Abstract
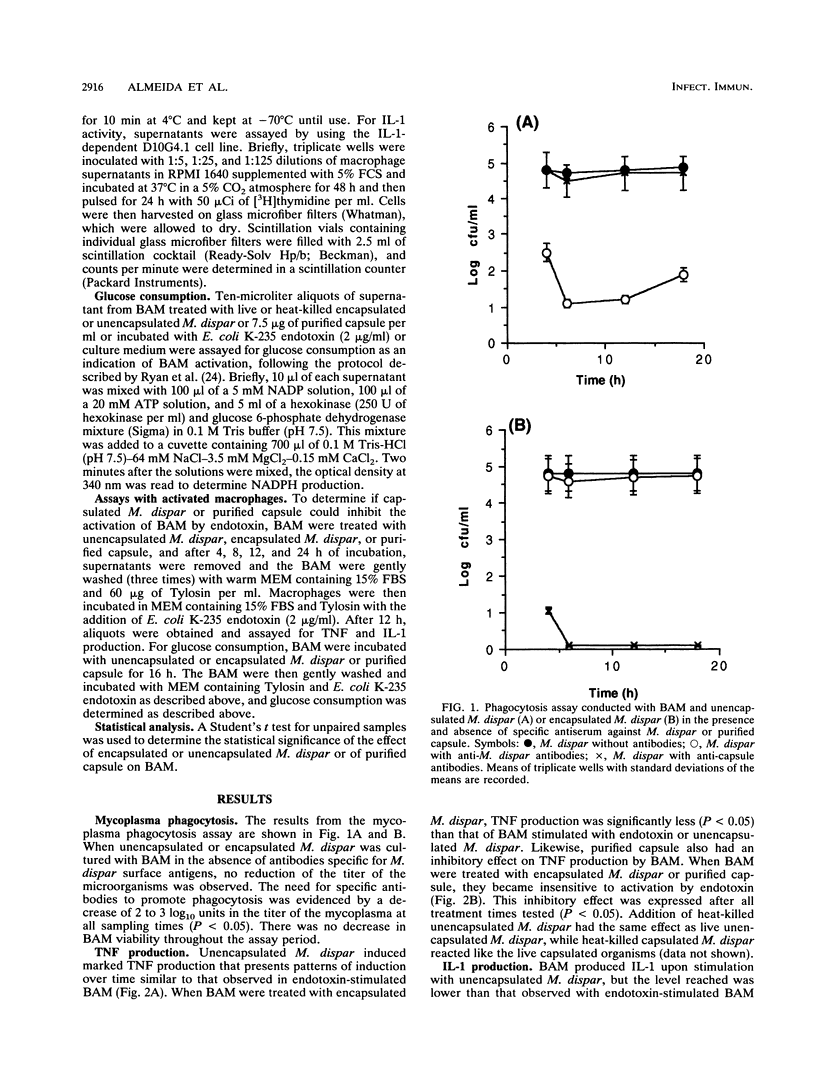
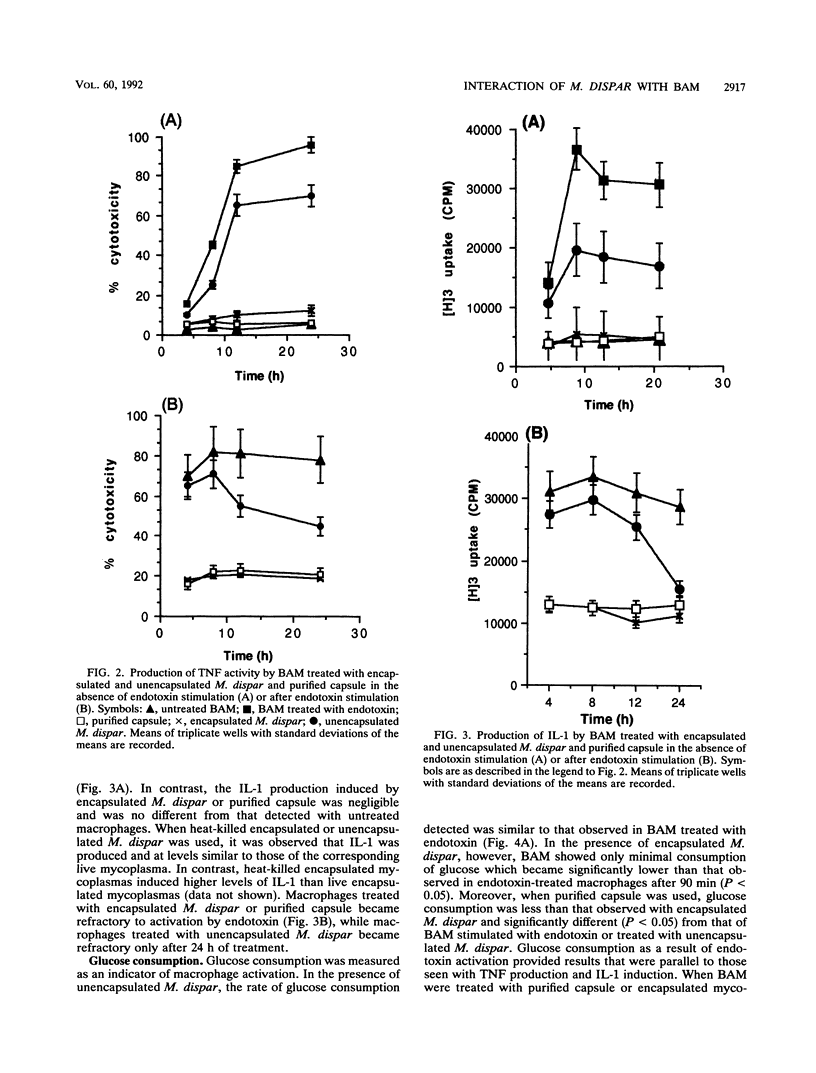
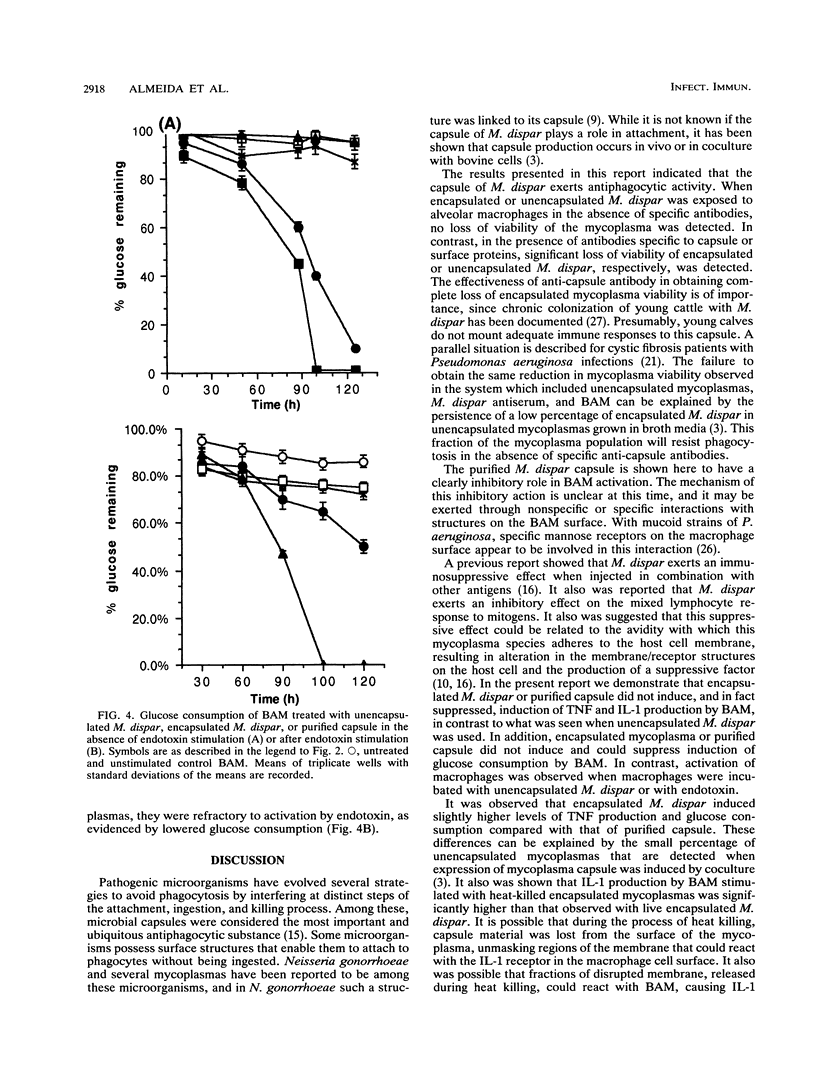
The capacity to avoid phagocytosis and the activation of bovine alveolar macrophages (BAM) by encapsulated Mycoplasma dispar or purified M. dispar capsule was investigated. Encapsulated and unencapsulated M. dispar were cocultured with BAM in the presence or absence of antisera prepared against unencapsulated M. dispar or purified capsule antiserum. Unopsonized mycoplasmas resisted phagocytosis, while only anti-capsule antibodies enhanced the phagocytosis of encapsulated mycoplasmas. BAM were cultured in the presence of purified M. dispar capsule or either live or heat-killed encapsulated or unencapsulated M. dispar. These BAM were then activated with Escherichia coli endotoxin or left without further activation. The supernatants of these cultures were assayed for tumor necrosis factor, interleukin 1, and glucose consumption as indicators of macrophage activation. Tumor necrosis factor and interleukin 1 were produced by BAM stimulated with unencapsulated M. dispar but not when encapsulated M. dispar or its purified capsule was used. Similarly, glucose consumption was increased in the presence of unencapsulated M. dispar, but not when BAM were cocultured with encapsulated M. dispar or purified capsule. When BAM were treated with purified capsule or encapsulated mycoplasmas, they could not be subsequently activated by endotoxin. These results indicate that encapsulated M. dispar or purified capsule exerts an inhibitory effect on the activity of BAM and prevents the activation of these cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Kaissi A., Alley M. R. Electron microscopic studies of the interaction between ovine alveolar macrophages and Mycoplasma ovipneumoniae in vitro. Vet Microbiol. 1983 Nov;8(6):571–584. doi: 10.1016/0378-1135(83)90006-8. [DOI] [PubMed] [Google Scholar]
