Abstract
A growing body of medical research has demonstrated that intensive control of serum glucose levels can minimize the development of diabetes-related complications. Success with insulin management ultimately depends on how closely a given regimen can mimic normal physiologic insulin release patterns. The new insulin analogs, including the rapid-acting analogs (aspart, lispro, glulisine), the long-acting basal analogs (glargine, detemir), and the premixed insulin analog formulations (75% neutral protamine lispro, 25% lispro; 50% neutral protamine lispro, 50% lispro; 70% protamine aspart, 30% aspart) have been formulated to allow for a closer replication of a normal insulin profile. The rapid-acting analogs can be administered at mealtimes and produce a rapid and short-lived insulin spike to address postprandial glucose elevations, while the long-acting analogs come close to the ideal of a smooth, relatively flat, 24-hour basal insulin supply, with less variability in action compared to NPH insulin. Despite these clear pharmacologic advantages, measurable clinical benefits in a complex disease such as diabetes can be hard to measure. To date, reviews of insulin analog studies have not found a dramatic overall improvement in glycosylated hemoglobin (HbA1c) outcomes compared to traditional human insulins, although all-analog basal-bolus regimens were associated with significantly lower HbA1c than all-human-insulin basal bolus regimens in some studies. Beyond HbA1c comparisons, however, insulin analogs have been shown in many instances to be associated with lower risks of hypoglycemia, lower levels of postprandial glucose excursions, better patient adherence, greater quality of life, and higher satisfaction with treatment. The long-acting basal analog insulin detemir has the additional advantage of producing less weight gain, which has been considered until now an almost inevitable consequence of insulin replacement.
Keywords: Diabetes complications, Diabetes mellitus, Hypoglycemia, Insulin, Patient non-adherence, Pharmacokinetics, Quality of life
Despite 85 years of research since Banting and Best’s isolation of insulin-containing extracts,1 diabetes still remains one of the most significant causes of morbidity and mortality in the world, and its global impact is likely to accelerate over the coming decades.2,3 Much of the medical and economic consequences of diabetes are attributable to its associated microvascular and macrovascular complications.4–6 Two classic large-scale clinical studies, the Diabetes Control and Complications Trial (DCCT)6 and the UK Prospective Diabetes Study (UKPDS),7 demonstrated that intensive blood-glucose control policies can decrease the frequency of complications, arguing that physicians and patients should strive to mimic, as closely as possible, the serum levels of insulin produced by a healthy person. Secretion of insulin by the pancreas, however, is under complex regulation that depends on the intake of nutrients, other gastrointestinal peptides (e.g., incretins), and overall metabolic levels (i.e., exercise versus rest).8 These physiological variables, which pose real challenges to the accurate metabolic replacement of insulin, are further complicated by the fact that diabetes requires self-management and therefore depends on the psychology, motivation, and understanding of the patient. While management of diabetes has greatly improved in recent years with newer strategies focusing on aggressive glucose control, traditional insulin products have fallen short of providing optimal therapy. The insulin analogs, relatively recent additions to the anti-diabetes armamentarium, have been designed to more closely mimic physiologic insulin profiles through improved pharmacokinetic characteristics, which result in either more rapid or prolonged pharmacodynamic effects. The purpose of this review is to examine the pharmacokinetic and clinical features of these new insulin analogs and to discuss current findings regarding their potential impact on overall treatment success, safety, patient satisfaction, and adherence to therapy compared to traditional human insulins.
Biochemical Characteristics of Insulin Analogs
Upon subcutaneous injection, insulin molecules form a depot from which absorption into the systemic circulation occurs. All insulin molecules have a tendency to self-aggregate into hexameric complexes and these clusters must dissociate into dimers and monomers to diffuse through interstitial fluid, penetrate the capillary wall, and enter the bloodstream.9 The unique pharmacologic features of individual insulin analog preparations largely alter the rate of hexamer dissociation and the subsequent movement of free insulin into the circulation, i.e., the time-concentration profile of activity.
Rapid-Acting Insulin Analogs
Rapid-acting insulin analogs are designed to offer a more rapid onset of action and shorter duration of activity than human soluble insulin. Currently, there are three commercially-available rapid-acting insulin analogs: insulin aspart, insulin lispro, and insulin glulisine (table 1 ▶). For each analog, the specific modifications made to the insulin molecule are relatively minor, involving only one or two amino acid alterations. These changes weaken the tendency to self-associate into hexamers, thereby facilitating more rapid absorption. However, the molecular changes do not alter the biological properties of the analogs in terms of binding to the insulin receptor, and the rapid-acting insulin analogs all possess the same glucose-lowering effects as human insulin.10–12
Table 1.
Rapid-acting, long-acting, and premixed insulin analogs.
| Analog | Trade name/manufacturer | Insulin molecule modifications |
| Rapid-acting analogs | ||
| Lispro | Humalog/Eli Lilly | Pro(B28)/Lys(B29) switched |
| Aspart | Novolog/Novo Nordisk | Asp replaces Pro(B28) |
| Glulisine | Apidra/Sanofi-Aventis | Asp(B3) replaced by Lys; Lys(B29) replaced by Glu |
| Long-acting analogs | ||
| Glargine | Lantus/Sanofi-Aventis | Asp(A21) replaced by Gly; 2 Arg added to C-terminus of B-chain |
| Detemirt | Levemir/Novo Nordisk | Thr(B30) omitted; C14 fatty acid chain added at B29 |
| Premixed analogs | ||
| 75% neutral protamine lispro, 25% lispro | 75/25 Humalog/Eli Lilly | NA |
| 50% neutral protamine lispro, 50% lispro | 50/50 Humalog/Eli Lilly | NA |
| 70% protamine aspart, 30% aspart | 70/30 Novolog/Novo Nordisk | NA |
Asp, aspartic acid; Arg, arginine; Glu, glutamic acid; Lys, lysine; Pro, proline; Thr, threonine; NA, not applicable.
Long-Acting Basal Insulin Analogs
There are currently two long-acting basal insulin analog preparations available for commercial use: insulin glargine and insulin detemir (table 1 ▶). Both analogs have been designed to provide consistent, relatively flat, and protracted basal insulin levels, features which result from their specific molecular modifications relative to the human insulin molecule.
Insulin glargine has been formulated with an amino acid substitution at position A21 (asparagine replaced by glycine) and two arginines at the C-terminus of the B-chain (B31 and B32). These changes shift the isoelectric point from 5.4 to 6.7, which make the agent most soluble at a slightly acidic pH and less soluble under neutral conditions. Upon injection, insulin glargine precipitates into stable hexamers within the physiologically pH-neutral environment, thereby prolonging its dissociation and subsequent absorption.9 The marketed formulation of insulin glargine also includes small amounts of added zinc, which further delay absorption.13
Insulin detemir has been modified from the human insulin structure through the addition of a C14 fatty acid side chain at position B29. This alteration serves to delay absorption through a combination of increased self-association at the injection site (hexamer stabilization) and a high degree of reversible albumin binding within subcutaneous tissue.14 In addition, the interaction between insulin detemir and albumin in the bloodstream after absorption may result in a more consistent time-action profile by buffering against sudden changes in the analog’s plasma concentration.
Premixed Analogs
Three types of fixed-ratio insulin analog mixes are currently available: a 75% insulin lispro protamine suspension with 25% insulin lispro, a 50% insulin lispro protamine suspension with 50% insulin lispro, and a 70% insulin aspart protamine suspension with 30% insulin aspart (table 1 ▶). These formulations have been developed to minimize the errors that can occur when patients self-mix insulin combinations. Fixed-mixed combinations may simplify the insulin regimen and reduce the number of daily injections.15 However, the fixed ratio of insulins in premixed combinations can provide suboptimal therapy for some patients, which may necessitate self-mixing for optimal glucose control.
