Abstract
Voriconazole is a broad-spectrum triazole antifungal agent indicated for invasive aspergillosis, refractory Candida infections, and other emerging invasive fungal infections. Adverse cutaneous reactions associated with voriconazole therapy occur in fewer than 10% of treated patients and range from mild erythematous eruptions to life-threatening reactions such as the Stevens-Johnson syndrome and toxic epidermal necrolysis. Photosensitivity reactions are an uncommon but characteristic dermatitis in voriconazole recipients, particularly following chronic administration. We report a case of voriconazole-induced phototoxicity in a 50-year-old male with Candida parapsilosis endocarditis that reversed on discontinuation of the drug.
Keywords: Antifungal agents, Candida parapsilosis, Phototoxicity
Case Report
A 50-year-old male heroin user with a previous mitral valve replacement presented with 2 weeks of fatigue, fevers, and chills. Physical examination was remarkable for a 2/6 holosystolic murmur in the left lower sternal border, with radiation to the apex. Laboratory tests revealed a white blood cell count of 25,500 cells/mm3, elevated Westergren erythrocyte sedimentation rate of 95 mm/h, and urinalysis showed 5 to 10 red blood cells/hpf. Multiple blood cultures grew Candida parapsilosis. A transesophageal echocardiogram showed a large vegetation on the mitral valve bioprosthesis. He received combination surgical and medical therapy for prosthetic mitral valve endocarditis due to C. parapsilosis. The patient underwent replacement of his prosthetic mitral valve and completed a 7-week course of parenteral liposomal amphotericin B daily combined with oral flucytosine. Because the C. parapsilosis had a relatively high minimum inhibitory concentration to fluconazole (MIC=4 μg/mL), the patient was placed on long-term oral voriconazole therapy 200 mg twice daily for suppressive treatment.
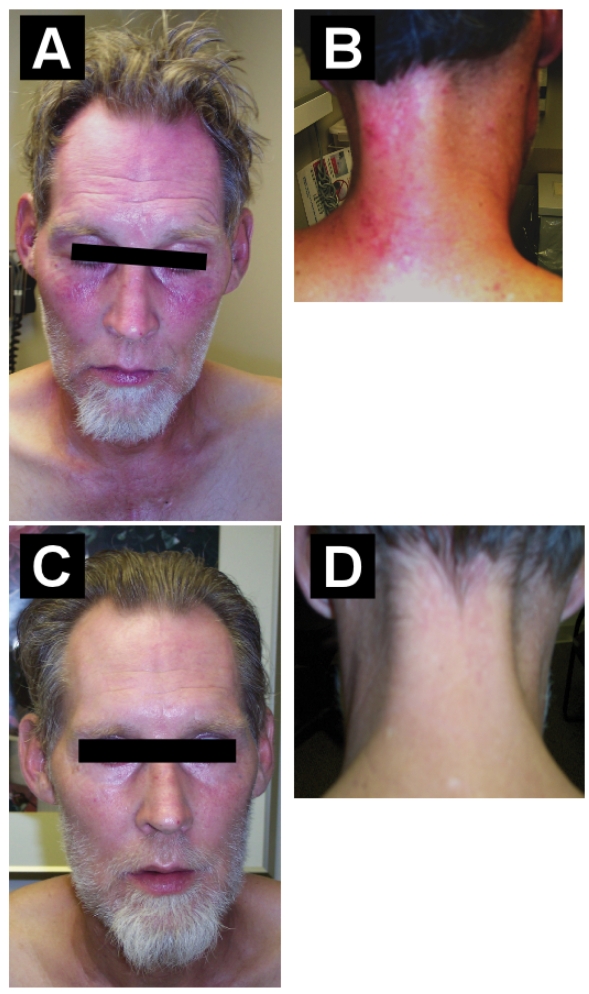
Five months into voriconazole therapy, he developed a non-pruritic, non-tender, erythematous, macular eruption of sun-exposed skin areas, particularly on the face, neck, and hand (figure 1 ▶, panels A and B). He had minimal sun exposure, wore long clothing, and drove his car with closed windows. The rash on the neck and hand developed on his left side, presumably from sun exposure while driving. This photosensitivity reaction resolved (figure 1 ▶, panels C and D) over 6 weeks, after fluconazole was substituted for voriconazole.
Figure 1.
Panels (A) and (B) show voriconazole-induced photosensitivity of the face, lips, and neck. Panels (C) and (D) show resolution of the photosensitivity reaction 6 weeks after stopping voriconazole.
