Abstract
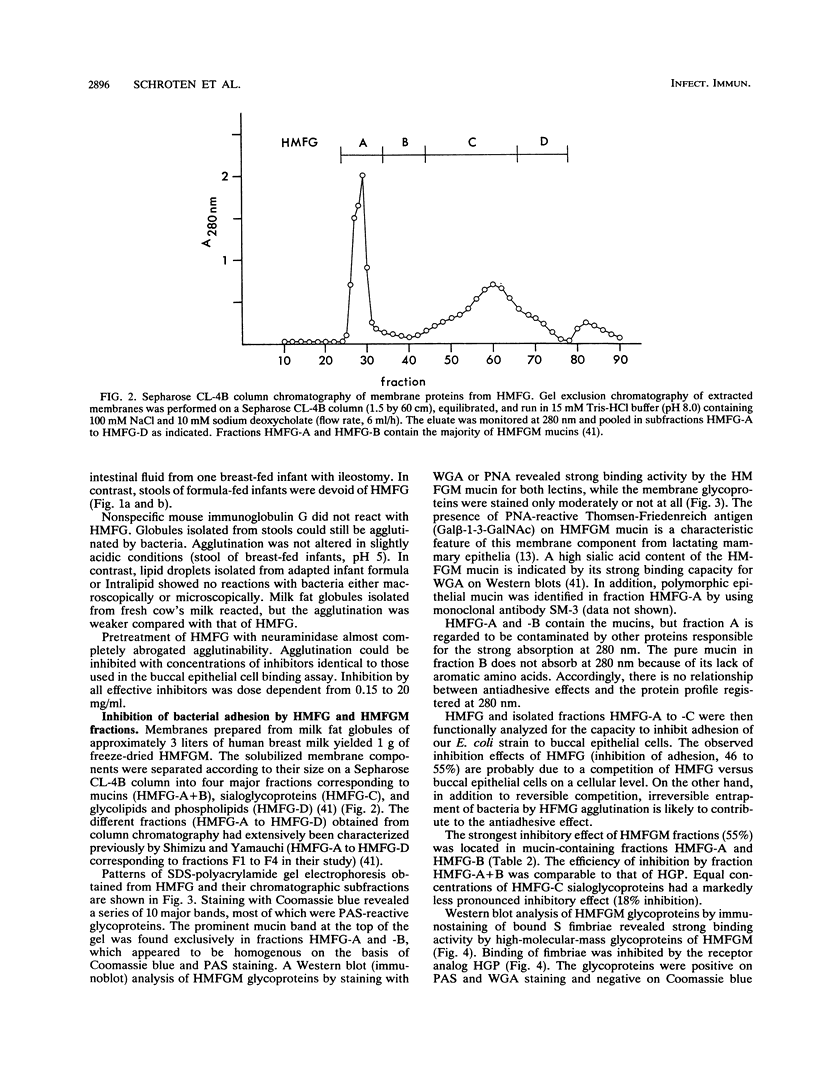
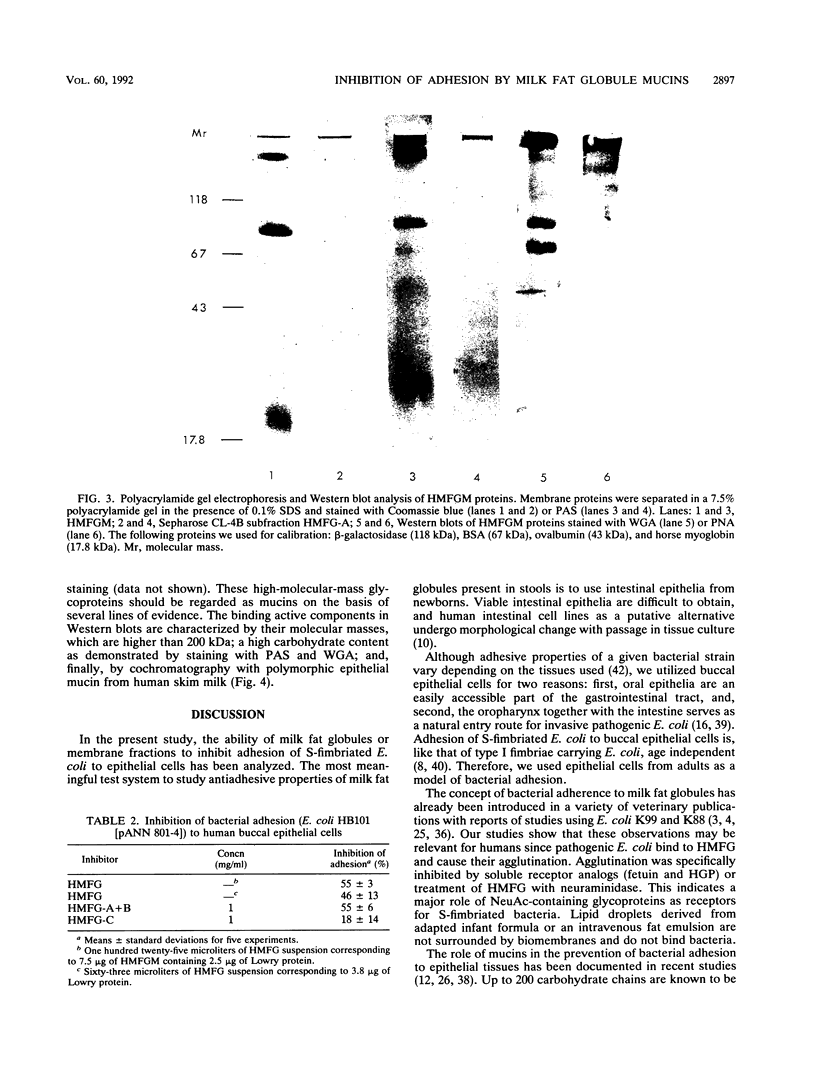
We investigated the presence of factors in human milk that inhibit invasion of pathogenic bacteria. The effect of human milk fat globule membrane (HMFGM) components on adhesion of cloned S-fimbriated Escherichia coli to human buccal epithelial cells was analyzed. S fimbriae are a common feature of E. coli strains causing sepsis and meningitis in newborns and are bound to epithelia via sialyl-(alpha-2-3)galactoside structures. Human milk fat globules (HMFG) could be agglutinated by the above-mentioned bacteria. Agglutination could be inhibited by fetuin, human glycophorin, and alpha 1-acid glycoprotein. In addition, pretreatment of HMFG with Vibrio cholerae neuraminidase markedly reduced bacterium-induced agglutinations, indicating the involvement of neuraminic acid-containing glycoproteins. In contrast, lipid droplets of infant formula or artificial lipid emulsions (Intralipid) could not be agglutinated. HMFG were present in stools of breast-fed neonates as shown by indirect immunofluorescence staining with a monoclonal antibody directed against carbohydrate residues present on HMFGM. These HMFG could be agglutinated by bacteria. HMFG inhibited E. coli adhesion to buccal epithelial cells. To further characterize relevant E. coli binding structures, HMFGM components were separated by gel chromatography. The mucin fraction showed the most pronounced inhibitory effect on adhesion of S-fimbriated E. coli to human buccal epithelial cells. Our data suggest that HMFG inhibit bacterial adhesion in the entire intestine and thereby may provide protection against bacterial infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Porras O., Hanson L. A., Lagergård T., Svanborg-Edén C. Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenzae by human milk and receptor oligosaccharides. J Infect Dis. 1986 Feb;153(2):232–237. doi: 10.1093/infdis/153.2.232. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S., Mirelman D. Nonimmunoglobulin fraction of human milk inhibits the adherence of certain enterotoxigenic Escherichia coli strains to guinea pig intestinal tract. Pediatr Res. 1987 Aug;22(2):130–134. doi: 10.1203/00006450-198708000-00004. [DOI] [PubMed] [Google Scholar]
- Atroshi F., Alaviuhkola T., Schildt R., Sandholm M. Fat globule membrane of sow milk as a target for adhesion of K88-positive Escherichia coli. Comp Immunol Microbiol Infect Dis. 1983;6(3):235–245. doi: 10.1016/0147-9571(83)90016-4. [DOI] [PubMed] [Google Scholar]
- Atroshi F., Schildt R., Sandholm M. K 88-mediated adhesion of E. coli inhibited by fractions in sow milk. Zentralbl Veterinarmed B. 1983 Jul;30(6):425–433. doi: 10.1111/j.1439-0450.1983.tb01864.x. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Burchell J., Gendler S., Taylor-Papadimitriou J., Girling A., Lewis A., Millis R., Lamport D. Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin. Cancer Res. 1987 Oct 15;47(20):5476–5482. [PubMed] [Google Scholar]
