus, all comparisons between dna2 mutant cells and wild-type cells are between strictly isogenic strains.
Sectoring Screen for Mutants Requiring Tor1p Overexpression
To identify mutants requiring a Tor1p overexpression plasmid for viability, we performed a red/white sectoring assay utilizing yeast expression plasmids and strains exactly as described (Zieler et al., 1995). Briefly, the strain background (YMW1) is ade2 ade3, which produces white colonies on rich media, but cells carrying a plasmid with the ADE3 gene (“indicator plasmid”) will form red colonies. During growth, the small percentage of cells that randomly lose the plasmid give rise to white “sectors” within an otherwise red colony. Any colony that is all red represents either yeast that have permanently converted to ADE3+ phenotype (via a plasmid recombination or gene conversion event) or yeast that require sequences on the plasmid for viability. By including an expression cassette for Tor1p on this plasmid, it is thus possible to screen for mutants that require this expression cassette (i.e., overexpression of Tor1p) for viability. The YMW1/2 strains were transformed with the a GAL1p→Tor1p overexpression indicator plasmid (pYDF77) and mutagenized with ethylmethane sulfonate (Sigma) to 50% viability as described (Lawrence, 1991). This plasmid gives functional overexpression of Tor1p as assessed by a 5-fold increase in rapamycin resistance when cells are grown in YPGal (our unpublished results). Mutagenized cells (stored in water at 4°C) were plated on 150-mm YPGal plates (to allow Tor1p overexpression) at a density of 500/plate and incubated at 30°C for 5–7 d. A total of 80,000 colonies were screened. Potential positive nonsectoring colonies were then screened for true Tor1p overexpression dependence as described (Zieler et al., 1995). Briefly, this included requiring that mutants: 1) die or grow very slowly on YPD; 2) die or grow very slowly on FOA-containing plates that select against the indicator plasmid; 3) are able to lose the indicator plasmid on YPGal if provided with an alternate GAL1p→TOR1 overexpression plasmid (pYDF88) not containing the ADE3 gene. All mutants were recessive and found to represent single gene defects as assessed by mating and tetrad analysis (our unpublished results).
Cloning of the ROT1/DNA2 Gene
Mutant 2–71 (rot1–1) containing pYDF88 (GAL1p→TOR1) was grown overnight in YPGal and transformed with 4 μg of a genomic DNA library in YCP50 (Rose et al., 1987), and transformants were selected on SCGal-ura plates (∼250,000 colonies total). After 2 d at 30°C, transformants were replica plated to YPD plates for 1 d at 30°C. Colonies demonstrating growth on YPD (120 total) were picked and restreaked on glucose-containing FOA plates for 2 d. Of these, two colonies that showed virtually no growth were then picked from the original SCGal-ura plates and plasmid DNA was extracted (Strathern and Higgins, 1991). Upon restriction mapping, it was apparent that both of the yeast colonies contained the identical library plasmid, and dideoxy sequencing analysis was carried out as described (Sambrook et al., 1989).
To confirm that a mutation in DNA2 is the relevant alteration in the rot1–1 mutant, a strain was constructed that contained the TRP1 gene integrated at the DNA2 locus by transforming yeast with a plasmid containing the TRP1 and DNA2 genes (pYDF117) that had been linearized by cutting with a single NdeI site within the DNA2 gene; this generated a genomic duplication of the DNA2 genes separated by the TRP1 gene (confirmed by Southern blotting). This strain (YDF26) was mated to the rot1–1 mutant, and, upon dissection of 14 tetrads onto YPD plates, all viable spores were found to be TRP1+, indicating that the mutation is tightly linked to DNA2 (our unpublished results).
Locating dna2 Mutations by Gap Repair
The location of the dna2–20 and dna2–21 mutations was determined by a gap repair method (Rothstein, 1991). This analysis was performed using gapped variants of a DNA2-containing ClaI/PstI genomic fragment cloned into PRS314 (Sikorski and Hieter, 1989) as templates for repair. The dna2 mutants carrying pYDF88 were transformed with these gapped variants and selected on SCGal-leu-trp (selecting for Tor1p overexpression as well as the repaired plasmid). Individual colonies (20) were picked and restreaked on YPD at 30°C and assayed for growth. The presence of only mutant colonies (i.e., inability to grow on YPD) was inferred to indicate that a particular template was missing the region mutated. This analysis located the presence of both the dna2–20 and dna2–21 mutations between a Bsm1 and PpuM1 site spanning amino acids 1076–1197. This region was amplified from mutant genomic DNA by the polymerase chain reaction, and sequence analysis demonstrated that each mutant contained only one mutation that would result in an amino acid alteration (our unpublished results).
Construction of dna2 Mutants
The dna2-Δ20 disruption strain was constructed by replacing nucleotides 366-4215 of the genomic DNA2 sequence with a BamHI fragment containing the TRP1 gene by using the one-step gene replacement technique (Rothstein, 1991) with a 2.7-kb ClaI/PstI disruption fragment. Diploid transformants were selected on SC-trp and disruptants screened by polymerase chain reaction with the following primers: ROT-1A (5′-GATCGTCGACGGGATCCCGAATTCCATGCCCGGAA-CGCCACAGAAG-3′); TRP-1B (CACGTGCTCAATAGTCACCAA-TGC). Of 13 screened, six were positive and one of these was chosen and called YDF27.
dna2 conditional alleles were isolated by a plasmid shuffle technique as described (Sikorski and Boeke, 1991). The recipient host strain was constructed by transforming YDF27 with pYDF113, dissecting tetrads, and selecting for a dna2 disruptant (i.e., TRP1+). Plasmid pYDF132 (containing a DNA2 genomic fragment in a LEU2-based centromeric vector) was the template for mutagenesis with hydroxylamine (Sigma). The efficiency of mutagenesis was assessed by the frequency of loss-of-function mutations in the LEU2 gene on the plasmid. Mutagenesis to 4–5% loss of leucine prototrophy (assessed in a leuB6 HB101 bacterial strain) was chosen, and this DNA was transformed directly into the recipient strain. Approximately 12,500 transformants were screened, resulting in 10 strains showing wild-type growth at 30°C and no growth at 37°C as assessed by colony-forming ability (our unpublished results). One of these, dna2–22, was selected and integrated into the yeast genome using the pop-in/pop-out gene replacement technique (Scherer and Davis, 1979).
Preparation of Anti-Dna2p Antibodies
Bacteria were transformed with a GST-ROT1p fusion constructed by cloning a NcoI/Bsm1 fragment of the DNA2 gene encoding aa 121–251 into the GEX2T expression vector (Smith and Johnson, 1988). GST fusion proteins were prepared and purified as described (Smith and Johnson, 1988) and injected into rabbits. Rabbits were boosted biweekly, and antisera were affinity purified using GST-Dna2p CNBr-activated columns (Pharmacia, Washington, DC) as described (Harlow and Lane, 1988). The reactivity of the antisera was assessed by comparing immunoblots of extracts from wild-type cells with those from cells overexpressing the Dna2p protein from the GAL1 promoter. A doublet representing polypeptides of molecular masses of approximately 170 and 190 kDa, which was detected only with immune serum and was expressed at approximately 20-fold excess over that of control cells, was assumed to represent the Dna2p polypeptide(s) (our unpublished results).
Preparation of Yeast Whole Cell Extracts
Log-phase yeast cells were spun down and washed in lysis buffer (20 mM Tris, pH 7.6, 150 mM NaCl, 2 mM EDTA, 0.2% TritonX-100), and 100 μl of cells were spun into each Eppendorf tube and resuspended in 150 μl lysis buffer with protease inhibitors (Boehringer Mannheim, Indianapolis, IN) (1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 100 μg/ml antipain, 10 μg/ml pepstatinA, 2 mM benzamidine, 10 μg/ml aprotinin, 65 μg/ml l-1-tosylamide-2-phenylethylchloromethyl, 65 μg/ml TLCK). Two hundred microliters of glass beads (425- to 600-μm, Sigma) were added, and the tubes were vortexed twice for 5 min. Cell lysis was defined by >80% of the cells being trypan blue permeable. The tubes were punctured in the bottom with a 26.5-gauge needle, and supernatants were spun into clean tubes at 2000 × g for 30 sec. The supernatants were then spun at 15,000 × g for 5 min, transferred to TLA.100.2 tubes, and spun at 100,000 × g for 20 min at 4°C in a Beckman TL-100 ultracentrifuge (Beckman, Fullerton, CA). Supernatants were assayed for protein concentration by the Bradford assay and either used immediately or made 30% (vol/vol) in glycerol and frozen at −80°C.
Immunoprecipitation and Immunoblotting
Protein A-Sepharose (Pharmacia) beads were washed several times in lysis buffer (see above) and, for each sample, 15 μl packed beads were incubated with 1 mg extract protein plus proper amount of antibody (1/30 μl of anti-Dna2p affinity purified antibodies or 1 μl 12CA5 anti-HA ascites), and the total volume was brought up to 500 μl in lysis buffer. Tubes were rotated at 4°C for 2 h and then spun for 1 min at 10,000 × g and washed three to four times in lysis buffer with protease inhibitors. After the final wash, pellets were resuspended in 1× SDS sample buffer and boiled 5 min. Immunoprecipitates or whole extracts were separated using SDS-PAGE and transferred to nitrocellulose as described (Harlow and Lane, 1988; Sambrook et al., 1989). Immunoblotting was carried out essentially as described using affinity-purified anti-Dna2p antibodies (1:10,000), anti-HA ascites (1:1000), anti-actin antisera (1:10,000) (a kind gift of D. Botstein, Stanford University). Horseradish peroxidase (HRP)-conjugated reagents include affinity-purified goat anti-rabbit-HRP antibodies (1:3000) (Jackson Immunoresearch, West Grove, PA), rabbit anti-mouse-HRP antibodies (1:1500) (Jackson) (Harlow and Lane, 1988), and Protein A-HRP (Jackson). Protein levels were quantified using a model 300A densitometer and ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Flow Cytometry
Haploid yeast were grown under the appropriate experimental conditions to approximately 1 × 107 cells/ml. Cells (5–10 ml) were sonicated for 20 sec on ice with a microtip sonicator on setting 2 to disperse clumps. Cells were washed once with Tris buffer (50 mM Tris-HCl, pH 7.5) and resuspended in 1.5 ml distilled water to which 3.5 ml 100% ethanol were added (70% final) and incubated for 1 h at room temperature. Cells were washed with 5 ml Tris buffer and resuspended in 1 ml of RNase (Sigma) buffer (10 mg/ml ribonuclease A in sodium acetate, pH 5.0, diluted 1:10 into Tris buffer). Cells were spun down and resuspended in 1 ml of freshly made pepsin (Sigma) solution (5 mg/ml in 0.05 N HCl) for 5 min at room temperature. Cells were spun down and washed in 2 ml propidium iodide (PI, Sigma) staining solution (0.05 mg/ml PI diluted into 200 mM Tris, pH 7.5, 200 mM NaCl, 70 mM MgCl2) and incubated for 1 h at room temperature and then overnight at 4°C in the dark. Cells were washed into 3 ml Tris buffer and analyzed on a FACScan machine (Becton-Dickinson, Lincoln Park, NJ). Twenty thousand events were analyzed for each sample.
