Abstract
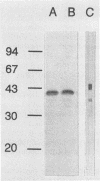
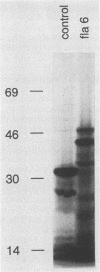
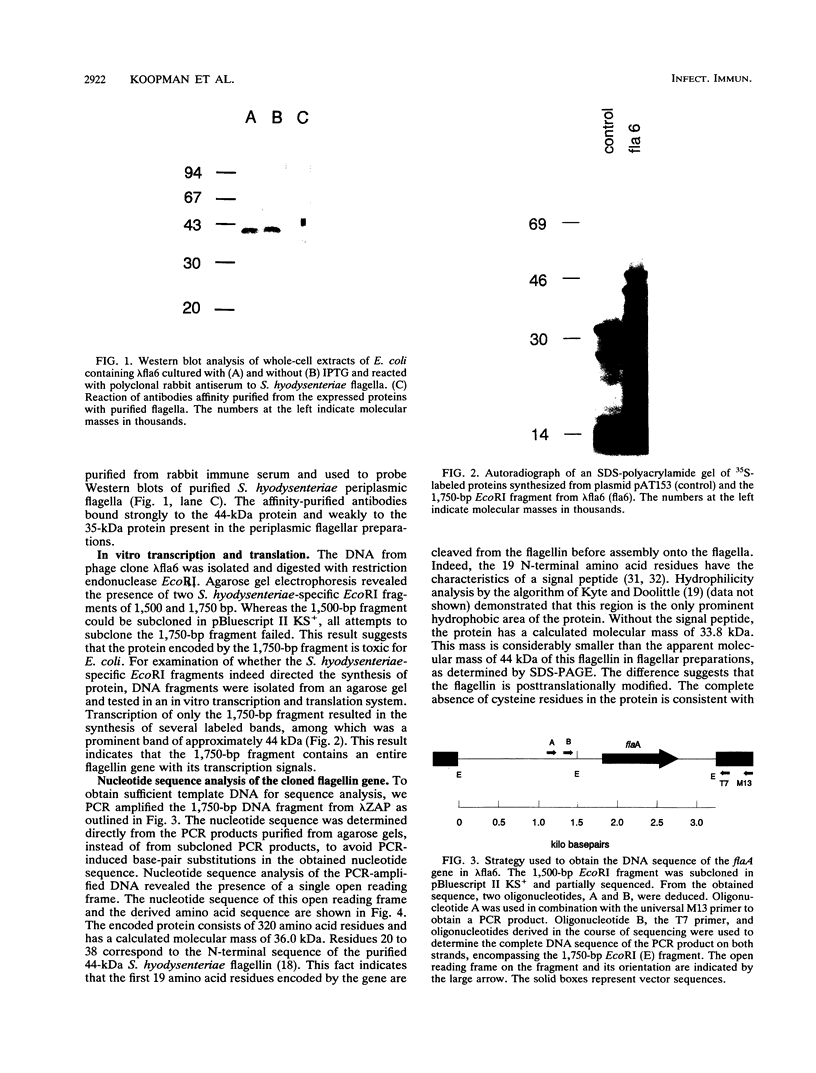
A Serpulina (Treponema) hyodysenteriae expression library was constructed in vector lambda ZAP and screened with a polyclonal antiserum raised against S. hyodysenteriae periplasmic flagella. A single immunoreactive plaque was chosen for further analysis. The recombinant phage from this plaque contained a gene encoding the 44-kDa protein that is on the outer layer (or sheath) of the periplasmic flagella. DNA sequence analysis showed that the gene encodes a protein of 320 amino acids. The protein is homologous to the flagellar sheath proteins of Treponema pallidum and Spirochaeta aurantia but not to any other flagellar proteins. We designated the cloned S. hyodysenteriae flagellar sheath protein gene flaA and the encoded protein FlaA. The 19 N-terminal amino acid residues of FlaA constitute a signal peptide that is cleaved from the protein before assembly onto the flagella in the periplasm. Amino acid residues 20 to 38 correspond to the N-terminal amino acid sequence of the native protein. Upstream from the gene, DNA motifs that are similar to the consensus Escherichia coli -35 and -10 promoter sequences and a ribosome binding site were identified. Downstream from the gene, two inverted repeat sequences that may serve as a rho-independent transcription termination signal are present.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander T. J., Taylor D. J. The clinical signs, diagnosis and control of swine dysentery. Vet Rec. 1969 Jul 19;85(3):59–63. doi: 10.1136/vr.85.3.59. [DOI] [PubMed] [Google Scholar]
- Boyden D. A., Albert F. G., Robinson C. S. Cloning and characterization of Treponema hyodysenteriae antigens and protection in a CF-1 mouse model by immunization with a cloned endoflagellar antigen. Infect Immun. 1989 Dec;57(12):3808–3815. doi: 10.1128/iai.57.12.3808-3815.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahamsha B., Greenberg E. P. Biochemical and cytological analysis of the complex periplasmic flagella from Spirochaeta aurantia. J Bacteriol. 1988 Sep;170(9):4023–4032. doi: 10.1128/jb.170.9.4023-4032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahamsha B., Greenberg E. P. Cloning and sequence analysis of flaA, a gene encoding a Spirochaeta aurantia flagellar filament surface antigen. J Bacteriol. 1989 Mar;171(3):1692–1697. doi: 10.1128/jb.171.3.1692-1697.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Chatfield S. N., Fernie D. S., Penn C., Dougan G. Identification of the major antigens of Treponema hyodysenteriae and comparison with those of Treponema innocens. Infect Immun. 1988 May;56(5):1070–1075. doi: 10.1128/iai.56.5.1070-1075.