Pharmacokinetic and Pharmacodynamic Characteristics of Insulin Analogs
Subcutaneous insulin preparations are primarily differentiated by the shape of their serum time-concentration profiles, which in turn translates into how rapid, how significant, and how protracted of an effect they will have on glucose levels. Equally important is the need for reproducible and non-variable activity, which has been a significant limitation with traditional insulin products.9
Rapid-Acting Analogs
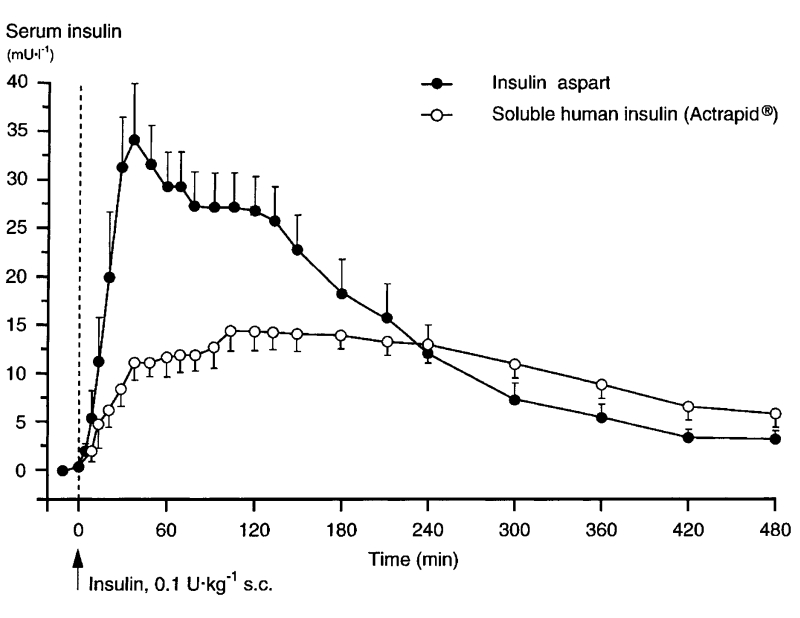
Pharmacokinetic studies of the three commercial rapid-acting analogs have consistently reported higher and more rapidly-attained peak values for serum insulin compared to human insulin, mimicking more closely a normal postprandial physiologic profile.16–19 In one study, for instance, insulin aspart was absorbed twice as quickly and attained more than double the serum concentrations compared to regular human insulin in healthy subjects (figure 1 ▶).20 Euglycemic clamp investigations in healthy subjects confirmed that the pharmacodynamic activity of insulin aspart was faster (glucose infusion rate [GIR] Tmax) and more extensive (GIR Cmax) than that of human insulin, regardless of injection site. One study, however, showed that abdominal injection of aspart was associated with a significantly shorter duration of effect compared to other sites.11 These studies also demonstrated a more rapid taper of metabolic activity of the insulin analogs relative to regular insulin. Finally, insulin aspart was found to have a lower range of pharmacokinetic and pharmacodynamic variability than human insulin (10% to 20%).21
Figure 1.
Serum insulin profiles (corrected for endogenous insulin) following a single dose of insulin aspart or regular human insulin injected under fasting conditions in 25 healthy male subjects.20 (Copyright 1999 Springer-Verlag. From Home PD, et al. Comparative pharmacokinetics and pharmacodynamics of the novel rapid-acting insulin analogue, insulin aspart, in healthy volunteers. Eur J Clin Pharmacol 1999;55:199–203. Reproduced with permission from Springer-Verlag.)
Similar results have been obtained in pharmacokinetic studies of insulin lispro and insulin glulisine. Thus, in a euglycemic clamp study in normal subjects, the peak serum insulin concentration was significantly higher (116 vs. 51 mU/mL [698 vs. 308 pmol/L]) and earlier (42 vs. 101 minutes) after the subcutaneous administration of insulin lispro than after regular insulin.22 The rates of glucose infusion needed to maintain euglycemia followed a similar pattern.22 Likewise, insulin glulisine reached twice the peak insulin concentration and had a time to peak concentration approximately twice as fast as that of regular human insulin.16 The time to 10% of total exposure (reflecting the onset of exposure) was about 30 minutes for insulin glulisine (0.15 U/kg) and about 55 minutes for regular human insulin, while the time to 90% of total exposure (reflecting completion of absorption) was about 3.5 and 6 hours for insulin glulisine and regular human insulin, respectively.16
When compared to each other, the short-acting insulin analogs appear to have relatively similar pharmacokinetic characteristics. A double-blind, two-period, crossover trial comparing insulin aspart and insulin lispro found no differences between them with regard to maximal insulin concentration, time to half-maximum insulin concentration, and time to 50% decrease of maximum insulin concentration.23 In a study of obese subjects without diabetes, insulin glulisine and insulin lispro were found to have similar, more rapid time-action profiles compared to human insulin, a difference that prevailed regardless of body mass index and subcutaneous fat thickness.24 However, in a randomized, double-blind, crossover study on lean to obese subjects without diabetes, insulin glulisine showed a somewhat greater early metabolic action than insulin lispro, an effect that was independent of body mass index and dose.25 The total metabolic effects over the entire study were not different between the two insulin analogs.25
The faster glucose-lowering effect of rapid-acting insulin analogs allows them to be dosed at mealtime when postprandial glucose absorption is highest. This imparts a significant advantage in convenience for patients relative to human insulin, which is recommended to be administered roughly 30 minutes prior to eating.20 In one study of children and adolescents with type 1 diabetes, a similar degree of glycemic control was achieved with insulin aspart, whether it was administered prior to or following a meal, although there was a tendency toward higher blood glucose levels 2 hours after the meal using the latter schedule.26 Analogous studies supporting meal-time dosing have been published for insulin glulisine27 and insulin lispro.28–32
Long-Acting Basal Analogs
Both insulin glargine and insulin detemir have been designed to approach the ideal characteristics for a basal insulin by having a relatively flat, reproducible, and long-acting concentration profile. By comparison, NPH insulin displays a peak in effect around 4 to 6 hours after injection and a duration of action ranging only from 12 to 16 hours,33 characteristics not observed with the long-acting analogs. Consequently, supper and/or bedtime dosing with NPH insulin may be associated with increased risk for hypoglycemia in the evening/early nighttime, as well as a waning effect in early morning hours when hepatic requirements and fasting plasma glucose (FPG) levels are increased (“dawn phenomenon”).9
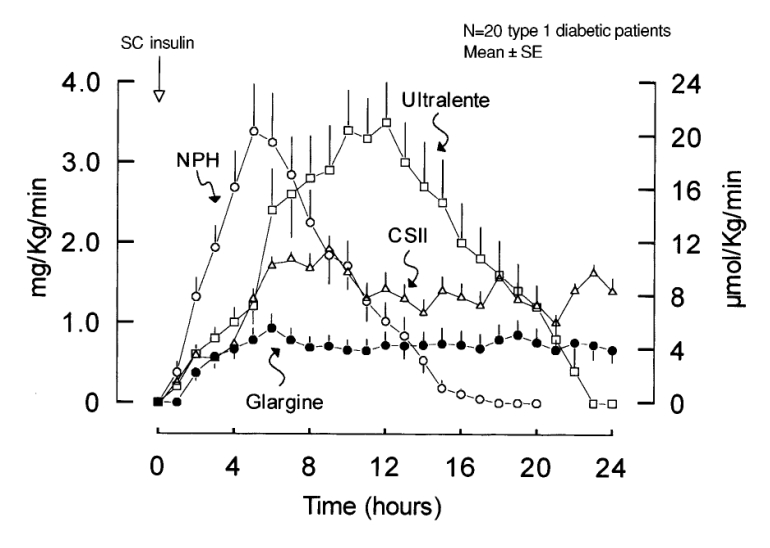
Pharmacokinetic/pharmacodynamic studies have confirmed that, compared to NPH insulin, both long-acting analogs have a more prolonged and consistent duration of action, without the marked post-injection peak characteristic of NPH insulin.34–38 Duration of action is most reliably measured through euglycemic clamp studies in patients with type 1 diabetes, rather than in healthy subjects or those with type 2 diabetes, because of the confounding influence of endogenous insulin secretion.39 Studies of this nature with insulin glargine have reported a duration of action of 20 to 24 hours after single dose administration35,36,40 and 24 to 25.6 hours at steady-state.40 The slight increase in duration of action at steady state may suggest an accumulation of insulin glargine after multiple doses, although a previous multiple-dose study reported no evidence of accumulation after 12 days of dosing.41 Compared with NPH insulin, insulin glargine is characterized by a smoother, relatively flat, GIR curve (figure 2 ▶). There appears to be little or no difference in the absorption rate of insulin glargine between various subcutaneous injection sites (e.g., arm, leg, abdomen),37 nor was absorption adversely affected by 30 minutes of intense exercise.42 Euglycemic clamp data gathered with insulin detemir in subjects with type 1 diabetes revealed a duration of action of 19.9 hours for a typical dose of 0.4 units/kg.38
Figure 2.