α Factor Synchronization/Release Protocol
Log-phase cells were resuspended in 1 ml YPD at OD 0.05, and α factor was added to 5 μg/ml and cells were cultured for 2.5 h at 30°C. Cell cycle arrest was monitored by accumulation of >95% unbudded cells as visualized by phase contrast microscopy. In some experiments cells were then placed in a 37°C shaking water bath, and 5 μg of α factor were added every hour for 1–3 h. Next, cells were spun down in an Eppendorf tube, washed twice in YPD, and diluted 1:20 into prewarmed YPD with 0.05 mg/ml pronase (Sigma) and 0.05 mg/ml proteinase K (Sigma) and cultured at the appropriate temperature. If indicated, HU and nocodazole were added at this time. At different times after α factor release, 75 μl were removed from the cultures and diluted into 425 μl cold YPD on ice to immediately halt cell cycle progression. Cells were sonicated with a microtip sonicator, diluted, and plated on duplicate YPD plates for 2 d at 30°C. A minimum of 150 colonies was counted for each time point.
Mitotic Recombination and Chromosome Loss Assay
Recombination and chromosome loss frequencies were quantified using the selection scheme of Hartwell and Smith (1985), essentially as described (Holm et al., 1989).
4,6-Diamidino-2-Phenylindole (DAPI) Staining
Cells were grown to an OD between 0.1 and 0.2 at the appropriate temperature before addition of 10% ultrapure formaldehyde (Polysciences, Inc., Warrington, PA) to a final concentration of 4%. After 10 min, cells were resuspended in 5 ml phosphate buffer (40 mM KPO4, pH 6.5, 0.5 mM MgCl2) with 4% formaldehyde and incubated for 1 h at the appropriate temperature. Next, cells were washed twice in phosphate buffer and resuspended in mounting medium, consisting of 10 mg/ml p-phenylenediamine (Sigma) in PBS, pH 9.0, in 90% glycerol with 20 ng/ml DAPI (4′, 6′-diamidino-2-phenylindole) (Accurate Chemical and Scientific Corp., Westbury, NY). Cells were visualized with Nomarski optics using a Zeiss Axioskop (Carl Zeiss, Thornwood, NY) with a 100×, 1.4 plan-neofluor lens.
RESULTS
A Screen for Mutants Requiring Elevated Levels of Tor1p
We have utilized a sectoring assay (Hartwell and Smith, 1985; Sanchez et al., 1996) that employs a TOR1+ strain and a GAL1p→TOR1 indicator plasmid to identify mutants that require the indicator plasmid (i.e., Tor1p overexpression) for optimal growth. On media containing galactose (YPGal) this plasmid gives functional overexpression of Tor1p when compared with cells grown with the plasmid in glucose (YPD), which suppresses Tor1p expression, and thus mutants were screened that require the plasmid on YPGal but cannot survive (or grow poorly) on YPD. This screen not only identifies mutants absolutely requiring Tor1p overexpression for viability but also those for which increased levels of Tor1p provide a relative growth advantage. Thus, a tor1 deletion mutant fails to display sectoring under these conditions, presumably due to the slight growth advantage of TOR1+ over tor1− cells in our strain background (Figure 4A; our unpublished results). A screen of ∼80,000 mutagenized yeast yielded 10 recessive mutants. Seven of these were tor1 mutants, as assessed by linkage to a tor1 deletion mutant (our unpublished results). Of the remaining three mutants, one was found to be in the TOR2 gene (our unpublished results). Since the purpose of this study was to identify novel genes in a putative TOR-signaling pathway, these mutants will not be described further.
Figure 4.
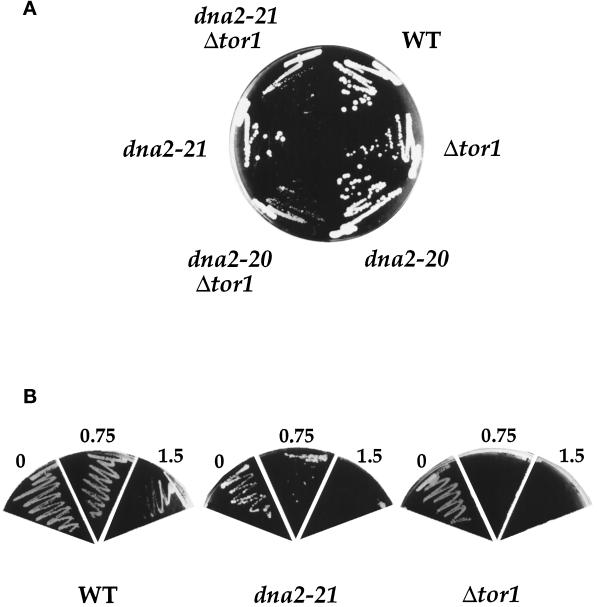
dna2 mutants show synthetic interactions with the TOR pathway. (A). dna2 tor1 mutants are slow growing. Strains YMW1 (WT), YDF20 (Δtor1), 2–71 (dna2–20), 2–174 (dna2–21), YDF117 (dna2–20 Δtor1), and YDF121 (dna2–21 Δtor1) were streaked on YPD plates containing 0.5 M sorbitol at 37°C for 60 h before photographing. (B) dna2 mutants show increased rapamycin sensitivity. Strains YMW1 (WT) and YDF20 (Δtor1), and 2–174 (dna2–21) were streaked on YPD plates containing indicated amounts of rapamycin at 30°C and photographed after 72 h of growth.
On the basis of complementation and linkage analysis, the final two mutants were found to be in the same gene which we named ROT1 (requires overexpression of Tor1p). This gene was cloned by complementation of the recessive rot1–1 mutant, and DNA sequencing revealed that the insert was a fragment from the right arm of chromosome VIII (Johnston et al., 1994). This insert complemented the growth defect of both rot1 mutants, and a deletion analysis of this fragment demonstrated that a 4.5-kb open reading frame encoded the complementing activity (our unpublished results). BLAST analysis (Altschul et al., 1990) revealed that this open reading frame corresponded to DNA2, a gene recently identified as being required for DNA replication in yeast and found to encode a protein with 3′→5′ DNA helicase activity (Budd et al., 1995; Budd and Campbell, 1995). As expected, the carboxyl-terminal one-third of the protein (amino acids 1072–1434) contains a region that closely matches a heptapartite consensus sequence that defines a superfamily of proteins with helicase activity (Schmid and Linder, 1992). That this gene may perform an essential function in all eukaryotic cells is underscored by the fact that a human cDNA clone has been identified that displays 34% overall identity (45% within the carboxyl-terminal 400 amino acids) to the yeast gene and is likely to encode the human homologue of DNA2 (Budd and Campbell, 1995). To avoid confusion, we have renamed the rot1–1 and rot1–2 mutants dna2–20 and dna2–21, respectively.
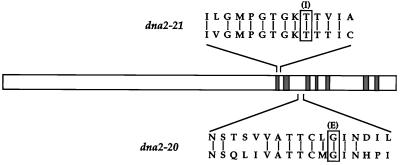
Since the sequence of DNA2 revealed at least one functional domain, we wished to determine whether the mutations in the dna2–20 and dna2–21 alleles are within this putative helicase region. The approximate location of the mutations was determined by a gap repair method and found to be similar in both mutants (see MATERIALS AND METHODS). Sequence analysis of this region revealed that the dna2–21 mutation is a C→T transition at nucleotide 3233, causing a thr1078→ile1078 change within region I, a domain known to comprise a critical part of the NTP-binding fold (Figure 1) (Walker et al., 1982). The dna2–20 mutation is a G→A transition at nucleotide 3500, causing a gly1167→glu1167 change in an area between regions II and III of the consensus helicase domain. These mutations are consistent with the fact that ethylmethane sulfonate mutagenesis exclusively causes transitions at G·C sites (Kohalmi and Kunz, 1988). Additionally, both of these mutations are in residues conserved between the yeast and putative human DNA2 genes (Budd and Campbell, 1995), and they occur within a region predicted to be essential for the helicase function of Dna2p. Indeed, mutations in the invariant lysine1080 in the NTP-binding fold of region I ablate the helicase and biological activity of DNA2 (Budd et al., 1995).
Figure 1.
Identification of the mutations in dna2–20 and dna2–21 alleles. Schematic drawing of the DNA2 sequence with the location of the seven helicase domains shown hatched and drawn to scale. Regions that contain the mutations are enlarged. The yeast and human DNA2 sequences are shown on the top and bottom, respectively, with conserved residues connected by bars. The mutated residue is boxed and the resulting amino acid shown in parentheses.