- Burchell J., Taylor-Papadimitriou J. Antibodies to human milk fat globule molecules. Cancer Invest. 1989;7(1):53–61. doi: 10.3109/07357908909038267. [DOI] [PubMed] [Google Scholar]
- Clegg H., Guerina N., Langermann S., Kessler T. W., Guerina V., Goldmann D. Pilus-mediated adherence of Escherichia coli K1 to human oral epithelial cells. Infect Immun. 1984 Jul;45(1):299–301. doi: 10.1128/iai.45.1.299-301.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. S., Jelliffe D. B., Jelliffe E. F. Breast-feeding and health in the 1980s: a global epidemiologic review. J Pediatr. 1991 May;118(5):659–666. doi: 10.1016/s0022-3476(05)80023-x. [DOI] [PubMed] [Google Scholar]
- Deneke C. F., McGowan K., Thorne G. M., Gorbach S. L. Attachment of enterotoxigenic Escherichia coli to human intestinal cells. Infect Immun. 1983 Mar;39(3):1102–1106. doi: 10.1128/iai.39.3.1102-1106.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowben R. M., Brunner J. R., Philpott D. E. Studies on milk fat globule membranes. Biochim Biophys Acta. 1967 Feb 1;135(1):1–10. doi: 10.1016/0005-2736(67)90002-8. [DOI] [PubMed] [Google Scholar]
- Fischer J., Klein P. J., Farrar G. H., Hanisch F. G., Uhlenbruck G. Isolation and chemical and immunochemical characterization of the peanut-lectin-binding glycoprotein from human milk-fat-globule membranes. Biochem J. 1984 Dec 1;224(2):581–589. doi: 10.1042/bj2240581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A. S., Thorpe L. W., Goldblum R. M., Hanson L. A. Anti-inflammatory properties of human milk. Acta Paediatr Scand. 1986 Sep;75(5):689–695. doi: 10.1111/j.1651-2227.1986.tb10275.x. [DOI] [PubMed] [Google Scholar]
- Guerina N. G., Kessler T. W., Guerina V. J., Neutra M. R., Clegg H. W., Langermann S., Scannapieco F. A., Goldmann D. A. The role of pili and capsule in the pathogenesis of neonatal infection with Escherichia coli K1. J Infect Dis. 1983 Sep;148(3):395–405. doi: 10.1093/infdis/148.3.395. [DOI] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch F. G., Uhlenbruck G., Peter-Katalinic J., Egge H., Dabrowski J., Dabrowski U. Structures of neutral O-linked polylactosaminoglycans on human skim milk mucins. A novel type of linearly extended poly-N-acetyllactosamine backbones with Gal beta(1-4)GlcNAc beta(1-6) repeating units. J Biol Chem. 1989 Jan 15;264(2):872–883. [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Ahrén C. Nonimmunoglobulin fraction of human milk inhibits bacterial adhesion (hemagglutination) and enterotoxin binding of Escherichia coli and Vibrio cholerae. Infect Immun. 1981 Jul;33(1):136–141. doi: 10.1128/iai.33.1.136-141.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebenthal E., Lee P. C., Heitlinger L. A. Impact of development of the gastrointestinal tract on infant feeding. J Pediatr. 1983 Jan;102(1):1–9. doi: 10.1016/s0022-3476(83)80276-5. [DOI] [PubMed] [Google Scholar]