- Albro P. W. Determination of protein in preparations of microsomes. Anal Biochem. 1975 Apr;64(2):485–493. doi: 10.1016/0003-2697(75)90458-3. [DOI] [PubMed] [Google Scholar]
- Almeida R. A., Rosenbusch R. F. Capsulelike surface material of Mycoplasma dispar induced by in vitro growth in culture with bovine cells is antigenically related to similar structures expressed in vivo. Infect Immun. 1991 Sep;59(9):3119–3125. doi: 10.1128/iai.59.9.3119-3125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain J. D. Lung macrophages: how many kinds are there? What do they do? Am Rev Respir Dis. 1988 Mar;137(3):507–509. doi: 10.1164/ajrccm/137.3.507. [DOI] [PubMed] [Google Scholar]
- Bredt W., Kist M., Jacobs E. Phagocytosis and complement action. Isr J Med Sci. 1981 Jul;17(7):637–640. [PubMed] [Google Scholar]
- Cole B. C., Ward J. R. Interaction of Mycoplasma arthritidis and other mycoplasmas with murine peritoneal macrophages. Infect Immun. 1973 May;7(5):691–699. doi: 10.1128/iai.7.5.691-699.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Delozier K. M., Asa D. K., Minion F. C., Cassell G. H. Interactions between murine alveolar macrophages and Mycoplasma pulmonis in vitro. Infect Immun. 1980 Aug;29(2):590–599. doi: 10.1128/iai.29.2.590-599.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980 Sep-Oct;2(5):817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Bartlema H. C. Role of the alveolar macrophage in pulmonary bacterial defense. Bull Eur Physiopathol Respir. 1977 Jan-Feb;13(1):57–67. [PubMed] [Google Scholar]