The dna2–20 Mutant is Specifically Rescued by Tor1p and Tor2p
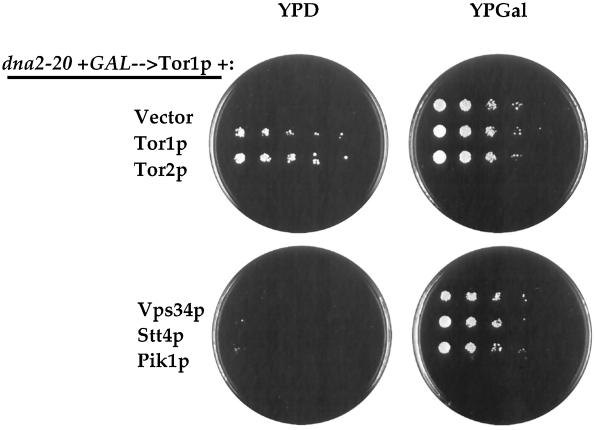
Because the purpose of this study was to learn more about the function that is shared by Tor1p and Tor2p, we tested the ability of Tor2p to rescue the dna2–20 mutant. In these experiments, the dna2–20 mutant carrying GAL1p→TOR1 is transformed with the overexpression plasmid to be tested and plated on either YPD (experimental) or YPGal (as a control for plating efficiency). As shown in Figure 2, TOR1 provided on a high-copy plasmid under control of the G6PD promoter effectively rescues the mutant on glucose-containing media, ruling out carbon source artifacts contributing to the original rescue with the GAL1p→TOR1 plasmid. In addition, a genomic fragment containing TOR2 (and its endogenous promoter) provided on a multicopy plasmid efficiently rescues the dna2–20 mutant (Figure 2). However, a similar multicopy vector containing a TOR1 genomic fragment is unable to suppress this mutant, which could be reflective of differences in either protein levels or potencies between Tor1p and Tor2p in this assay (our unpublished results). In addition, neither the wild-type TOR1 nor a rapamycin-resistant allele of TOR1 (ser1972→ile1972) when present on a centromeric plasmid is able to rescue the dna2–20 or dna2–21 mutants (our unpublished results).
Figure 2.
Rescue of dna2–20 is TOR-specific. The dna2–20 mutant carrying pYDF88 (GAL1p→TOR1) was transformed with the following plasmids: pYDF18 (vector), pYDF66 (Tor1p), pJK3–3 (Tor2p) (Kunz et al., 1993), 324.34 (Vps34p) (Herman and Emr, 1990), YEp352-STT4 (Stt4p) (Yoshida et al., 1994), and YEp352-PIK1 (Pik1p) (Flanagan et al., 1993). All of these plasmids are 2μ-based and contain the genes under control of their endogenous promoters, except for the Tor1p expression construct that utilizes the GPD promoter (see MATERIALS AND METHODS). Transformants were selected on SCGal-leu minus the appropriate amino acid to also select for the various plasmids. Cells were scraped off plates (representing ∼100 colonies), resuspended in water, sonicated, and spotted onto YPD and YPGal plates. Ten thousand cells were spotted on the leftmost region with successive fivefold dilutions moving to the right. The plates were incubated for 3 d at 30°C and photographed.
The specificity of this rescue was addressed by testing whether other yeast proteins that contain a lipid kinase domain could suppress the dna2–20 mutant. As shown in Figure 2, Vps34p (a PI 3-kinase involved in vacuolar protein sorting), Stt4p (a PI 4-kinase involved in cell wall integrity), and Pik1p (a nuclear PI 4-kinase) were all unable to rescue the dna2–20 mutant on YPD when present on multicopy plasmids. A GAL1p→MEC1 plasmid was also unable to rescue the dna2–20 mutant on galactose, suggesting that other PIK-related family members do not have this activity (our unpublished results).
Regions of Tor1p Essential for Rescue of dna2 Mutants
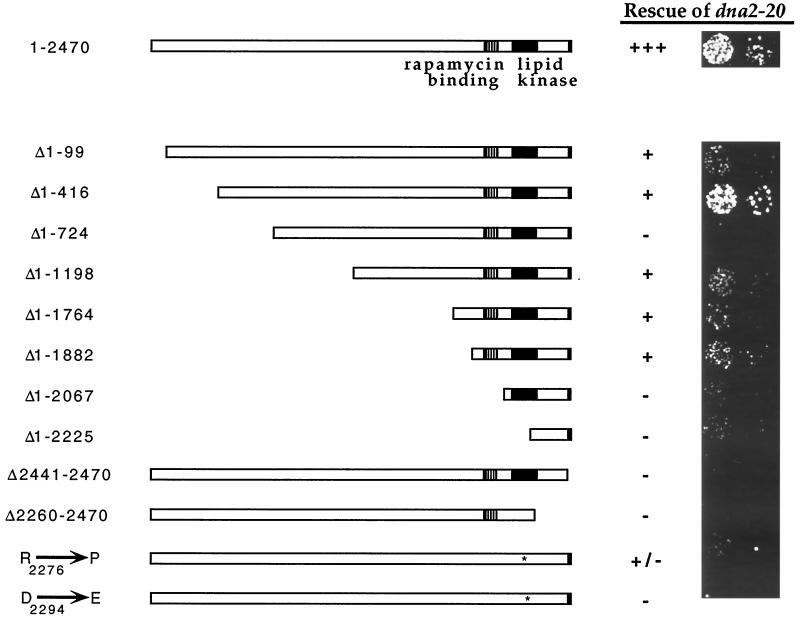
A deletion analysis of Tor1p was conducted to determine whether the rescuing function could be localized within the protein. As is shown in Figure 3, a 99-amino acid N-terminal deletion ablates much of the rescuing activity, but the residual function is removed only by N-terminal truncations that delete the FKBP-12-rapamycin binding (FRB) domain (Δ1–2067 in Figure 3). A 29-amino acid truncation at the C terminus completely abolishes rescuing activity and is of interest in that it removes a highly conserved domain that is found in the TOR/FRAP proteins as well as other PIK-related family members (Keith and Schreiber, 1995). The issue of whether the putative kinase activity is required for the suppression was addressed by employing the use of two kinase domain mutants, arg2276→pro2276 and asp2294→glu2294, which have been shown to be inactive with regards to their cell cycle function (Zheng et al., 1995). As shown in Figure 3, these mutants are virtually devoid of rescuing activity, although the arg2276→pro2276 is slightly active. Thus, reminiscent of their roles in G1 progression, both the amino- and carboxyl-terminal domains as well as the kinase activity of Tor1p are required for suppression of the dna2–20 mutant (Helliwell et al., 1994; Zheng et al., 1995).
Figure 3.
Regions of Tor1p required for rescue of dna2–20 mutant. The dna2–20 mutant carrying pYDF88 (GAL1p→>TOR1) was transformed with various Tor1p deletion constructs (see MATERIALS AND METHODS). Cells were scraped off transformation plates (∼100 colonies), resuspended in water, sonicated, and spotted onto YPD plates. Spots represent 1200 and 150 cells applied. Plates were incubated at 30°C for 3 d. The apparently elevated activity seen in the Δ1–416 mutant is not reproducible and is thus represented as +. The absence of suppression by the Δ1–724 construct may be due to low protein expression, as this mutant protein is not detected by immunoblot analysis (our unpublished results).
dna2 Mutants Are Compromised by a tor1 Deletion
Since they are rescued by elevated TOR activity, we next tested whether dna2 mutants are uniquely susceptible to a diminution in TOR function. To do this, we first needed to find conditions whereby the dna2 mutants are viable with wild-type levels of Tor1p. Both the dna2–20 and dna2–21 mutants are rescued for growth on YPD by osmotic stabilizers (e.g., 1 M sorbitol, 0.5 M NaCl) or growth at temperatures above 32°C (Figure 4A; our unpublished observations). Using these conditions, we first examined the effect of a tor1 deletion on the viability of dna2 mutants. It should be noted that tor1 cells grow slightly more slowly than wild-type cells at 30°C but are inviable at 37°C in our strain background unless grown on plates containing 0.5–1.0 M sorbitol (our unpublished observations). As seen in Figure 4A, both the dna2–20 and dna2–21 mutants form colonies on plates containing 0.5 M sorbitol at 37°C but grow very slowly when combined with a tor1 deletion even under conditions where the tor1 strain grows reasonably well. In addition, the dna2–21 mutant is synthetically lethal with a tor1 deletion at 32–35°C on YPD (our unpublished observations).
We also tested whether dna2 mutants show increased sensitivity to rapamycin. Since the dna2–21 mutant grows very slowly on YPD (i.e., in the absence of Tor1p overexpression), we tested this mutant (and the tor1 deletion for comparison) for sensitivity to the drug on YPD at 30°C. When compared with wild-type, the dna2–21 mutant is approximately twofold more sensitive to rapamycin, while a tor1 deletion strain is approximately three- to fourfold more sensitive (Figure 4B; our unpublished observations). In addition, the dna2–20 and dna2–21 mutants are 1.5- to twofold more sensitive to rapamycin when grown on YPD at 37°C (our unpublished observations).
Phenotype of dna2 Deletion Mutants
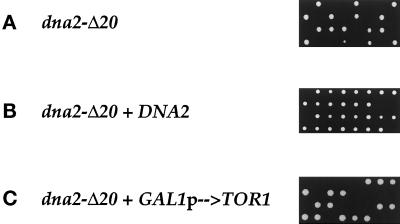
The DNA2 gene was disrupted by homologous recombination (see MATERIALS AND METHODS). DNA2+/dna2-Δ20 cells are viable and show no growth defects at 24°, 30°, or 37°C (our unpublished observations). Upon sporulation and tetrad dissection, these diploids gave rise to only two viable cells in each tetrad (Figure 5A), consistent with the previous observation that DNA2 is an essential gene (Budd and Campbell, 1995). Upon microscopic examination of the dna2-Δ20 cells that did not form colonies (15 tetrads examined), there were an average of 11 cells (ranging from 1 to 27) generated by each spore with >97% of the cells having a large budded morphology (our unpublished observations). Because the spores were able to divide, this demonstrates that the DNA2 gene is essential for viability (and not simply germination) and also indicates that the cell contains a relative excess of functional Dna2p protein. Next, DNA2+/dna2-Δ20 diploids were transformed with plasmids containing either the DNA2 gene or the GAL1p→TOR1 cassette and tetrads were dissected. Although the dna2 disruptants are rescued by a Dna2p expression plasmid (Figure 5B), none are rescued by the Tor1p overexpression plasmid (Figure 5C). dna2 deletion mutants are also not rescued by elevated temperatures or 1 M sorbitol (our unpublished observations).
Figure 5.
A dna2-Δ20 deletion mutant is inviable and not suppressed by Tor1p. YDF27 (dna2-Δ20/DNA2+) (A), or YDF27 transformed with pYDF113 (DNA2) (B), or pYDF77 (GAL1p→TOR1) (C) were sporulated and tetrads dissected on either YPD (A), (B), or YPGal (C), and plates were incubated at 30°C for 4 d and photographed.