Rates of glucose infusion needed to maintain plasma glucose at the target value of 130 mg/dL after subcutaneous injection of glargine, NPH, and ultralente and after continuous subcutaneous insulin infusion (CSII) of lispro in patients with type 1 diabetes.36 (Copyright 2000 American Diabetes Association. From Diabetes Care 2000; 49:2142–2148. Reprinted with permission from The American Diabetes Association.)
A recent meta-analysis of insulin analog pharmacodynamic data applied a common definition of duration of action (time from injection to plasma glucose >8.3 mmol/L).39 While the duration of action for both insulin detemir and insulin glargine displayed dose dependency, duration of action was close to 24 hours in a clinically relevant dosing range of 0.35 U/kg to 0.8 U/kg in patients with type 1 diabetes. In a comparative study of insulin detemir and insulin glargine in type 2 patients, the two insulin analogs had similar durations of actions.43 The authors concluded that both long-acting analogs are suitable for once daily administration. One key difference in this and many other studies is that insulin detemir demonstrated significantly less variability in metabolic effect than both NPH insulin and insulin glargine, which is an issue of potential clinical relevance.
Traditional long-acting insulin products are subject to a high rate of pharmacokinetic and pharmacodynamic variability.21 Variability in blood glucose levels as a result of this variability are linked to increased risk of complications and should be considered alongside FPG and glycosylated hemoglobin (HbA1c) levels when monitoring glycemic control (see Clinical Effects of Insulin Analogs, below). Moreover, variability in response can contribute to patient fears of hypoglycemia and discourage aggressive glycemic control efforts. Insulin detemir, formulated as a clear neutral solution, does not rely on proper resuspension technique or dissolution of crystals at the injection site, as does NPH insulin.9
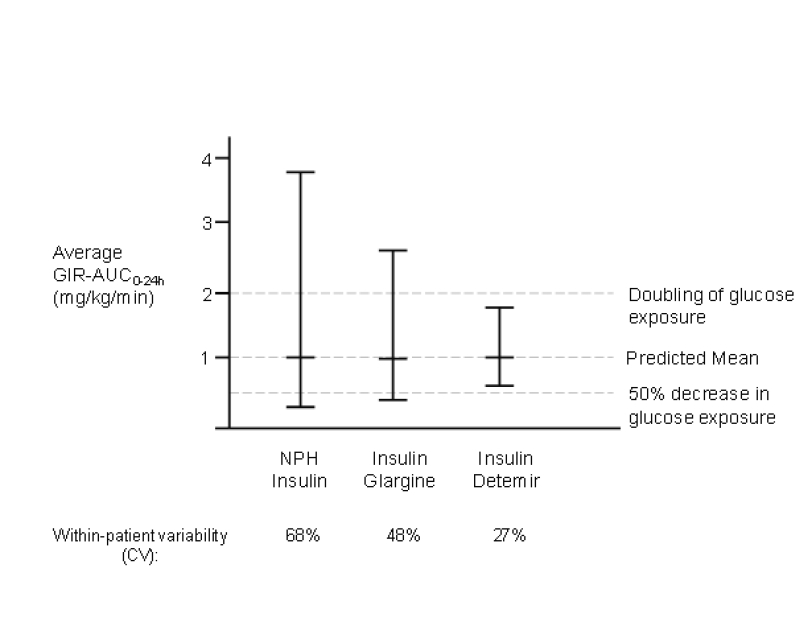
The degree of variability in response to insulin therapy, whether between different patients (inter-individual) or within the same patient from dose to dose (intra-individual), is commonly expressed as the coefficient of variation (CV) of the time required for 50% of injection-site insulin to disappear or as the CV for a particular pharmacodynamic measurement.9 Intra-individual variability is of the greatest clinical concern. The degree to which an individual’s glucose-lowering response differs from one injection to another can become a risk factor for hypoglycemia and a limiting factor in the pursuit of tight control.39 A randomized, double-blind, euglycemic clamp study of 54 type 1 diabetes subjects found a significantly lower CV for GIRAUC[0–12h] with insulin detemir (27%) compared with both NPH insulin (59%; P<0.001) and insulin glargine (46%; P<0.001) for GIRAUC[12–24h]. The CVs for detemir, NPH, and glargine were 27%, 68%, and 48%, respectively (P<0.001, all comparisons) (figure 3 ▶).35 Data gathered in patients with type 2 diabetes also revealed a significantly lower CV for GIRAUC[total] with insulin detemir versus insulin glargine (47% vs. 215%; P<0.001), as well as for GIRmax (40% vs. 147%; P<0.001).39
Figure 3.
Reproducibility of glucose-lowering effect with repeated injections of NPH insulin, insulin glargine, and insulin detemir.35 Figure contains prediction intervals illustrating potential day-to-day variability in glycemic response. The probability that a subject with a mean GIR over 24 hours of 1 mg/kg/min would experience an effect of less than half of usual (i.e., <0.5 mg/kg/min) is 16% with NPH insulin, 7% with insulin glargine, and 0.7% with insulin detemir. (Copyright 2006 American Diabetes Association. From Diabetes 2004;53:1614–1620. Reprinted with permission from The American Diabetes Association.)
Premixed Analogs
Premixes of conventional regular human insulins have an onset of action of approximately 0.5 to 2 hours, usually plateau at 3 to 6 hours, and last up to 24 hours. By comparison, the premixed insulin analogs have a more rapid onset of action (approximately 15 minutes) and reach a peak biological action more rapidly (1 to 4 hours). Like the conventional human insulin premixes, effects of the premixed insulin analogs last up to 24 hours. Studies also indicate that the pharmacokinetic properties of the premixed insulin analogs have less intra-individual variability than the premixed conventional human insulins.15
Clinical Effects of Insulin Analogs
HbA1c
The monitoring of HbA1c levels has become both a standard measure for evaluating individual treatment success and compliance, as well as a benchmark parameter for establishing treat-to-target goals. HbA1c levels provide an indirect measure of overall glycemic exposure over the preceding 2 to 3 months, with about 50% of the effect influenced by the previous 30 days.44,45 The American Diabetes Association (ADA) advocates maintaining HbA1c levels <7%,46 while the American Association of Clinical Endocrinologists47 and the International Diabetes Federation48 recommend a more aggressive target value of ≤6.5%.