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie D. S., Ripley P. H., Walker P. D. Swine dysentery: protection against experimental challenge following single dose parenteral immunisation with inactivated Treponema hyodysenteriae. Res Vet Sci. 1983 Sep;35(2):217–221. [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Isaacs R. D., Hanke J. H., Guzman-Verduzco L. M., Newport G., Agabian N., Norgard M. V., Lukehart S. A., Radolf J. D. Molecular cloning and DNA sequence analysis of the 37-kilodalton endoflagellar sheath protein gene of Treponema pallidum. Infect Immun. 1989 Nov;57(11):3403–3411. doi: 10.1128/iai.57.11.3403-3411.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs R. D., Radolf J. D. Expression in Escherichia coli of the 37-kilodalton endoflagellar sheath protein of Treponema pallidum by use of the polymerase chain reaction and a T7 expression system. Infect Immun. 1990 Jul;58(7):2025–2034. doi: 10.1128/iai.58.7.2025-2034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Harris D. L., Baum D. H. Immunity to Swine dysentery in recovered pigs. Am J Vet Res. 1979 Oct;40(10):1352–1354. [PubMed] [Google Scholar]
- Kent K. A., Sellwood R., Lemcke R. M., Burrows M. R., Lysons R. J. Analysis of the axial filaments of Treponema hyodysenteriae by SDS-PAGE and immunoblotting. J Gen Microbiol. 1989 Jun;135(6):1625–1632. doi: 10.1099/00221287-135-6-1625. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J., Guerry P. Evidence for posttranslational modification and gene duplication of Campylobacter flagellin. J Bacteriol. 1989 Jun;171(6):3031–3038. doi: 10.1128/jb.171.6.3031-3038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Charon N. W., Cook R. G., Fuentes M. D., Limberger R. J. Antigenic relatedness and N-terminal sequence homology define two classes of periplasmic flagellar proteins of Treponema pallidum subsp. pallidum and Treponema phagedenis. J Bacteriol. 1988 Sep;170(9):4072–4082. doi: 10.1128/jb.170.9.4072-4082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. D. Clinical and pathological observations on the experimental passage of swine dysentery. Can J Comp Med. 1974 Jan;38(1):7–13. [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Alexander T. J. The production of dysentery in swine by feeding cultures containing a spirochaete. Br Vet J. 1971 Nov;127(11):58–61. doi: 10.1016/s0007-1935(17)37282-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland F., Paul G., Sumper M. Halobacterial flagellins are sulfated glycoproteins. J Biol Chem. 1985 Dec 5;260(28):15180–15185. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wergifosse P., Coene M. M. Comparison of the genomes of pathogenic treponemes of human and animal origin. Infect Immun. 1989 May;57(5):1629–1631. doi: 10.1128/iai.57.5.1629-1631.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]