Dna2p and Tor1p Do Not Physically Interact
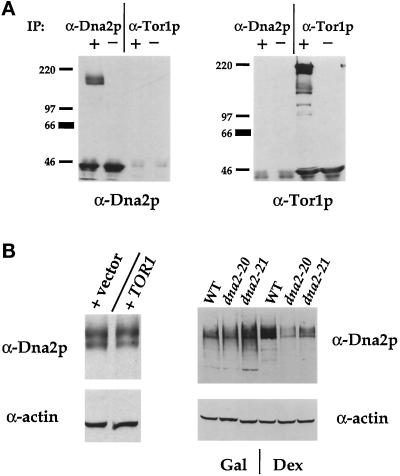
One explanation for the observation that the Tor1p-dependent suppression of dna2 mutants requires the Dna2p protein (Figure 5C) is that Tor1p and Dna2p physically interact. To test this, wild-type yeast was transformed with a plasmid expressing HA-Tor1p under the control of the GAL1 promoter; this construct is active in dna2–20–rescuing activity (our unpublished observations). Yeast extracts were prepared and immunoprecipitated with either anti-Dna2p antibodies or an anti-HA monoclonal antibody. The anti-Dna2p antibodies specifically recognize two polypeptides of Mr 170 and 190 kDa, respectively, a doublet that is consistent with the predicted molecular mass of Dna2p (see MATERIALS AND METHODS). Figure 6A shows that, although the anti-Dna2p antibodies efficiently immunoprecipitate Dna2p, immunoprecipitates with the anti-HA antibody do not show any associated Dna2p. Similarly, although the anti-HA antibody precipitates the HA-Tor1p, none is seen associated with anti-Dna2p immunoprecipitates.
Figure 6.
(A) Dna2p and Tor1p do not interact in vitro. Extracts from CB018 transformants were prepared and immunoprecipitations (labeled “IP”) performed with either affinity-purified anti-Dna2p antibodies (α-Dna2p) or 12CA5 (α-Tor1p) ascites. Washed immunoprecipitates were loaded onto 6% SDS-PAGE gels and immunoblotted with either anti-Dna2p (left) or anti-HA (right) antibodies. + and − indicate the presence and absence, respectively, of extract in the immunoprecipitate. (B). Effect of Tor1p overexpression on Dna2p levels. Left panel, strain CB018 was transformed with either PRS315 (vector) or pYDF125 (TOR1), and immunoblots were probed with either affinity-purified anti-Dna2p antibodies or anti-actin antisera. Right panel, strains YMW1(WT), 2–71(dna2–20), and 2–174(dna2–21) were transformed with pYDF77 (GAL1p→TOR1) and grown for 5 h in YPGal and either prepared for extracts (Gal) or shifted to YPD for 9 h (Dex) before preparation of extracts. Equal amounts of protein were loaded onto 8% gels and immunoblots probed with either anti-Dna2p (top) or anti-actin (bottom) antibodies. Protein levels were quantified by densitometry and normalized to actin levels.
Effect of Tor1p Overexpression on Dna2p Protein Levels
In view of the role of Tor1p and Tor2p in protein translation, one explanation for our observations is that Tor1p overexpression increases the level of Dna2p within the cell. As shown in Figure 6B (left panel), wild-type yeast transformed with a GAL1p→TOR1 plasmid or a control plasmid contains similar levels of Dna2p when cultured in YPGal, despite equal levels of protein loading as assessed by probing the same blot with anti-hexokinase (Hxk2P) antisera. To test the possibility that Tor1p overexpression specifically stabilizes the dna2–20 and dna2–21 mutant alleles, we compared levels of Dna2p in wild-type and dna2 cells (carrying the GAL1p→TOR1 plasmid) grown in galactose or 9 h after a transfer to glucose-containing media; at this time the mutants contain >80% large budded cells as visualized by microscopy (our unpublished observations). When normalized to actin, the levels of wild-type Dna2p increase by 25% after transfer to YPD while levels of Dna2p from dna2–20 and dna2–21 decrease by 38% and 43%, respectively (Figure 6B). Similar results are seen 6 h after transfer to YPD (our unpublished observations).
Phenotype of dna2 Ts Mutants
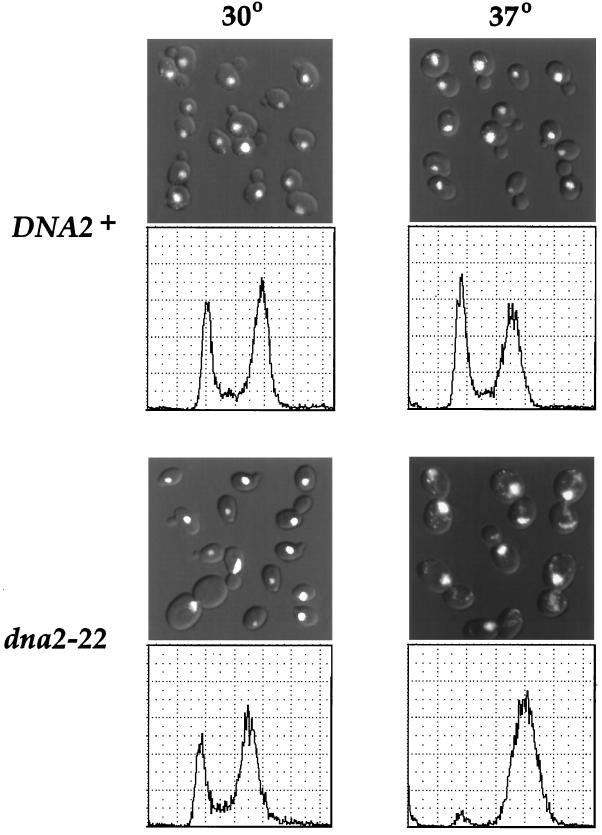
When placed in YPD to repress Tor1p expression, the dna2–20 and dna2–21 mutants arrest with a >80% of the cells with a large budded phenotype as assessed microscopically (our unpublished results); the dna2-Δ1 deletion mutants showed a similar phenotype (see above). Because these results suggest that Dna2p has a role in cell cycle progression, we constructed conditional alleles in which the function of Dna2p could be more easily manipulated. Ten temperature-sensitive (Ts) alleles were generated by in vitro mutagenesis of the DNA2 gene (see MATERIALS AND METHODS). All mutants were recessive with regard to their ability to grow at 37°C, and all arrested with a large budded phenotype within two to three cell cycles after temperature shift (our unpublished results). With regard to their interaction with Tor1p, only two of the mutants showed a slight growth advantage in the presence of Tor1p overexpression, even when tested at semipermissive temperatures; however, this growth advantage was subtle and did not result in the nonsectoring phenotype seen in the original dna2 mutants (our unpublished results). One of these mutants (dna2–22) was selected and integrated into the genome for further study (see MATERIALS AND METHODS). Figure 7 demonstrates that dna2–22 mutants arrest with a large budded morphology with the nucleus at or near the bud neck. The excess DAPI staining in the mutant is likely due to the increase in mitochondrial DNA despite the cell cycle arrest. In addition, antitubulin staining reveals that these cells have a short mitotic spindle, typical of cells arrested in medial nuclear division before mitosis (our unpublished results) (Hartwell, 1974). Analysis of DNA content by flow cytometry demonstrates that the dna2–22 mutant arrests with replicated DNA (Figure 7). In addition, dna2–20 and dna2–21 mutants, as well as the remaining nine dna2 Ts mutants, also arrest with replicated DNA (our unpublished results). These data reveal that dna2 mutants display all of the characteristics of cells arrested at the G2/M border (Pringle and Hartwell, 1981).
Figure 7.
Phenotype of the dna2–22 Ts mutant. Strains YDF100 (DNA2+) and YDF102 (dna2–22) were grown at 30°C in YPD to a density of 1.5 × 106 cells/ml, and cultures were split and incubated at either 30°C or 37°C for 4 h. Top panel, DAPI staining. Shown are DIC images with DAPI staining overlaid using Adobe Photoshop 3.0. Bottom panel, cells were processed for propidium iodide (PI) staining and flow cytometric analysis.
dna2 Mutants Have Defects in DNA Integrity
Arrest at G2/M is often due to activation of the DNA checkpoint system, a signaling pathway activated by the presence of damaged or incompletely replicated DNA (Hartwell and Weinert, 1989). One way of documenting that dna2 mutants arrest with damaged or incompletely replicated DNA is to assess their propensity for recombination. Mutants in genes that have a role in DNA replication and/or repair show an increase in recombination and chromosome loss (Hartwell and Smith, 1985). After a brief incubation at 37°C, dna2–22/dna2–22 diploids have an approximately 10-fold increase in both mitotic recombination and chromosome loss events when compared with wild-type cells (Table 2). As with the defect in growth at 37°C, this phenotype is recessive (Table 2).
Table 2.
Mitotic recombination and chromosome loss in dna2 mutants
| Strain | Temperature | Recomb. (×104) | Monosomes (×105) |
|---|---|---|---|
| DNA2+/DNA2+ | 30°C | 7.48 ± 1.89 | 7.04 ± 1.43 |
| 37°C | 6.21 ± 0.83 | 16.3 ± 2.81 | |
| DNA2+/dna2-22 | 30°C | 7.96 ± 1.45 | 7.83 ± 0.52 |
| 37°C | 6.73 ± 0.38 | 16.5 ± 3.49 | |
| dna2-22/dna2-22 | 30°C | 8.75 ± 2.73 | 10.92 ± 5.3 |
| 37°C | 88.7 ± 16.1 | 183.2 ± 23.0 |
Strains YDF104 (DNA2+/DNA2+), YDF105 (DNA2+/dna2-22) and YDF106 (dna2-22/dna2-22) were grown to log phase in YPD to OD = .1 (∼3 × 107 cells/ml) and cultured at either 30°C or 37°C for 2 h. This gives ∼80% large budded arrest as assayed microscopically and results in a 50% reduction in viability as assessed by comparing microscopic counts with colony counts (our unpublished results). The frequency of mitotic recombinants and monosomic cells was determined as described (see MATERIALS AND METHODS). Numbers represent the averages and SDs from three independent experiments.