HbA1c is clearly influenced by indices of glucose tolerance such as FPG and postprandial plasma glucose (PPG), although the relative influence of each factor on HbA1c continues to be debated. In one recent prospective study involving 164 type 2 diabetes patients with unsatisfactory glycemic control, a 3-month forced-titration intensified treatment program produced a mean decrease in HbA1c from 8.7% to 6.5%. Multiple linear regression analyses determined that decreases in PPG explained about twice as much of the HbA1c improvement as decreases in FPG.45 Conversely, a study performed in 262 treatment-naïve type 2 diabetes patients found that HbA1c correlated more strongly with FPG (r=0.85) than PPG exposure (r=0.539).44 The discrepancy between studies in this field might be explained by the fact that the relative contribution of PPG to HbA1c may predominate in relatively well-controlled patients, whereas FPG may come to predominate as the disease worsens.49 Biological variation in A1C alleles may also play a significant role in determining HbA1c glycosylation levels.50
The effects of rapid-acting and long-acting insulin analogs on HbA1c levels have been the subject of two recent Cochrane reviews.51,52 In a review of 49 randomized clinical studies comparing rapid-acting insulin analogs with regular human insulin, a weighted mean difference in HbA1c of −0.1% was determined in favor of analogs in patients with type 1 diabetes. Among patients with type 2 diabetes, there was no difference in HbA1c between rapid-acting analogs and regular human insulin.52 In a similar review of eight studies comparing long-acting insulin analogs with NPH insulin in patients with type 2 diabetes, there was no clinically meaningful difference in HbA1c results between the two insulin types.51 It should be noted that basal insulin trials, in particular, are generally designed to titrate dosing as needed to achieve preset HbA1c targets, and similar HbA1c outcomes should therefore not be entirely unexpected.53
Three clinical trials compared all-analog insulin regimens to all-human insulin regimens.54–56 In the largest, a basal-bolus regimen of insulin aspart/insulin detemir was compared with NPH insulin/regular insulin in 595 patients with type 1 diabetes for 18 weeks.54 At study end, mean HbA1c was lower in the aspart/detemir group compared with the NPH/regular insulin group (7.88% vs. 8.11%; P<0.001). In a smaller but longer study of 56 type 1 subjects, a regimen of glargine plus lispro was associated with a mean HbA1c of 7.5% after 32 weeks compared to 8.0% with a regimen of NPH plus unmodified human insulin.56 In the third study, a crossover design study of 28 adolescent subjects, there was no significant difference in HbA1c between subjects treated with glargine/lispro and those treated with NPH/regular insulin, each for 16 weeks (8.7% vs. 9.1%; P=0.13).55
A number of studies have assessed HbA1c levels in patients treated with premixed human insulins versus those treated with premixed insulin analogs, including premixed insulin lispro formulations57–59 and insulin aspart formulations.60–62 While one study showed small but significant improvements in HbA1c levels after treatment with 50/50 premixed insulin lispro relative to premixed human insulins,57 other studies failed to find similar advantages favoring premixed insulin analogs.58,60,62 Consistently, however, patients receiving premixed insulin analogs exhibited improved PPG control relative to premixed human insulins, reflecting the higher absorption rate of the analogs.58–62
PPG
The ADA defines PPG as a measurement of glucose 2 hours after the start of a meal.63 For those patients whose pre-meal glucose values are within target but whose HbA1c targets are not, the ADA recommends treatment to reduce average PPG to <180 mg/dL.63 The American Association of Clinical Endocrinologists and the International Diabetes Foundation advocate a PPG treatment goal of <140 mg/dL.47,64
While improvements in HbA1c levels have been correlated with reduced appearance or progression of microvascular complications such as neuropathy, nephropathy, and retinopathy,6 macrovascular sequelae (e.g., ischemic heart disease, cerebrovascular disease, peripheral vascular disease) may be linked more closely to postprandial hyperglycemia, particularly in type 2 diabetes.65 In fact, isolated postprandial hyperglycemia (PPG >140 mg/dL or 7.8 mmol/L) in patients with normal FPG and optimal HbA1c (<6.1%) increases the risk of death from cardiovascular disease by 2-fold.65 Moreover, in large studies of populations consisting of both non-diabetic and diabetic patients, PPG (and FPG) levels have been associated with risk of all-cause and cardiovascular disease mortality.66,67
In a review of published studies comparing rapid-acting insulin analogs used in basal-bolus therapy to human insulin, the rapid-acting analogs were noted to have a greater impact on PPG in every study, with values ranging between 0.6 and 2.0 mmol/L (10.8 and 36.0 mg/dL) lower with analog versus human insulin.53 In one of the longest trials (32 weeks) comparing an all-analog basal-bolus regimen (lispro/glargine) with an all-human insulin regimen (NPH/human insulin), the all-analog regimen was associated with a 15% lower PPG AUC (75 vs. 88 mmol/L/h; P=0.002).56
Hypoglycemia Risk
Fear of hypoglycemia and its associated risks of accident, coma, or death remains a major psychological obstacle to the aggressive pursuit of tight glycemic control. In the DCCT, the incidence of severe hypoglycemia was 3-fold higher in the intensive treatment group compared to the conventional treatment cohort (P<0.001).6 Moreover, the risk of severe hypoglycemia increased as monthly HbA1c values declined.
As an interesting follow-up to the DCCT, one large diabetes center continued to follow diabetes care parameters and HbA1c data from 1993 to 1998 for 884 patients with type 1 diabetes.68 From 1993 to 1996, HbA1c continued to decline significantly, but this trend was again associated with a significant increase in the number of severe hypoglycemic events (P<0.001). Starting in the autumn of 1996, a majority (676) of the patients switched to insulin lispro when it first became available. HbA1c levels continued to exhibit significant (P<0.001) improvement in the patients switched to lispro, but there was no corresponding increase in the rate of severe hypoglycemia in these subjects (P=0.26). Furthermore, HbA1c levels did not show further significant improvement in the subjects who remained on regular insulin. These findings suggest that intensive therapy with insulin analogs may not be associated with the same risks of hypoglycemia as older regimens with human insulin.68
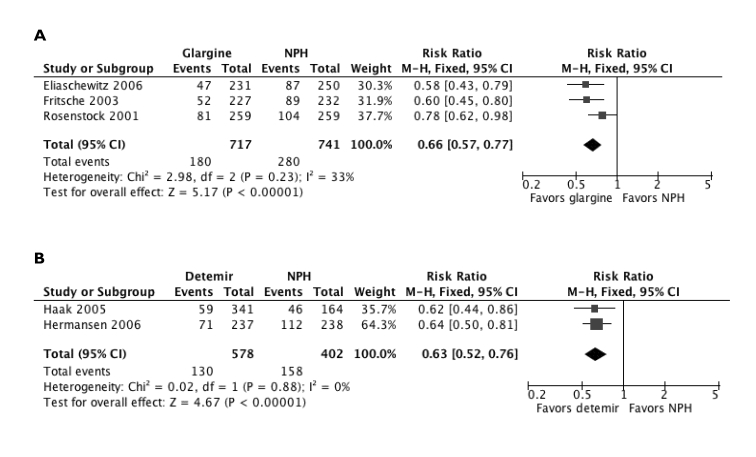
The Cochrane review of study data with rapid-acting insulin analogs also found a lower median incidence of severe hypoglycemic episodes per 100 person-years (21.8; range, 0 to 247.4) compared with regular insulin (46.1; range, 0 to 544).52 Likewise, the Cochrane review of basal insulin analog trials found significantly lower risks of nocturnal hypoglycemia with glargine (P=0.00003) and detemir (P<0.00001) relative to NPH insulin (figure 4 ▶).51 The risk of symptomatic hypoglycemia was also lower with both glargine versus NPH (P=0.005) and detemir versus NPH (P=0.00003).51 The rate of severe hypoglycemia was lower with both basal insulin analogs compared to NPH, although statistical significance was not reached (both P=0.2).
Figure 4.
Comparison of nocturnal hypoglycemic risk with long-acting insulin analogs (a) glargine and (b) detemir versus NPH insulin.51 Data were modified from the original published description by using a fixed effect, rather than a random effect, meta-analytic model. Analysis was conducted using Review Manager [RevMan] [Computer program] Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008. (Copyright 2007 Cochrane Collaboration, reproduced with permission. From Horvath K, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;(2):CD005613.)