Comment
Voriconazole, a broad-spectrum triazole antifungal agent, is the first line therapy for invasive aspergillosis.1 It is also indicated for refractory Candida infections and other emerging invasive fungal infections, such as fusariosis and Scedosporium apiospermum.2–4 Similar to the other azole agents, the mechanism of action of voriconazole is inhibition of cytochrome P450-dependent 14α-lanosterol demethylation, a vital step in cell membrane ergosterol synthesis by fungi. The most common adverse effect of voriconazole therapy is reversible disturbance of vision (photopsia), occurring in 30% of patients.2
Dermatologic reactions, mostly mild rashes, are the second most common adverse consequence of voriconazole, occurring in fewer than 10% of treated patients.5,6 Severe reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported.2,7 In contrast to the other azole antifungal agents, photosensitivity is a more common dermatologic complication of voriconazole and is most often seen with prolonged treatment duration.5,8–10 Voriconazole-induced photosensitivity reactions include erythematous eruptions of sun-exposed areas, such as facial erythema, chelitis, hyperpigmentation of the hands, exfoliative dermatitis, discoid lupus erythematosus, and pseudoporphyria.5,8–14 In general, such reactions are rapidly reversible upon discontinuation of the drug. Our patient developed a mild phototoxicity reaction of the face, neck, and upper extremities that normalized soon after stopping voriconazole. The development of multifocal facial squamous cell carcinomas due to a severe photosensitivity reaction from long-term voriconazole therapy was recently described.15
The etiology of voriconazole-induced photosensitivity remains unclear, but may be due to indirect retinoid effects or direct phototoxic effects of voriconazole or one of its metabolites.5,9–11 Photosensitivity reactions appear to be idiosyncratic, rather than dose-dependent.2 Counseling and recommendations regarding avoidance of sun exposure and use of sunscreens may lessen or resolve a majority of cases of voriconazole-induced photosensitivity despite drug continuation.
In summary, we present a case of reversible voriconazole-induced photosensitivity in a patient receiving chronic antifungal therapy for an invasive Candida infection. This case highlights the importance of recognizing adverse dermatologic reactions noted with voriconazole, an azole antifungal agent whose clinical use continues to expand.
Potential Conflicts of Interest: Dr. Aronoff has received honoraria from AstraZeneca, Merck, and Pfizer for speaking.
References
- 1.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF; Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008; 46:327–360. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LB, Kauffman CA. Voriconazole: a new triazole antifungal agent. Clin Infect Dis 2003;36:630–637. [DOI] [PubMed] [Google Scholar]
- 3.Malani AN, Kauffman CA. Changing epidemiology of rare mould infections: implications for therapy. Drugs 2007;67:1803–1812. [DOI] [PubMed] [Google Scholar]
- 4.Perfect JR, Marr KA, Walsh TJ, Greenberg RN, DuPont B, de la Torre-Cisneros J, Just-N, bling G, Schlamm HT, Lutsar I, Espinel-Ingroff A, Johnson E. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis 2003;36:1122–1131. [DOI] [PubMed] [Google Scholar]
- 5.Denning DW, Griffiths CE. Muco-cutaneous retinoid-effects and facial erythema related to the novel triazole antifungal agent voriconazole. Clin Exp Dermatol 2001;26:648–653 [DOI] [PubMed] [Google Scholar]
- 6.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B; Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002;347:408–415. [DOI] [PubMed] [Google Scholar]
- 7.Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E, Haas A, Ruhnke M, Lode H. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis 2002;34:563–571. [DOI] [PubMed] [Google Scholar]
- 8.Kwong WT, Hsu S. Pseudoporphyria associated with voriconazole. J Drugs Dermatol 2007;6:1042–1044. [PubMed] [Google Scholar]
- 9.Rubenstein M, Levy ML, Metry D. Voriconazole-induced retinoid-like photosensitivity in children. Pediatr Dermatol 2004;21:675–678. [DOI] [PubMed] [Google Scholar]
- 10.Vandecasteele SJ, Van Wijngaerden E, Peetermans WE. Two cases of severe phototoxic reactions related to long-term outpatient treatment with voriconazole. Eur J Clin Microbiol Infect Dis 2004;23:656–657. [DOI] [PubMed] [Google Scholar]
- 11.Racette AJ, Roenigk HH Jr, Hansen R, Mendelson D, Park A. Photoaging and phototoxicity from long-term voriconazole treatment in a 15-year-old girl. J Am Acad Dermatol 2005;52(5 suppl 1):S81–S85. [DOI] [PubMed] [Google Scholar]
- 12.Tolland JP, McKeown PP, Corbett JR. Voriconazole-induced pseudoporphyria. Photodermatol Photoimmunol Photomed 2007;23:29–31. [DOI] [PubMed] [Google Scholar]
- 13.Dolan CK, Hall MA, Blazes DL, Norwood CW. Pseudoporphyria as a result of voriconazole use: a case report. Int J Dermatol 2004;43:768–771. [DOI] [PubMed] [Google Scholar]
- 14.Walsh TJ, Lutsar I, Driscoll T, Dupont B, Roden M, Ghahramani P, Hodges M, Groll AH, Perfect JR. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr Infect Dis J 2002;21:240–248. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy KL, Playford EG, Looke DF, Whitby M. Severe photosensitivity causing multifocal squamous cell carcinomas secondary to prolonged voriconazole therapy. Clin Infect Dis 2007;44:e55–e56. [DOI] [PubMed] [Google Scholar]