We next tested whether the G2/M arrest in dna2 mutants is dependent upon RAD9 and MEC1, two genes involved in the DNA checkpoint-signaling pathway (Weinert and Hartwell, 1988; Weinert et al., 1994). Dna2–22 rad9 and dna2–22 mec1–1 double mutants were generated and compared with wild-type and single mutants with regard to their viability and cell cycle arrest after a brief incubation at either 24°C or 37°C. As a control, we included the cdc2, cdc13, cdc9, and cdc17 (not shown) mutants, which are known to arrest at G2/M in a checkpoint-dependent manner (Weinert and Hartwell, 1993; Weinert et al., 1994). Table 3 demonstrates that dna2–22 mutants lose >97% viability after the temperature shift; this loss of viability is largely unchanged in dna2–22 mec1–1 double mutants and slightly improved in dna2–22 rad9 double mutants. As expected, the percentage of large budded cells after incubation at the restrictive temperature drops from more than 85% in the dna2–22 single mutants to 41% and 45% in the dna2–22 rad9 and dna2–22 mec1–1 double mutants, respectively. In contrast, although the other cdc mutants also lose viability after the temperature shift, a rad9 mutation exacerbates this effect, consistent with previous results (Weinert and Hartwell, 1993). Of noteworthy exception is cdc9 (a DNA ligase mutant), which, similar to the dna2 mutant, displays a fourfold increase in viability after temperature shift if combined with the rad9 mutation. These experiments demonstrate that the cell cycle arrest seen in dna2 mutants is due to activation of the DNA checkpoint and suggests that dna2 mutants sustain some level of DNA damage.
Table 3.
Effect of checkpoint mutations on dna2-22 viability and G2/M arrest
| Strain | Viability
|
%
Large
Budded
|
||
|---|---|---|---|---|
| 24°C | 37°C | 30°C | 37°C | |
| WT | 97.5 ± .94 | 96.4 ± 2.3 | 30.0 ± 5.6 | 24.0 ± 3.6 |
| mec1 | 92.3 ± 1.1 | 77.4 ± 5.8 | 29.0 ± 2.65 | 27.3 ± 2.52 |
| rad9 | 94.6 ± .49 | 94.8 ± 1.9 | 25.7 ± 1.53 | 23.3 ± 2.31 |
| dna2-22 | 94.6 ± 1.7 | 2.77 ± 1.6 | 26.7 ± 3.79 | 86.3 ± 5.13 |
| dna2-22 mec1-1 | 89.3 ± 2.0 | 3.48 ± 1.4 | 27.3 ± 0.58 | 4.50 ± 2.65 |
| dna2-22 rad9 | 93.7 ± 1.3 | 6.53 ± 2.4 | 26.3 ± 3.21 | 40.7 ± 0.58 |
| cdc2 | 92.7 ± .45 | 7.7 ± 1.5 | ND | ND |
| cdc2 rad9 | 43.6 ± .38 | .09 ± .02 | ND | ND |
| cdc13 | 92.7 ± .86 | 40.7 ± 2.9 | ND | ND |
| cdc13 rad9 | 70.6 ± 4.0 | 2.2 ± 1.9 | ND | ND |
| cdc9 | 91.2 ± 1.1 | 7.4 ± .50 | ND | ND |
| cdc9 rad9 | 92.1 ± .66 | 28.1 ± 2.7 | ND | ND |
Indicated strains were incubated in YPD at 24° to OD = .02 (approx. 8 × 106 cells/ml). Cultures were split and incubated at 24° or 37° for 4 hr. Cells were sonicated, visualized for large budded morphology, and plated on YPD at 24° for 16 hr before assaying for viability microscopically. Yeast giving rise to colonies containing >30 cells after 16 hr were considered viable. A minimum of 250 colonies was counted for each strain. Numbers represent the averages and standard deviations from 3 independent experiments.
dna2 Mutants Are Rescued by Checkpoint Mutations
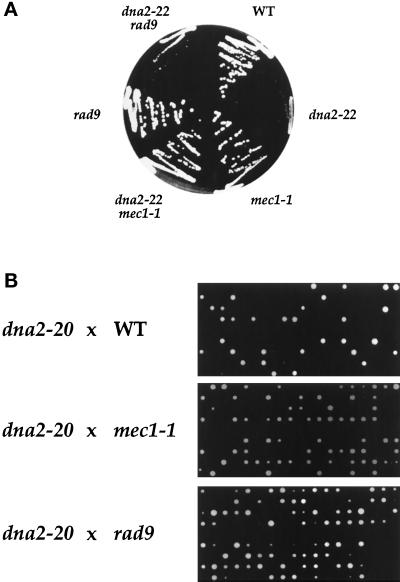
Because of the paradoxical increase in viability of the Ts dna2–22 rad9 mutant over the dna2–22 mutant (see Table 3), we next wished to test whether introduction of checkpoint mutations would also increase the colony- forming ability of dna2 mutants under semipermissive conditions. Indeed, we find that a dna2–2 mec1 mutant (but not a dna2–22 rad9 mutant) forms colonies at 37°C in the presence of osmotic stabilizers while the dna2–22 mutant is inviable (Figure 8A). As bypass of a checkpoint might explain Tor1-dependent rescue, we next tested whether such mutations might rescue the original dna2–20 and dna2–21 mutants by crossing them into our strains and examining the products of meiosis by tetrad analysis. The results for the dna2–20 mutant are shown in Figure 8B, while similar results were found for the dna2–21 mutant (our unpublished results). Many tetrads containing >two live cells are seen in both the dna2 rad9 and dna2 mec1 crosses, whereas the wild-type cross never produces a tetrad with greater than two viable spores. Although W303 strains (the dna2 mutants) are being crossed to A364 strains (wild-type and checkpoint mutants) in these experiments (which might account for the low spore viability), it should be noted that the wild-type and checkpoint strains are strictly isogenic, and thus the observed rescue is due solely to the checkpoint mutations.
Figure 8.
dna2 mutants are rescued by DNA checkpoint mutations. (A) Strains YDF92 (mec1–1), YDF94 (dna2–22 mec1–1), YDF96 (rad9), YDF98 (dna2–22 rad9), YDF100 (WT), and YDF102 (dna2–22) were streaked on YPD plates containing 1 M sorbitol at 37°C for 3 d before photographing. (B) Strain 2–71 (dna2–20) was mated to strains YDF100 (WT), YDF92 (mec1–1), and YDF96 (rad9). After sporulation of diploids, tetrads were dissected on YPD. Plates were incubated at 30°C for 3 d before photographing.
Dna2p Executes Its Function during Late S Phase
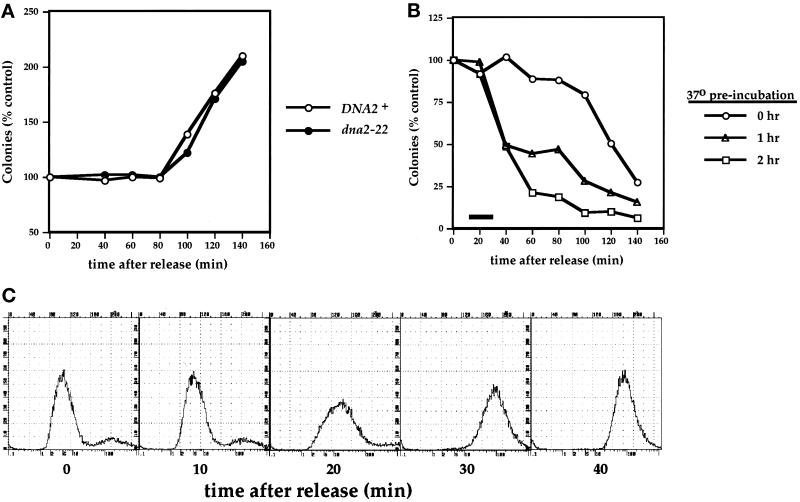
Since defects in DNA metabolism seem to be the cause for the cell cycle arrest of dna2 mutants (Tables 2 and 3), we hypothesized that Dna2p might act during S phase. We chose to use loss of viability at 37°C as a marker for when dna2 mutants pass the Dna2p execution point in the absence of functional protein. Specifically, synchronized cells were subjected to downshifts in temperature to determine the latest point at which Dna2p can act in the cell cycle. Cells were arrested in G1 with α mating factor at 30°C, released from the arrest at either 30°C or 37°C, and viability was assayed by colony-forming ability at the permissive temperature. Figure 9A shows the results of such an experiment when cells are released from α factor arrest at 30°C. Both wild-type and dna2–22 cells are effectively synchronized and undergo mitosis (as assessed by both a sudden increase in colony-forming ability as well as by microscopic examination) between 80 and 120 min after release. When cells are released at 37°C, wild-type cells again display a sudden increase in cell number at 80 min (our unpublished results), while the dna2–22 mutant shows a decrease in colony-forming ability at around this time (Figure 9B). The extent and timing of this effect could be manipulated by including a 37°C preincubation before release from α factor. Prolonged preincubation at 37°C results in a decrease in viability between 20 and 60 min after release, in contrast to the cell death that occurs just after mitosis in cells that have not been preincubated (Figure 9B). That this “late” death truly follows mitosis is supported by the observation that it is prevented by delayed addition of α factor, which allows released cells to enter mitosis but prevents their subsequent entry into S phase (our unpublished results); presumably, these cells are dying during the Dna2p execution point of the following cell cycle. We next wished to determine whether this timing is consistent with an S phase event, and thus the DNA content of the cells was examined at various times after α factor release. Figure 9C demonstrates that, for cells that were incubated for 1 h at 37°C before release, bulk DNA synthesis occurs between 10 and 30 min after release from α factor. The kinetics of DNA synthesis for the cells arrested for 2 h could not accurately be measured due to the accumulation of mitochondrial DNA resulting from the prolonged α factor arrest, which interfered with chromosomal DNA analysis (our unpublished results). These results are consistent with Dna2p having a role in late S phase.
Figure 9.
Dna2p executes its function at around the time of late S phase. (A and B) dna2–22 cells lose viability at 37°C but not 30°C. (A) Strains YDF100 (DNA2+) and YDF102 (dna2–22) were synchronized with α factor and released at 30°C. In panel B, YDF102 (dna2–22) cells were synchronized as in panel A but then preincubated at 37°C for various times before α factor release. Aliquots were taken at the times shown and assayed for colony-forming ability at 30°C. All numbers are normalized to values at t = 0 after α factor release. The black bar in panel B represents the time of DNA synthesis (see panel C) in the 1-h preincubation samples. (C) Kinetics of DNA synthesis in the dna2–22 mutant. Aliquots of cells from the 1-h preincubation protocol in panel B were taken every 10 min after α factor release and analyzed for DNA content by FACS analysis.