Adherence
Effective use of any therapy in real-world settings is ultimately dependent on appropriate and compliant usage by individual patients. For a chronic disease in particular, adherence to pharmacologic treatment is essential for improving long-term prognosis, yet can be difficult to achieve in practice.69–71 Diabetes has a number of characteristics that make adherence to treatment especially difficult: it is a lifelong, chronic disorder that requires daily, if not hourly attention; comprehensive disease control necessitates lifestyle adjustments along with medication in many cases; insulin treatment in particular may be considered complex, intrusive, and inconvenient; and the goal is prevention, rather than symptom reduction or cure. Furthermore, aside from the demands of insulin administration, many patients with diabetes take many other medications, including oral glucose-lowering agents, and treatments for comorbid conditions, such as dyslipidemia, high blood pressure, and depression.72,73
Studies confirm that adherence to diabetes regimens remains unsatisfactory. A recent systematic review was performed of published investigations of adherence with oral hypoglycemic agents (OHAs) (20 studies) and insulin therapy (3 studies) in patients with type 2 diabetes.74 Retrospective data revealed adherence rates to OHA therapy ranged from 36% to 93%, while prospective trials using electronic monitoring reported compliance with 67% to 85% of prescribed OHA doses. Mean insulin adherence was 63% in a large retrospective database including both long-term and new-start insulin users, a lower adherence than seen with OHAs (73% to 86%). A number of variables have been causally correlated with poor adherence including clinical depression,75,76 alcohol use,76 twice daily versus once-daily OHA regimens,77 polytherapy versus monotherapy,78 poor comprehension of the treatment regimen,72 poor perception of treatment benefits,72 medication costs,72 and fear of weight gain or other side effects.79,80
Poor adherence has a measurably negative impact on disease control. In one analysis, a significant inverse association was apparent between HbA1c and an adherence index (P<0.001).81 Poor adherence was also related to higher rates of hospital admissions for diabetic ketoacidosis (P<0.001) and all admissions for acute diabetes-related complications (P=0.008). Conversely, glycemic control has been shown to improve progressively as medication adherence improves (P<0.0001).82 Relatively few studies have addressed whether insulin analogs are associated with better patient adherence than human insulins. One study showed that fear and rate of nocturnal hypoglycemia, a major factor for non-adherence, was lower in patients using glargine compared with those using NPH insulin.83 Consequently, more patients on glargine were inclined to adjust their doses to achieve FPG targets.
Treatment Satisfaction
Several studies have reported that patients with diabetes using insulin analogs describe greater satisfaction with their insulin treatment compared to those using regular human insulin products. In a prospective, multicenter, randomized, open-label, parallel-group study involving 423 type 1 diabetes patients treated with basal-bolus therapy, participants were randomly assigned to the use of human insulin or insulin aspart as their bolus insulin for 64 weeks.84 Treatment satisfaction was assessed using the World Health Organization Diabetes Treatment Satisfaction Questionnaire. Scores pertaining to perceived hyperglycemia were lower in the group using insulin aspart, indicating that subjects using aspart perceived high blood glucose levels to be less marked than the patients using human insulin (P=0.005). The insulin aspart group also indicated a greater degree of flexibility with their treatment compared to those using human insulin (P=0.022).
In another prospective, 6-month study conducted in 88 centers in 8 European countries, 1070 adults with type 1 diabetes were randomized 2:1 to insulin aspart or human insulin before meals, each in conjunction with NPH insulin as a basal insulin supply.85 Treatment satisfaction at the end of the 6 months, as evidenced by the diabetes treatment satisfaction questionnaire scores, was significantly better in the group using insulin aspart (baseline-adjusted difference in scores between groups, 2.3 points; P<0.001).
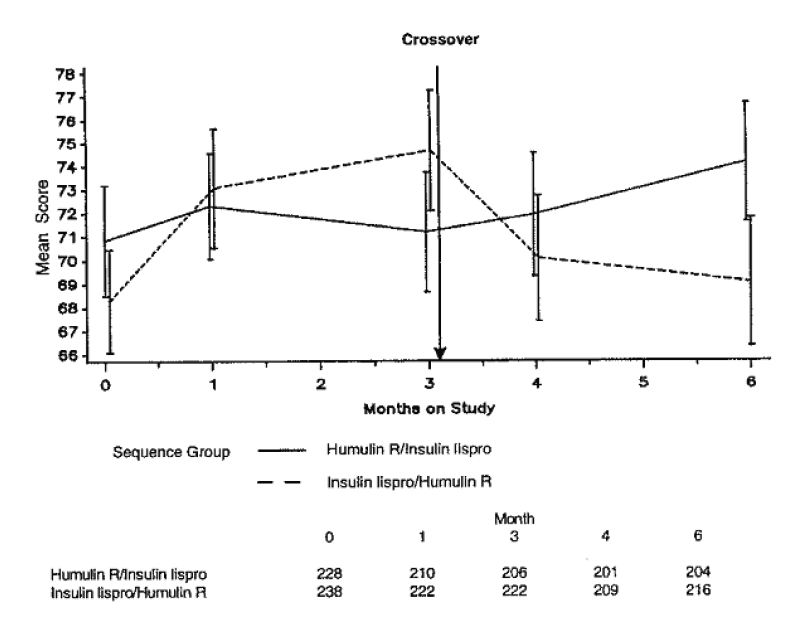
A third study compared quality-of-life issues in a crossover-design study involving 468 patients with type 1 diabetes, all of whom underwent 3 months of treatment using insulin lispro or regular human insulin as part of a basal-bolus regimen.86 Treatment satisfaction in the groups treated with insulin lispro increased significantly (P<0.001) compared with human insulin use (figure 5 ▶). Scores for treatment flexibility were also significantly greater with insulin lispro (P=0.001).
Figure 5.
Mean treatment satisfaction scores (95% CI shown by vertical bars) at each visit in a crossover study involving patients with type 1 diabetes treated with insulin lispro and regular human insulin. Satisfaction decreased in patients when switched from lispro to human insulin, and increased when patients were switched from human insulin to insulin lispro.86 (Copyright 1997 American Diabetes Association. From Diabetes Care 1997;20:948–958. Reprinted with permission from The American Diabetes Association.)
Weight Gain
Numerous studies have documented that improvements in glycemic control wrought by insulin and/or OHAs are frequently accompanied by undesirable increases in body weight.6,87–89 Weight gain in type 2 patients occurs through increases in both fat and fat-free mass.88,89 In the DCCT, even modest weight increases had a negative impact on lipid profiles and systolic blood pressure.90 Similar findings in type 2 patients were noted in a report from the Swedish National Diabetes Register.91 Intensive insulin therapy has also been correlated with complex changes in inflammation markers, possibly influenced by degree of weight gain, which could pose a risk for atherosclerosis.92 Weight gain is particularly unwelcome in type 2 diabetes, considering that 80% to 90% of such individuals are already overweight.93 Weight gain can also interfere with treatment compliance and success.80
Insulin-mediated weight gain has been attributed to reduced urinary glucose excretion (calorie retention) and a lowering of metabolic rate, both of which are direct consequences of improved glucose metabolism.93–95 It has also been recently postulated that weight gain observed in association with improvements in HbA1c in both type 1 and type 2 diabetes may be considered “catch-up” weight re-gain, i.e., is reflective of the natural body weight of the patients had they not had diabetes.96 While these phenomena are common in patients using either insulin or sulfonylurea therapy, data from the UKPDS identified a greater burden of weight gain among insulin-users (4.0 kg) compared with those using chlorpropamide (2.6 kg) or glibenclamide (1.7 kg), despite similar levels of metabolic control.7 The inherent anabolic activity of insulin on both adipose and muscle tissue may be responsible for prolonged periods of weight gain, even beyond the initial phase of “glucose control-related’ weight gain, or re-gain.88 So-called “defensive snacking” behaviors, driven by fears of hypoglycemia, can also contribute to weight gain in patients using insulin. Ironically, patients who are at the greatest risk for insulin-related weight gain are typically those with the greatest need for insulin (poorly controlled on oral agents), those who are eager to undertake intensive insulin management, and those who respond well to treatment.93 In fact, some of the main predictors of weight gain include high initial glycemia and degree of improvement in glycemic control.95–97 Another interesting, but unfortunate, characteristic of this phenomenon is that the rate of weight gain is often greatest during the early months of therapy when glycemic control is also undergoing dramatic correction.93 This can interfere with the patient’s adjustment to insulin therapy and possibly create a barrier for continued treatment adherence.