- Gourlay R. N., Howard C. J., Thomas L. H., Wyld S. G. Pathogenicity of some Mycoplasma and Acholeplasma species in the lungs of gnotobiotic calves. Res Vet Sci. 1979 Sep;27(2):233–237. [PubMed] [Google Scholar]
- Henson P. M., Larsen G. L., Henson J. E., Newman S. L., Musson R. A., Leslie C. C. Resolution of pulmonary inflammation. Fed Proc. 1984 Oct;43(13):2799–2806. [PubMed] [Google Scholar]
- Hesse R. A., Toth T. E. Effects of bovine parainfluenza-3 virus on phagocytosis and phagosome-lysosome fusion of cultured bovine alveolar macrophages. Am J Vet Res. 1983 Oct;44(10):1901–1907. [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of microorganisms. Rev Infect Dis. 1982 Jan-Feb;4(1):104–123. doi: 10.1093/clinids/4.1.104. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Gourlay R. N. Immune response of calves following the inoculation of Mycoplasma dispar and Mycoplasma bovis. Vet Microbiol. 1983 Feb;8(1):45–56. doi: 10.1016/0378-1135(83)90018-4. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction in vitro of Mycoplasma pulmonis with mouse peritoneal macrophages and L-cells. J Exp Med. 1971 Feb 1;133(2):231–259. doi: 10.1084/jem.133.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudtson W. U., Reed D. E., Daniels G. Identification of Mycoplasmatales in pneumonic calf lungs. Vet Microbiol. 1986 Feb;11(1-2):79–91. doi: 10.1016/0378-1135(86)90009-x. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Saunders J. M., Ames P., Edwards M. S., Auerbach H., Goldfarb J., Speert D. P., Hurwitch S. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N Engl J Med. 1987 Sep 24;317(13):793–798. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- Rosenbusch R. F. Influence of mycoplasma preinfection on the expression of Moraxella bovis pathogenicity. Am J Vet Res. 1983 Sep;44(9):1621–1624. [PubMed] [Google Scholar]