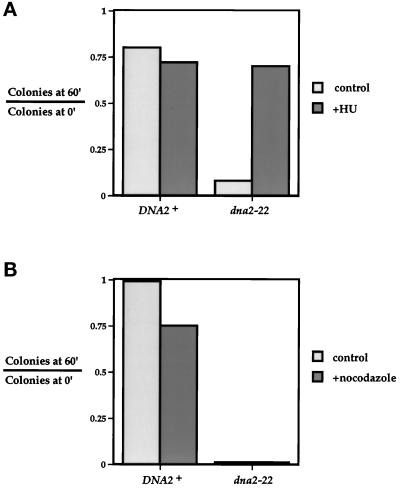
If Dna2p does indeed perform a function during DNA synthesis, then specific inhibition of this event should prevent the loss of viability of dna2 mutants. HU is an inhibitor of ribonucleotide reductase that arrests cells in early S phase due to a depletion of nucleotide precursors necessary for DNA synthesis (Slater, 1973). Synchronized cells were released at 37° in the presence or absence of HU and then tested for viability 60 min later. Figure 10A shows that wild-type cells are not affected by the addition of HU after α factor release at 37°C. It should be noted that cells are plated before the first mitosis occurs, and therefore the HU-induced arrest does not result in a decrease in colony-forming ability. Most notably, HU effectively restores the viability of the dna2–22 mutant. Figure 10B shows that, unlike HU, a mitotic inhibitor such as nocodazole is unable to rescue the loss of viability seen in the dna2–22 mutant. Thus, the Dna2p-dependent step requires DNA synthesis but not mitosis, and, along with the kinetic data presented, likely places the execution point during late S phase.
Figure 10.
The Dna2p execution step depends upon S phase but not mitosis. (A) DNA2 executes its function after the HU-dependent step. Strains YDF100 (DNA2+) and YDF102 (dna2–22) were synchronized with α factor, shifted to 37°C for 3 h, and released from α factor at 37°C into YPD (control) or YPD+0.2 mM HU (+HU). At t = 0 and 60 min after release, cells were diluted into YPD and plated at 30°C. Colonies were counted after 2 d. (B) DNA2 executes its function independent of the nocodazole-dependent step. Cells were treated as in panel A except that, after α factor release, the strains were incubated in either YPD (control) or YPD+15 μg/ml nocodazole +nocodazole). Cells were then diluted and plated as in panel A. Results are expressed as the number of colonies formed at t = 60 min after α factor release divided by the number of colonies formed at t = 0 min after release.
dna2 Mutants Replicate the Bulk of Their DNA
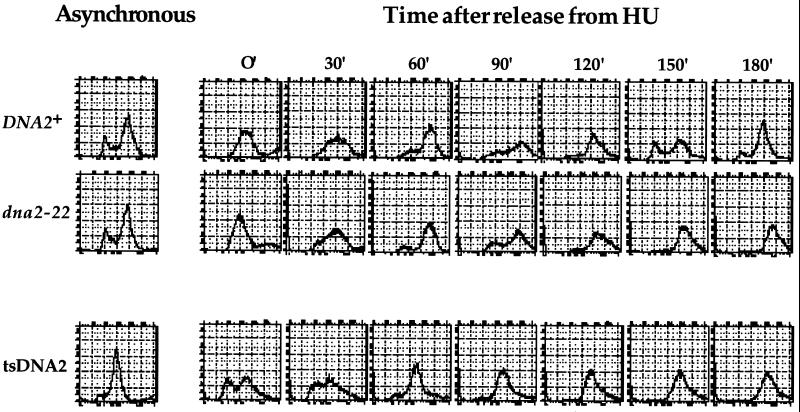
In light of the fact that DNA2 encodes a putative helicase and that dna2 mutants have defects during S phase, we wondered whether the gene might encode a protein absolutely required for replication fork progression; specifically, we wished to determine whether Dna2p is required for bulk DNA synthesis. Although the data presented suggest that dna2 mutants are able to replicate their DNA (see Figures 7 and 9C), these experiments might be misleading in light of the fact that it takes several hours at 37°C for the protein in the dna2–22 mutant to be rendered nonfunctional (Figure 9B); thus, residual Dna2p activity may allow DNA replication in these experiments. We attempted to address this by arresting dna2 mutants in G1, maintaining this arrest while shifting cells to 37°C for several hours, and then testing for ability of the mutants to replicate their DNA after α factor release. To this end, we included HU in the α factor arrest protocol because HU inhibits the mitochondrial DNA synthesis that interferes with quantitation of chromosomal DNA in cells subjected to a prolonged G1 arrest. The results of such an analysis with both our dna2–22 strain as well as the isogenic tsDNA2 mutant studied by Budd and Campbell (1995) are shown in Figure 11. The inefficient G1 arrest in the tsDNA2 mutant is likely due to the preponderance of cells in G2 (even in an unsynchronized population) in combination with its extremely slow growth rate (Figure 11). Despite this, both the dna2–22 mutant as well as the tsDNA2 mutant are similar to wild-type cells with respect to the kinetics and extent of DNA replication. A similar type of experiment was performed using the usual α factor arrest protocol on mitochondrial deficient (ρ°) yeast and yielded similar results (our unpublished results).
Figure 11.
dna2 mutants replicate the bulk of their DNA. Strains YDF100 (DNA2+), YDF102 (dna2–22), and tsDNA2 (Budd and Campbell, 1995) were grown to OD 0.25 in YPD and arrested at 30°C with 10 μg/ml α factor. After 3.5 h, cultures were spun down and resuspended in prewarmed (37°C) YPD+0.2 mM HU and incubated at 37°C for 3 h. Cells were then washed and resuspended in prewarmed (37°C) YPD, and 5-ml aliquots were taken and analyzed for DNA content.
DISCUSSION
In a screen to identify novel members of a putative TOR-signaling pathway, we have identified three classes of mutants that require elevated levels of Tor1p for optimal growth. Two of these classes were tor1 and tor2 mutants, confirming the ability of this screen to identify genes relevant to our studies. The third class of mutants contained alterations in the DNA2, a gene encoding a helicase required for DNA synthesis (Budd and Campbell, 1995). In this report we have attempted both to characterize the nature of the Tor1-dependent rescue as well as to further clarify the role of Dna2p in the cell.
As predicted, if the rescuing function is indeed related to the redundant G1 progression function, both Tor1p and Tor2p should suppress dna2 mutants; this is indeed the case (Figure 2). Similarly, as with their role in cell cycle progression, the N terminus as well as the kinase activity of Tor1p are required for efficient rescue of dna2 mutants (Figure 3). Curiously, unlike tor mutants, dna2 mutants do not show a G1 arrest but, instead, are blocked in G2/M. Most attempts to explain this would include the notion that the functions of Dna2p and Tor1p might be overlapping but not identical. It appears that the activity of Dna2p is not a limiting target of rapamycin treatment, however, because cells transformed with GAL1p→DNA2, although producing approximately 20-fold higher than physiologic levels of Dna2p, do not acquire any measure of resistance to rapamycin (our unpublished results).
What might be the mechanism of the Tor-dependent rescue? Any hypothesis must take into account the fact that Tor1p does not rescue null mutations in dna2 (Figure 5C), and therefore complete bypass of Dna2p function is not likely. Because Tor1p cannot rescue any of the more severe Ts dna2 mutants that we have generated, one possibility is that the effectiveness of the rescue is inversely correlated with the severity of the dna2 mutation; however, this simple explanation is less likely with the observation that none of the Ts dna2 mutants are suppressed even at semipermissive temperatures (our unpublished results). This degree of allele specificity is surprising and suggested to us that the two proteins might indeed physically interact, but by both biochemical and two-hybrid analysis we fail to find any evidence for this interaction (Figure 6A; our unpublished results). Aside from physical interaction, allele specificity might occur if Dna2p has multiple functions, only a subset of which are suppressed by the TOR pathway, and only the dna2–20 and dna2–21 mutations specifically compromise this function(s). However, we have no evidence that any of our mutants differ with regard to their essential defects.
In light of their G1 progression function (i.e., via effects on translation), one possibility is that the TORs specifically stabilize certain alleles of dna2. Although Tor1p overexpression does not affect levels of wild-type Dna2p, we do find that it results in a 40% increase in levels of both the dna2–20 and dna2–21 mutant proteins (Figure 6B). However, these data are complicated by the fact that the cells lose viability in the absence of Tor1p overexpression, and thus, even though we use actin as a control, it is possible that Rot1p has an especially short half-life in dying cells. Interestingly, Berg and co-workers (Zieler et al., 1995) have found that mutations in a gene they call WEB2 (which is identical to DNA2) are rescued by overexpression of the adenovirus E1A protein . Because E1A is known to elicit most, if not all, of its effects on host cells via changes in transcription, the mechanism of this rescue is probably indirect. One obvious possibility is that E1A induces transcription of DNA2, although the authors find that DNA2 transcript levels are unaffected by E1A overexpression. However, it is conceivable that these studies have revealed that a unique feature of dna2 mutants is the dramatic phenotypic consequences that can result from very small changes in levels of Dna2p.
However, there is another potential mechanism for the TOR-dependent rescue of dna2 mutants—namely, bypass of a DNA checkpoint. Our data support the notion that mild defects in dna2, although resulting in a checkpoint-dependent arrest, actually are ameliorated by a defective DNA checkpoint (Figure 8 and Table 3). Indeed, we have direct evidence that both the dna2–20 and dna2–21 mutants, those rescued by Tor1p overexpression, are suppressed by introduction of either rad9 or mec1 mutations (Figure 8). When overexpressed, perhaps Tor1p and Tor2p, due to their sequence similarity to regions of Mec1p, are able to interfere with Mec1p-dependent functions by binding regulators and/or effectors of Mec1p. This would predict an alteration in checkpoint-dependent activity that would rescue very subtle dna2 mutants. However, one prediction of this hypothesis is that wild-type cells overproducing Tor1p should be checkpoint deficient, and we find that cells overexpressing Tor1p are no more sensitive to UV or MMS damage than wild-type cells (our unpublished results).