- Lindahl M. Binding of F41 and K99 fimbriae of enterotoxigenic Escherichia coli to glycoproteins from bovine and porcine colostrum. Microbiol Immunol. 1989;33(5):373–379. doi: 10.1111/j.1348-0421.1989.tb01985.x. [DOI] [PubMed] [Google Scholar]
- Mantle M., Basaraba L., Peacock S. C., Gall D. G. Binding of Yersinia enterocolitica to rabbit intestinal brush border membranes, mucus, and mucin. Infect Immun. 1989 Nov;57(11):3292–3299. doi: 10.1128/iai.57.11.3292-3299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Korhonen T. K., Pere A., Hacker J., Soinila S. Binding sites in the rat brain for Escherichia coli S fimbriae associated with neonatal meningitis. J Clin Invest. 1988 Mar;81(3):860–865. doi: 10.1172/JCI113395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Rogers G. N., Korhonen T., Dahr W., Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986 Oct;54(1):37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton S., Borgström B., Stemberger B. H., Welsch U. Release of membrane from milk fat globules by conjugated bile salts. J Pediatr Gastroenterol Nutr. 1986 Mar-Apr;5(2):262–267. [PubMed] [Google Scholar]
- Patton S., Keenan T. W. The milk fat globule membrane. Biochim Biophys Acta. 1975 Oct 31;415(3):273–309. doi: 10.1016/0304-4157(75)90011-8. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford B. A., Thomas V. L., Ramsay M. A. Binding of staphylococci to mucus in vivo and in vitro. Infect Immun. 1989 Dec;57(12):3735–3742. doi: 10.1128/iai.57.12.3735-3742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarff L. D., McCracken G. H., Schiffer M. S., Glode M. P., Robbins J. B., Orskov I., Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975 May 17;1(7916):1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Yamauchi K. Isolation and characterization of mucin-like glycoprotein in human milk fat globule membrane. J Biochem. 1982 Feb;91(2):515–524. doi: 10.1093/oxfordjournals.jbchem.a133724. [DOI] [PubMed] [Google Scholar]
- Sojar H. T., Bahl O. P. A chemical method for the deglycosylation of proteins. Arch Biochem Biophys. 1987 Nov 15;259(1):52–57. doi: 10.1016/0003-9861(87)90469-3. [DOI] [PubMed] [Google Scholar]
- Varian S. A., Cooke E. M. Adhesive properties of Escherichia coli from urinary-tract infections. J Med Microbiol. 1980 Feb;13(1):111–119. doi: 10.1099/00222615-13-1-111. [DOI] [PubMed] [Google Scholar]
- Wevers P., Picken R., Schmidt G., Jann B., Jann K., Golecki J. R., Kist M. Characterization of pili associated with Escherichia coli O18ac. Infect Immun. 1980 Aug;29(2):685–691. doi: 10.1128/iai.29.2.685-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]