- Ryan J. L., Glode L. M., Rosenstreich D. L. Lack of responsiveness of C3H/HeJ macrophages to lipopolysaccharide: the cellular basis of LPS-stimulated metabolism. J Immunol. 1979 Mar;122(3):932–935. [PubMed] [Google Scholar]
- Sher T., Rottem S., Gallily R. Mycoplasma capricolum membranes induce tumor necrosis factor alpha by a mechanism different from that of lipopolysaccharide. Cancer Immunol Immunother. 1990;31(2):86–92. doi: 10.1007/BF01742371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Wright S. D., Silverstein S. C., Mah B. Functional characterization of macrophage receptors for in vitro phagocytosis of unopsonized Pseudomonas aeruginosa. J Clin Invest. 1988 Sep;82(3):872–879. doi: 10.1172/JCI113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart P. M., Cassell G. H., Woodward J. G. Induction of class II MHC antigen expression in macrophages by Mycoplasma species. J Immunol. 1989 May 15;142(10):3392–3399. [PubMed] [Google Scholar]
- Tanskanen R. Transmission of Mycoplasma dispar among calves collected and reared for beef production. Acta Vet Scand. 1987;28(2):241–248. doi: 10.1186/BF03548246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Schorlemmer H. U., Furr P. M., Allison A. C. Macrophage secretion and the complement cleavage product C3a in the pathogenesis of infections by mycoplasmas and L-forms of bacteria and in immunity to these organisms. Clin Exp Immunol. 1978 Sep;33(3):486–494. [PMC free article] [PubMed] [Google Scholar]
- Tinant M. K., Bergeland M. E., Knudtson W. U. Calf pneumonia associated with Mycoplasma dispar infection. J Am Vet Med Assoc. 1979 Oct 15;175(8):812–813. [PubMed] [Google Scholar]