Regardless of the mechanism, a major question is whether Tor1p-dependent rescue of dna2 mutants is representative of a physiological relationship or whether Tor1p assumes an alternative role in the cell when overexpressed—the latter might be expected to occur after Tor1p mislocalization and/or indiscreet protein-protein interactions. Increased sensitivity of dna2 mutants to a diminution in TOR activity would support a common biological role for these two proteins. Indeed, we find that compromising TOR function with either a tor1 deletion or treatment with rapamycin reveals synthetic lethal interactions between TOR1/2 and DNA2 (Figure 4).
Notwithstanding its relationship to the TORs, we were initially intrigued that dna2 mutants not only arrest at G2/M but lose viability. However, it is clear that this is not a unique property of cdc mutants that arrest in G2/M because several other mutants of this class lose viability (to a varying degree) after a 4-h arrest (Table 3). Another peculiar observation is that, after a brief incubation at their restrictive temperatures, both dna2 rad9 and dna2 mec1 double mutants display similar or even increased viabilities when compared with dna2 mutants (Table 3). This is unusual for cdc mutants of this class, as most mutants with defects in DNA metabolism show at least a 10-fold decrease in viability when combined with checkpoint mutations (Table 3 and Weinert et al., 1994). Interestingly, the only other mutant displaying this paradoxical increase in viability is cdc9 (DNA ligase). This may suggest that dna2 and cdc9 mutants incur a similar type of DNA damage. In the same vein, we find it curious that dna2 mutants, when grown under semipermissive conditions, are actually rescued by introduction of DNA checkpoint mutations (Figure 8). Again, this is unprecedented for this type of cdc mutant, with the exception of the cdc13 mutation (Weinert and Hartwell, 1993; Weinert et al., 1994). Our dna2 mec1 mutants do not acquire lethal amounts of DNA damage, in that they can be restreaked many times, supporting the notion that checkpoint mutations truly rescue the dna2 mutants and do not simply allow several “suicide” divisions before dying. We are again left with the notion that an intact checkpoint is a liability for dna2 mutants, especially at semipermissive temperatures. Indeed, there is precedence for DNA checkpoint activation causing undue damage to a cell (Lydall and Weinert, 1995).
We used the loss of viability of dna2 mutants to determine that Dna2p acts late in S phase, an approach that successfully defined the execution point of topoisomerase II in yeast (Holm et al., 1985). This conclusion is supported by the time of action of the Dna2p (Figure 9) as well as the observation that its function requires ongoing DNA synthesis (Figure 10). Because it has been previously suggested that Dna2p might be required for leading strand synthesis (Budd and Campbell, 1995), we found it curious that dna2 mutants arrest with a G2 level of DNA (Figure 7). However, one explanation for our observations is that we are not able to completely inactivate the function of Dna2p using our temperature-shift protocol. Indeed, our experiments indicate that it requires approximately 3 h at 37°C to completely abolish Dna2p activity in the dna2–22 mutant (Figure 9B). However, even using an extended temperature-shift protocol, we still find no difference between dna2 and wild-type cells with regard to the extent and kinetics of DNA synthesis (Figure 11). Nevertheless, it is still possible that we are unable to incompletely inactivate Dna2p function, and thus mutant cells are synthesizing damaged DNA that is subsequently detected by the DNA checkpoint.
One possible explanation for our data and the observation that the DNA in dna2 mutants is of low molecular weight (Budd and Campbell, 1995) is that dna2 mutants are unable to process and/or join Okazaki fragments. Lagging strand synthesis requires that the RNA primer is separated from the DNA template and cleaved from the DNA strand to allow the incoming DNA polymerase to complete synthesis of the upstream fragment. This has been shown to be catalyzed by two proteins, RNaseH1 and FEN1 (also known as MF1/Dnase IV/calf 3′→5′ exonuclease) (Goulian et al., 1990; Turchi et al., 1994). The yeast homolog of FEN1, Rth1p/Rad27p, is essential for viability at 37°C, with mutants arresting at the G2/M border (Reagan et al., 1995; Sommers et al., 1995). Furthermore, it has been shown recently that Dna2p interacts physically and genetically with yeast FEN-1 (Budd and Campbell, 1997). Since FEN-1 is required for Okazaki fragment processing in many in vitro models of lagging strand DNA synthesis, Dna2p might also have a role in the maturation of Okazaki fragments. Further support for this notion may come with an in vitro model of eukaryotic DNA synthesis whose dependence on cellular factors accurately reflects the situation in vivo.
Table 1.
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| YMW1 | MATα ade2-1 ade3Δ22 his3-11,15 leu2-3,112 trp1-1 can1-100 ura3-1 | Zieler et al., 1995 |
| YDF20 | YMW1 tor1-Δ10::TRP1 | This study |
| CB018 | MATa Δpep4::HIS3 Δprb1 Δprc1 ura3-52 ade2 trp1 leu2 can1 ciro | C. Brenner, unpublished |
| YDF27 | YMW1 MATa dna2-Δ20 | This study |
| MATαDNA2+ | ||
| 2-71 | YMW1 MATa dna2-20 | This study |
| 2-174 | YMW1 MATa dna2-21 | This study |
| YDF117 | YMW1 MATa dna2-20 tor1-Δ10::TRP1 | This study |
| YDF121 | YMW1 MATa dna2-21 tor1-Δ10::TRP1 | This study |
| YDF92 | MATa mec1-1 ura3 trp1 his7 | This study |
| YDG94 | MATa mec1-1 ura3 trp1 his7 dna2-22 | This study |
| YDF96 | MATa ura3 trp1 his7 leu2 rad9Δ::LEU2 | This study |
| YDF98 | MATa ura3 trp1 leu2 rad9Δ::LEU2 dna2-22 | This study |
| YDF100 | MATa ura3 trp1 his7 leu2 | This study |
| YDF102 | MATa ura3 trp1 his7 leu2 dna2-22 | This study |
| DBY1034 | MATa his4-539 lys2-801 ura3-52 | D. Botstein, unpublished |
| DBY1511 | MATα ura3-52 hom3 leu2 lys2-801 can1-51 | D. Botstein, unpublished |
| YDF104 | MATa his4-539 LEU2+ lys2-801 ura3-52 CAN1+ HOM3+ DNA2+ | This study |
| MATα HIS4+ leu2LYS+ura3-52 can1-51 hom3DNA2+ | ||
| YDF105 | MATa his4-539 LEU2+ lys2-801 ura3-52 CAN1+ HOM3+ dna2-22 | This study |
| MATα HIS4+ leu2LYS+ura3-52 can1-51 hom3DNA2+ | ||
| YDF106 | MATa his4-539 LEU2+ lys2-801 ura3-52 CAN1+ HOM3+ dna2-22 | This study |
| MATα HIS4+ leu2LYS+ura3-52 can1-51 hom3dna2-22 |
ACKNOWLEDGMENTS
We wish to thank Steve Biggar, Steffan Ho, Jamison Nourse, Neil Clipstone, and all members of the Crabtree laboratory for stimulating discussion and advice. We especially thank Luika Timmerman for countless hours of FACS assistance. We are indebted to David Amberg, Kristy Richards, Pam Foreman, and Helge Zieler for both their technical and intellectual expertise. We thank the laboratories of David Botstein, Martha Cyert, Ted Weinert, Judith Campbell, Jeremy Thorner, Scott Emr, Y. Anraku, and Stuart Schreiber for yeast strains and plasmids.