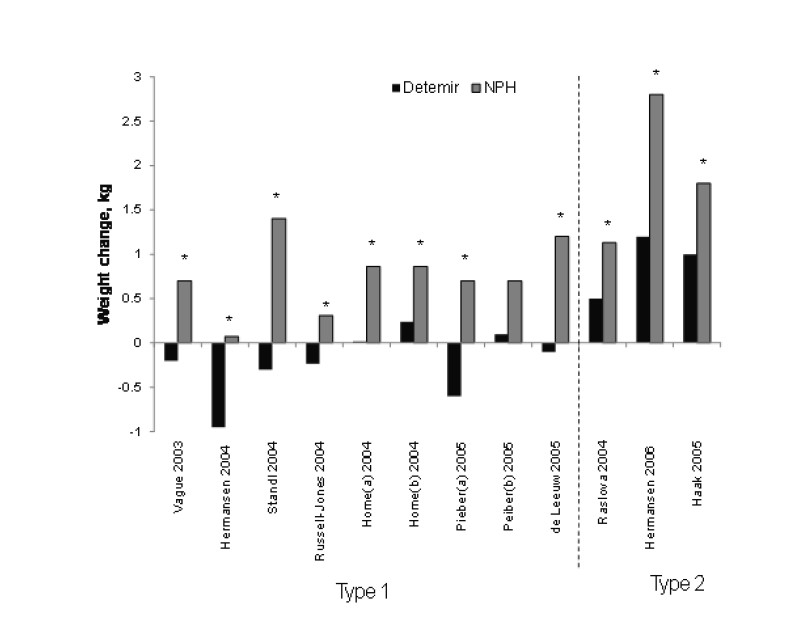
Until recently, weight gain had been assumed to be an almost unavoidable consequence of insulin therapy. However, for reasons not yet fully understood, a large body of evidence indicates that the long-acting insulin analog detemir actually has a weight-sparing effect. Data from at least 10 clinical trials in patients with type 1 and type 2 diabetes have reported significantly less weight gain with the use of insulin detemir compared with NPH insulin (figure 6 ▶). The weight-sparing effects of insulin detemir may be most pronounced in patients who are the most obese.98,99
Figure 6.
Mean changes in body weight reported with insulin detemir versus NPH insulin in seven studies in type 1 diabetes and three studies in type 2 diabetes. Studies ranged in duration from 16 weeks to 52 weeks. The difference in weight change between insulin detemir and NPH insulin was statistically significant (*P<0.05) in all but one analysis.
This weight-sparing phenomenon appears to be unique for insulin detemir. While some studies have shown that patients treated with insulin glargine initially gain less weight relative to those treated with NPH,100,101 no difference between insulin glargine and NPH was noted in patients treated for 1 year.97 Moreover, in a recently reported study, patients with type 2 diabetes were switched from NPH insulin or insulin glargine to insulin detemir for their basal insulin supply in combination with oral anti-diabetic drugs. Fourteen weeks after the switch to detemir, mean body weight was significantly reduced in patients previously using both NPH insulin (−0.7 kg; P<0.01) and insulin glargine (−0.5 kg; P<0.05).102 Other studies have documented weight increases or weight stasis after treatment with glargine, although the weight increases, when observed, tended to be less than those occurring with NPH insulin.103–114
The mechanisms behind the weight-sparing trait of insulin detemir have yet to be fully clarified. Insulin detemir is associated with a lower risk of hypoglycemia relative to other basal insulin preparations,99,108,115 consistent with its greater pharmacokinetic predictability and smooth time-action profile.35 This, in turn, could minimize the extent of “defensive snacking” behaviors that patients often exhibit when concerned about possible hypoglycemia.99,116 While this theory is plausible, it is not likely the sole mechanism. Insulin glargine has also been shown to reduce the risk of hypoglycemia compared with NPH, but nonetheless remains associated with weight gain.117
One other theory posits that the high degree of albumin binding by insulin detemir may influence its relative hepatic versus peripheral effects. Preliminary data suggest that insulin detemir may interact with hepatocytes to a greater degree than peripheral tissues, thus effectively suppressing hepatic glucose output without promoting lipogenesis in the periphery.116,118 Insulin detemir may also be more effective than human insulin in restoring impaired satiety signaling within the central nervous system, thereby suppressing appetite and excess eating.116
Quality of Life
Insulin therapy of any type has the potential to affect quality of life in both negative and positive ways.119 Clearly, quality of life can be impeded by concerns about needles/pain, hassles of frequent injections, and fears of hypoglycemia, weight gain, and other potential adverse events.119,120 On the other hand, improved glycemic control can have positive ramifications, including reductions in morbid complications.119 Low quality of life is a relevant issue not simply for the emotional well-being of the patient, but also because it can interfere further with treatment compliance.86
In a study involving teenagers with diabetes, those not meeting glucose control goals through multiple daily insulin injections of regular human insulin were given the option of switching to insulin lispro. After 12 months, teens who had switched to lispro evidenced similar metabolic control but less difficulty coping with diabetes, less negative impact of diabetes on quality of life, and fewer diabetes-related worries than those using regular insulin.121
In a cohort of Japanese children and adolescents with type 1 diabetes, switching to a rapid-acting insulin analog improved quality-of-life in the majority of patients (305/389; 78%).122 Various reasons for quality-of-life enhancement were cited, including meal flexibility (analog could be injected immediately before food intake or right after eating), decreased frequency of preprandial and nocturnal hypoglycemia, and freedom to schedule injections according to lifestyle.
Cost Effectiveness of Insulin Analogs
Assessing the overall cost effectiveness of insulin analogs relative to older human insulins requires sophisticated economic modeling that factors in not only direct medication expense, but also the expense of hypoglycemic episodes (emergency department/follow-up clinic costs), complications (microvascular, macrovascular, etc.), loss of work time, etc. One study estimated the average cost of complications in patients with type 2 diabetes at $47,240 per patient per 30 years (in 2000 U.S. dollars).123 Hence, potential therapeutic benefits associated with lower rates of hypoglycemic episodes and improved PPG and FPG control might be expected to offset the higher initial medication costs of insulin analogs. A number of U.S. health economic modeling studies have carried out this type of analysis comparing detemir, glargine, and NPH insulins,124 insulin glargine and reference therapy,125 and insulin lispro and regular insulin.126,127 General conclusions from these studies suggest that total direct healthcare costs may be relatively similar between insulin analog and older insulin treatments, while treatment with insulin analogs may be associated with increases in quality-adjusted life expectancy.
Summary
While HbA1c has become a valuable marker for monitoring diabetes treatment success, it should be evaluated in a broader context of treatment-related outcomes and issues. A comprehensive appreciation of the potential value of a diabetes intervention should take into account other factors, particularly those that may impact patient satisfaction and quality of life issues. Once the patient leaves the physician’s office, success hinges almost completely on patient acceptance of and adherence to the treatment protocol. From this standpoint, insulin analogs have been shown to have less pharmacologic variability, lower hypoglycemia risk, and greater impact on quality of life and treatment satisfaction compared with traditional insulin formulations, all of which would be expected to improve adherence. In addition, insulin detemir has an apparently unique weight-sparing effect that might have secondary implications for improved quality of life, treatment adherence, and comorbid disease progression.
Acknowledgments
The author thanks David Norris, PhD for editorial assistance provided during the preparation of this manuscript. This assistance was paid for through educational funding supplied by Novo Nordisk A/S to Ecosse Medical Communications (Princeton, NJ).
References
- 1.Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus: preliminary report. 1922. CMAJ 1991;145:1281–1286. [PMC free article] [PubMed] [Google Scholar]
- 2.Bonow RO, Smaha LA, Smith SC Jr, Mensah GA, Lenfant C. World Heart Day 2002: the international burden of cardiovascular disease: responding to the emerging global epidemic. Circulation 2002;106:1602–1605. [DOI] [PubMed] [Google Scholar]
- 3.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414–1431. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Diabetes complications. 2007. Available from: http://www.cdc.gov/diabetes/statistics/complications_national.htm. Accessed July 18, 2008.
- 5.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853. [PubMed] [Google Scholar]
- 8.Zinman B. The physiologic replacement of insulin. An elusive goal. N Engl J Med 1989;321:363–370. [DOI] [PubMed] [Google Scholar]
- 9.Guerci B, Sauvanet JP. Subcutaneous insulin: pharmacokinetic variability and glycemic variability. Diabetes Metab 2005;31(4 Pt 2):4S7–4S24. [DOI] [PubMed] [Google Scholar]
- 10.Apidra [package insert]. Bridgewater, NJ: Sanofi Aventis; 2003.
- 11.Mudaliar SR, Lindberg FA, Joyce M, Beerdsen P, Strange P, Lin A, Henry RR. Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care 1999; 22:1501–1506. [DOI] [PubMed] [Google Scholar]
- 12.Humalog [package insert]. Indianapolis, IN: Eli Lilly; 2007.
- 13.Gillies PS, Figgitt DP, Lamb HM. Insulin glargine. Drugs 2000;59:253–260. [DOI] [PubMed] [Google Scholar]
- 14.Havelund S, Plum A, Ribel U, Jonassen I, Vølund A, Markussen J, Kurtzhals P. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res 2004;21:1498–1504. [DOI] [PubMed] [Google Scholar]
- 15.Mooradian AD, Bernbaum M, Albert SG. Narrative review: a rational approach to starting insulin therapy. Ann Intern Med 2006;145:125–134. [DOI] [PubMed] [Google Scholar]
- 16.Becker RH, Frick AD. Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin Pharmacokinet 2008;47:7–20. [DOI] [PubMed] [Google Scholar]
- 17.Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmacodynamics of insulin aspart. Clin Pharmacokinet 2001;40:641–659. [DOI] [PubMed] [Google Scholar]
- 18.Holleman F, Hoekstra JB. Insulin lispro. N Engl J Med. 1997;337:176–183. [DOI] [PubMed] [Google Scholar]
- 19.Noble SL, Johnston E, Walton B. Insulin lispro: a fast-acting insulin analog. Am Fam Physician 1998; 57:279–286, 289–292. [PubMed] [Google Scholar]
- 20.Home PD, Barriocanal L, Lindholm A. Comparative pharmacokinetics and pharmacodynamics of the novel rapid-acting insulin analogue, insulin aspart, in healthy volunteers. Eur J Clin Pharmacol 1999;55:199–203. [DOI] [PubMed] [Google Scholar]
- 21.Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care 1998;21:1910–1914. [DOI] [PubMed] [Google Scholar]
- 22.Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes 1994;43:396–402. [DOI] [PubMed] [Google Scholar]
- 23.Plank J, Wutte A, Brunner G, Siebenhofer A, Semlitsch B, Sommer R, Hirschberger S, Pieber TR. A direct comparison of insulin aspart and insulin lispro in patients with type 1 diabetes. Diabetes Care 2002;25:2053–2057. [DOI] [PubMed] [Google Scholar]
- 24.Becker RH, Frick AD, Burger F, Potgieter JH, Scholtz H. Insulin glulisine, a new rapid-acting insulin analogue, displays a rapid time-action profile in obese non-diabetic subjects. Exp Clin Endocrinol Diabetes 2005;113:435–443. [DOI] [PubMed] [Google Scholar]
- 25.Heise T, Nosek L, Spitzer H, Heinemann L, Niemöller E, Frick AD, Becker RH. Insulin glulisine: a faster onset of action compared with insulin lispro. Diabetes Obes Metab 2007;9:746–753. [DOI] [PubMed] [Google Scholar]
- 26.Danne T, Aman J, Schober E, Deiss D, Jacobsen JL, Friberg HH, Jensen LH; ANA 1200 Study Group. A comparison of postprandial and preprandial administration of insulin aspart in children and adolescents with type 1 diabetes. Diabetes Care 2003;26:2359–2364. [DOI] [PubMed] [Google Scholar]
- 27.Rave K, Klein O, Frick AD, Becker RH. Advantage of premeal-injected insulin glulisine compared with regular human insulin in subjects with type 1 diabetes. Diabetes Care 2006;29:1812–1817. [DOI] [PubMed] [Google Scholar]
- 28.Tupola S, Komulainen J, Jääskeläinen J, Sipilä I. Post-prandial insulin lispro vs. human regular insulin in prepubertal children with type 1 diabetes mellitus. Diabet Med 2001;18:654–658. [DOI] [PubMed] [Google Scholar]
- 29.Rassam AG, Zeise TM, Burge MR, Schade DS. Optimal administration of lispro insulin in hyperglycemic type 1 diabetes. Diabetes Care 1999;22:133–136. [DOI] [PubMed] [Google Scholar]
- 30.Schernthaner G, Wein W, Sandholzer K, Equiluz-Bruck S, Bates PC, Birkett MA. Postprandial insulin lispro. A new therapeutic option for type 1 diabetic patients. Diabetes Care 1998;21:570–573. [DOI] [PubMed] [Google Scholar]
- 31.Anderson JH Jr, Brunelle RL, Keohane P, Koivisto VA, Trautmann ME, Vignati L, DiMarchi R. Mealtime treatment with insulin analog improves postprandial hyperglycemia and hypoglycemia in patients with non-insulin-dependent diabetes mellitus. Multicenter Insulin Lispro Study Group. Arch Intern Med 1997;157:1249–1255. [PubMed] [Google Scholar]
- 32.Anderson JH Jr, Brunelle RL, Koivisto VA, Trautmann ME, Vignati L, DiMarchi R. Improved mealtime treatment of diabetes mellitus using an insulin analogue. Multicenter Insulin Lispro Study Group. Clin Ther 1997;19:62–72. [DOI] [PubMed] [Google Scholar]
- 33.Peterson GE. Intermediate and long-acting insulins: a review of NPH insulin, insulin glargine and insulin detemir. Curr Med Res Opin 2006;22:2613–2619. [DOI] [PubMed] [Google Scholar]
- 34.Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 2000;23:644–649. [DOI] [PubMed] [Google Scholar]
- 35.Heise T, Nosek L, Rønn BB, Endahl L, Heinemann L, Kapitza C, Draeger E. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004;53:1614–1620. [DOI] [PubMed] [Google Scholar]
- 36.Lepore M, Pampanelli S, Fanelli C, Porcellati F, Bartocci L, Di Vincenzo A, Cordoni C, Costa E, Brunetti P, Bolli GB. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000;49:2142–2148. [DOI] [PubMed] [Google Scholar]
- 37.Owens DR, Coates PA, Luzio SD, Tinbergen JP, Kurzhals R. Pharmacokinetics of 125I-labeled insulin glargine (HOE 901) in healthy men: comparison with NPH insulin and the influence of different subcutaneous injection sites. Diabetes Care 2000;23:813–819. [DOI] [PubMed] [Google Scholar]
- 38.Plank J, Bodenlenz M, Sinner F, Magnes C, Görzer E, Regittnig W, Endahl LA, Draeger E, Zdravkovic M, Pieber TR. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care 2005;28:1107–1112. [DOI] [PubMed] [Google Scholar]
- 39.Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab 2007;9:648–659. [DOI] [PubMed] [Google Scholar]
- 40.Porcellati F, Rossetti P, Ricci NB, Pampanelli S, Torlone E, Campos SH, Andreoli AM, Bolli GB, Fanelli CG. Pharmacokinetics and pharmacodynamics of the long-acting insulin analog glargine after 1 week of use compared with its first administration in subjects with type 1 diabetes. Diabetes Care 2007;30:1261–1263. [DOI] [PubMed] [Google Scholar]
- 41.Heise T, Bott S, Rave K, Dressler A, Rosskamp R, Heinemann L. No evidence for accumulation of insulin glargine (LANTUS): a multiple injection study in patients with type 1 diabetes. Diabet Med 2002;19:490–495. [DOI] [PubMed] [Google Scholar]
- 42.Peter R, Luzio SD, Dunseath G, Miles A, Hare B, Backx K, Pauvaday V, Owens DR. Effects of exercise on the absorption of insulin glargine in patients with type 1 diabetes. Diabetes Care 2005;28:560–565. [DOI] [PubMed] [Google Scholar]
- 43.Klein O, Lynge J, Endahl L, Damholt B, Nosek L, Heise T. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab 2007;9:290–299. [DOI] [PubMed] [Google Scholar]
- 44.Peter R, Luzio SD, Dunseath G, Pauvaday V, Mustafa N, Owens DR. Relationship between HbA1c and indices of glucose tolerance derived from a standardized meal test in newly diagnosed treatment naive subjects with type 2 diabetes. Diabet Med 2006;23:990–995. [DOI] [PubMed] [Google Scholar]
- 45.Woerle HJ, Neumann C, Zschau S, Tenner S, Irsigler A, Schirra J, Gerich JE, Göke B. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes. Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract 2007;77:280–285. [DOI] [PubMed] [Google Scholar]
- 46.Executive summary: standards of medical care in diabetes -2008. Diabetes Care 2008;31(suppl 1):S5–S11. [DOI] [PubMed] [Google Scholar]
- 47.AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007;13(suppl 1):1–68. [DOI] [PubMed] [Google Scholar]
- 48.International Diabetes Federation. Global guideline for type 2 diabetes. Chapter 6: Glucose control levels. 2005. Available from: http://www.idf.org/webdata/docs/GGT2D%2006%20Glucose%20control%20levels.pdf. Accessed July 18, 2008.