REFERENCES
- Altschul S, Gish W, Miller W, Myers E, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Barbet N, Schneider U, Helliwell S, Stansfield I, Tuite M, Hall M. TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell, 1996;7:25–42. doi: 10.1091/mbc.7.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas EE, Chen PH, Leszyk J, Biswas SB. Biochemical and genetic characterization of a replication protein A dependent DNA helicase from the yeast, Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1995;206:850–856. doi: 10.1006/bbrc.1995.1121. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Budd M, Campbell J. A yeast replicative helicase, Dna2 helicase, interacts with yeast FEN-1 nuclease in carrying out its essential function. Mol Cell Biol. 1997;17:2136–2142. doi: 10.1128/mcb.17.4.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd ME, Campbell JL. A yeast gene required for DNA replication encodes a protein with homology to DNA helicases. Proc Natl Acad Sci USA. 1995;92:7642–7646. doi: 10.1073/pnas.92.17.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd ME, Choe WC, Campbell JL. DNA2 encodes a DNA helicase essential for replication of eukaryotic chromosomes. J Biol Chem. 1995;270:26766–26769. doi: 10.1074/jbc.270.45.26766. [DOI] [PubMed] [Google Scholar]
- Cardenas M, Heitman J. FKBP-12-rapamycin target TOR2 is a vacuolar protein with an associated phosphatidylinositol-4 kinase activity. EMBO J. 1995;14:5892–5907. doi: 10.1002/j.1460-2075.1995.tb00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CL, Cantley LC. Phosphoinositide kinases. Biochemistry. 1990;29:11147–11156. doi: 10.1021/bi00503a001. [DOI] [PubMed] [Google Scholar]
- Chiu MI, Katz H, Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci USA. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Di Como C, Arndt K. Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes & Dev. 1996;10:1904–1916. doi: 10.1101/gad.10.15.1904. [DOI] [PubMed] [Google Scholar]
- Drebot MA, Johnston GC, Singer RA. A yeast mutant conditionally defective only for reentry into the mitotic cell cycle from stationary phase. Proc Natl Acad Sci USA. 1987;84:7948–7952. doi: 10.1073/pnas.84.22.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan CA, Schnieders EA, Emerick AW, Kunisawa R, Admon A, Thorner J. Phosphatidylinositol 4-kinase: gene structure and requirement for yeast cell viability. Science. 1993;262:1444–1448. doi: 10.1126/science.8248783. [DOI] [PubMed] [Google Scholar]
- Giasson E, Meloche S. Role of p70 S6 protein kinase in angiotensin II-induced protein synthesis in vascular smooth muscle cells. J Biol Chem. 1995;270:5225–5231. doi: 10.1074/jbc.270.10.5225. [DOI] [PubMed] [Google Scholar]
- Gorbalenya AE, Koonin EV, Donchenko AP, Blinov VM. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989;17:4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M, Richards SH, Heard CJ, Bigsby BM. Discontinuous DNA synthesis by purified mammalian proteins [published erratum appears in J. Biol. Chem. 1990 Dec 25;265(36):22569] J Biol Chem. 1990;265:18461–18471. [PubMed] [Google Scholar]
- Graves LM, Bornfeldt KE, Argast GM, Krebs EG, Kong X, Lin TA, Lawrence JC., Jr cAMP- and rapamycin-sensitive regulation of the association of eukaryotic initiation factor 4E and the translational regulator PHAS-I in aortic smooth muscle cells. Proc Natl Acad Sci USA. 1995;92:7222–7226. doi: 10.1073/pnas.92.16.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Hartwell LH. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974;38:164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Smith D. Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics. 1985;110:381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Helliwell SB, Wagner P, Kunz J, Deuter-Reinhard M, Henriquez R, Hall MN. TOR1 and TOR2 are structurally and functionally similar but not identical phosphatidylinositol kinase homologues in yeast. Mol Biol Cell. 1994;5:105–118. doi: 10.1091/mbc.5.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman PK, Emr SD. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:6742–6754. doi: 10.1128/mcb.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey JW. Protein phosphorylation controls translation rates. J Biol Chem. 1989;264:20823–20826. [PubMed] [Google Scholar]
- Holm C, Goto T, Wang JC, Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985;41:553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- Holm C, Stearns T, Botstein D. DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage. Mol Cell Biol. 1989;9:159–168. doi: 10.1128/mcb.9.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. When is a lipid kinase not a lipid kinase? When it is a protein kinase. Cell. 1995;83:1–4. doi: 10.1016/0092-8674(95)90225-2. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies HB, Reinhard C, Kozma SC, Thomas G. Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family. Proc Natl Acad Sci USA. 1994;91:4441–4445. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M, Andrews S, Brinkman R, Cooper J, Ding H, Dover J, Du Z, Favello A, Fulton L, Gattung S. Complete nucleotide sequence of Saccharomyces cerevisiae chromosome VIII. Science. 1994;265:2077–2082. doi: 10.1126/science.8091229. [DOI] [PubMed] [Google Scholar]
- Keith CT, Schreiber SL. PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science. 1995;270:50–51. doi: 10.1126/science.270.5233.50. [DOI] [PubMed] [Google Scholar]
- Kohalmi SE, Kunz BA. Role of neighbouring bases and assessment of strand specificity in ethylmethanesulphonate and N-methyl-N′-nitro-N-nitrosoguanidine mutagenesis in the SUP4-o gene of Saccharomyces cerevisiae. J Mol Biol. 1988;204:561–568. doi: 10.1016/0022-2836(88)90355-5. [DOI] [PubMed] [Google Scholar]
- Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Movva NR, Hall MN. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993;73:585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Lawrence C. Classical mutagenesis techniques. In: Guthrie C, Fink G, editors. Guide to Yeast Genetics and Molecular Biology. San Diego, CA: Academic Press, Inc.; 1991. pp. 273–281. [Google Scholar]
- Li X, Yoder B, Burgers PM. A Saccharomyces cerevisiae DNA helicase associated with replication factor C. J Biol Chem. 1992a;267:25321–25327. [PubMed] [Google Scholar]
- Li X, Yoder B, Burgers PM. Three new DNA helicases from Saccharomyces cerevisiae. Chromosoma. 1992b;102:S93–S99. doi: 10.1007/BF02451791. [DOI] [PubMed] [Google Scholar]
- Lin TA, Kong X, Saltiel AR, Blackshear PJ, Lawrence JC., Jr Control of PHAS-I by insulin in 3T3–L1 adipocytes. Synthesis, degradation, and phosphorylation by a rapamycin-sensitive and mitogen-activated protein kinase-independent pathway. J Biol Chem. 1995;270:18531–18538. doi: 10.1074/jbc.270.31.18531. [DOI] [PubMed] [Google Scholar]
- Lydall D, Weinert T. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science. 1995;270:1488–1491. doi: 10.1126/science.270.5241.1488. [DOI] [PubMed] [Google Scholar]
- Matson SW, Bean DW, George JW. DNA helicases: enzymes with essential roles in all aspects of DNA metabolism. Bioessays. 1994;16:13–22. doi: 10.1002/bies.950160103. [DOI] [PubMed] [Google Scholar]
- Pringle J, Hartwell L. The Saccharomyces cerevisiae cell cycle. In: Broach J, Strathern J, Jones E, editors. Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1981. pp. 97–142. [Google Scholar]
- Reagan MS, Pittenger C, Siede W, Friedberg EC. Characterization of a mutant strain of Saccharomyces cerevisiae with a deletion of the RAD27 gene, a structural homolog of the RAD2 nucleotide excision repair gene. J Bacteriol. 1995;177:364–371. doi: 10.1128/jb.177.2.364-371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD, Novick P, Thomas JH, Botstein D, Fink GR. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. In: Guthrie C, Fink G, editors. Guide to Yeast Genetics and Molecular Biology. San Diego, CA: Academic Press, Inc.; 1991. pp. 281–301. [DOI] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Sabatini DM, Pierchala BA, Barrow RK, Schell MJ, Snyder SH. The rapamycin and FKBP12 target (RAFT) displays phosphatidylinositol 4-kinase activity. J Biol Chem. 1995;270:20875–20878. doi: 10.1074/jbc.270.36.20875. [DOI] [PubMed] [Google Scholar]
- Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sanchez Y, Desany BA, Jones WJ, Liu Q, Wang B, Elledge SJ. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways [see comments] Science. 1996;271:357–360. doi: 10.1126/science.271.5247.357. [DOI] [PubMed] [Google Scholar]
- Schena M, Picard D, Yamamoto K. Vectors for constitutive and inducible gene expression in yeast. In: Guthrie C, Fink G, editors. Guide to Yeast Genetics and Molecular Biology. San Diego, CA: Academic Press, Inc.; 1991. pp. 389–398. [DOI] [PubMed] [Google Scholar]
- Scherer S, Davis R. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci USA. 1979;76:4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SR, Linder P. D-E-A-D protein family of putative RNA helicases. Mol Microbiol. 1992;6:283–291. doi: 10.1111/j.1365-2958.1992.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Kunz J, Hall M. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc Natl Acad Sci USA. 1996;93:13780–13785. doi: 10.1073/pnas.93.24.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Bickle M, Beck T, Hall M. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell. 1997;88:531–542. doi: 10.1016/s0092-8674(00)81893-0. [DOI] [PubMed] [Google Scholar]
- Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Schulz V, Zakian V. The Saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell. 1994;76:145–155. doi: 10.1016/0092-8674(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. In: Guthrie C, Fink G, editors. Guide to Yeast Genetics and Molecular Biology. San Diego, CA: Academic Press, Inc.; 1991. pp. 3–21. [Google Scholar]
- Sherman F, Hicks J. Micromanipulation and dissection of asci. In: Guthrie C, Fink G, editors. Guide to Yeast Genetics and Molecular Biology. San Diego, CA: Academic Press, Inc.; 1991. pp. 21–37. [Google Scholar]
- Shimizu K, Sugino A. Purification and characterization of DNA helicase III from the yeast Saccharomyces cerevisiae. J Biol Chem. 1993;268:9578–9584. [PubMed] [Google Scholar]
- Sikorski R, Boeke J. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. In: Guthrie C, Fink G, editors. Guide to Yeast Gentics and Molecular Biology. San Diego, CA: Academic Press, Inc.; 1991. pp. 302–318. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater ML. Effect of reversible inhibition of deoxyribonucleic acid synthesis on the yeast cell cycle. J Bacteriol. 1973;113:263–270. doi: 10.1128/jb.113.1.263-270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Johnson K. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutatihione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sommers CH, Miller EJ, Dujon B, Prakash S, Prakash L. Conditional lethality of null mutations in RTH1 that encodes the yeast counterpart of a mammalian 5′- to 3′-exonuclease required for lagging strand DNA synthesis in reconstituted systems. J Biol Chem. 1995;270:4193–4196. doi: 10.1074/jbc.270.9.4193. [DOI] [PubMed] [Google Scholar]
- Strathern J, Higgins D. Recovery of plasmids from yeast into Esherichia coli: shuttle vectors. In: Guthrie C, Fink G, editors. Guide to Yeast Genetics and Molecular Biology. San Diego, CA: Academic Press, Inc.; 1991. pp. 319–329. [DOI] [PubMed] [Google Scholar]
- Terada N, Lucas JJ, Szepesi A, Franklin RA, Domenico J, Gelfand EW. Rapamycin blocks cell cycle progression of activated T cells prior to events characteristic of the middle to late G1 phase of the cycle. J Cell Physiol. 1993;154:7–15. doi: 10.1002/jcp.1041540103. [DOI] [PubMed] [Google Scholar]
- Terada N, Patel HR, Takase K, Kohno K, Nairn AC, Gelfand EW. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc Natl Acad Sci USA. 1994;91:11477–11481. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi JJ, Huang L, Murante RS, Kim Y, Bambara RA. Enzymatic completion of mammalian lagging-strand DNA replication. Proc Natl Acad Sci USA. 1994;91:9803–9807. doi: 10.1073/pnas.91.21.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert TA, Hartwell LH. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988;241:317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- Weinert TA, Hartwell LH. Cell cycle arrest of cdc mutants and specificity of the RAD9 checkpoint. Genetics. 1993;134:63–80. doi: 10.1093/genetics/134.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert TA, Kiser GL, Hartwell LH. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes & Dev. 1994;8:652–665. doi: 10.1101/gad.8.6.652. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ohya Y, Goebl M, Nakano A, Anraku Y. A novel gene, STT4, encodes a phosphatidylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J Biol Chem. 1994;269:1166–1172. [PubMed] [Google Scholar]
- Zheng XF, Florentino D, Chen J, Crabtree GR, Schreiber SL. TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell. 1995;82:121–130. doi: 10.1016/0092-8674(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Zieler HA, Walberg M, Berg P. Suppression of mutations in two Saccharomyces cerevisiae genes by the adenovirus E1A protein. Mol Cell Biol. 1995;15:3227–3237. doi: 10.1128/mcb.15.6.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]