- 49.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003; 26:881–885. [DOI] [PubMed] [Google Scholar]
- 50.McCarter RJ, Hempe JM, Chalew SA. Mean blood glucose and biological variation have greater influence on HbA1c levels than glucose instability: an analysis of data from the Diabetes Control and Complications Trial. Diabetes Care 2006; 29:352–355. [DOI] [PubMed] [Google Scholar]
- 51.Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, Kaiser T, Pieber TR, Siebenhofer A. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;(2):CD005613. [DOI] [PubMed]
- 52.Siebenhofer A, Plank J, Berghold A, Jeitler K, Horvath K, Narath M, Gfrerer R, Pieber TR. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev 2006;(2):CD003287. [DOI] [PubMed]
- 53.Gough SC. A review of human and analogue insulin trials. Diabetes Res Clin Pract 2007;77:1–15. [DOI] [PubMed] [Google Scholar]
- 54.Hermansen K, Fontaine P, Kukolja KK, Peterkova V, Leth G, Gall MA. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia 2004;47:622–629. [DOI] [PubMed] [Google Scholar]
- 55.Murphy NP, Keane SM, Ong KK, Ford-Adams M, Edge JA, Acerini CL, Dunger DB. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care 2003;26:799–804. [DOI] [PubMed] [Google Scholar]
- 56.Ashwell SG, Amiel SA, Bilous RW, Dashora U, Heller SR, Hepburn DA, Shutler SD, Stephens JW, Home PD. Improved glycaemic control with insulin glargine plus insulin lispro: a multicentre, randomized, cross-over trial in people with Type 1 diabetes. Diabet Med 2006;23:285–292. [DOI] [PubMed] [Google Scholar]
- 57.Yamada S, Watanabe M, Kitaoka A, Shiono K, Atsuda K, Tsukamoto Y, Kawana Y, Irie J. Switching from premixed human insulin to premixed insulin lispro: a prospective study comparing the effects on glucose control and quality of life. Intern Med 2007;46:1513–1517. [DOI] [PubMed] [Google Scholar]
- 58.Roach P, Yue L, Arora V. Improved postprandial glycemic control during treatment with Humalog Mix25, a novel protamine-based insulin lispro formulation. Humalog Mix25 Study Group. Diabetes Care 1999;22:1258–1261. [DOI] [PubMed] [Google Scholar]
- 59.Koivisto VA, Tuominen JA, Ebeling P. Lispro Mix25 insulin as premeal therapy in type 2 diabetic patients. Diabetes Care 1999;22:459–462. [DOI] [PubMed] [Google Scholar]
- 60.Mortensen H, Kocova M, Teng LY, Keiding J, Bruckner I, Philotheou A. Biphasic insulin aspart vs. human insulin in adolescents with type 1 diabetes on multiple daily insulin injections. Pediatr Diabetes 2006;7:4–10. [DOI] [PubMed] [Google Scholar]
- 61.Schmoelzer I, de Campo A, Pressl H, Stelzl H, Dittrich P, Oettl K, Wascher TC. Biphasic insulin aspart compared to biphasic human insulin reduces postprandial hyperlipidemia in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 2005;113:176–181. [DOI] [PubMed] [Google Scholar]
- 62.Boehm BO, Home PD, Behrend C, Kamp NM, Lindholm A. Premixed insulin aspart 30 vs. premixed human insulin 30/70 twice daily: a randomized trial in type 1 and type 2 diabetic patients. Diabet Med 2002;19:393–399. [DOI] [PubMed] [Google Scholar]
- 63.American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care 2007;30(suppl 1):S4–S41. [DOI] [PubMed] [Google Scholar]
- 64.International Diabetes Foundation. Guideline for management of postmeal glucose. Available at: http://www.idf.org/home/index.cfm?unode=185108C7-1E27-4A03-9B73-01D54087E32E. Accessed July 17, 2008.
- 65.Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med 2003;163:1306–1316. [DOI] [PubMed] [Google Scholar]
- 66.DECODE Study Group, European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 2003;26:688–696. [DOI] [PubMed] [Google Scholar]
- 67.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999;22:233–240. [DOI] [PubMed] [Google Scholar]
- 68.Chase HP, Lockspeiser T, Peery B, Shepherd M, MacKenzie T, Anderson J, Garg SK. The impact of the diabetes control and complications trial and humalog insulin on glycohemoglobin levels and severe hypoglycemia in type 1 diabetes. Diabetes Care 2001;24:430–434. [DOI] [PubMed] [Google Scholar]
- 69.Haynes RB, Yao X, Degani A, Kripalani S, Garg A, McDonald HP. Interventions to enhance medication adherence. Cochrane Database Syst Rev 2005;(4):CD000011. [DOI] [PubMed]
- 70.Haynes RB, McKibbon KA, Kanani R. Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications. Lancet 1996;348:383–386. [DOI] [PubMed] [Google Scholar]
- 71.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA 2002;288:2868–2879. [DOI] [PubMed] [Google Scholar]
- 72.Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med 2005;118 (suppl 5A):27S–34S. [DOI] [PubMed] [Google Scholar]
- 73.Piette JD, Heisler M, Wagner TH. Problems paying out-of-pocket medication costs among older adults with diabetes. Diabetes Care 2004;27:384–391. [DOI] [PubMed] [Google Scholar]
- 74.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care 2004;27:1218–1224. [DOI] [PubMed] [Google Scholar]
- 75.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med 2000;160:3278–3285. [DOI] [PubMed] [Google Scholar]
- 76.Lerman I, Lozano L, Villa AR, Hernández-Jiménez S, Weinger K, Caballero AE, Salinas CA, Velasco ML, Gómez-Pérez FJ, Rull JA. Psychosocial factors associated with poor diabetes self-care management in a specialized center in Mexico City. Biomed Pharmacother 2004;58:566–570. [DOI] [PubMed] [Google Scholar]
- 77.Dezii CM, Kawabata H, Tran M. Effects of once-daily and twice-daily dosing on adherence with prescribed glipizide oral therapy for type 2 diabetes. South Med J 2002;95:68–71. [PubMed] [Google Scholar]
- 78.Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycaemic medication in a population of patients with type 2 diabetes: a retrospective cohort study. Diabet Med 2002;19:279–284. [DOI] [PubMed] [Google Scholar]
- 79.Chao J, Nau DP, Aikens JE. Patient-reported perceptions of side effects of antihyperglycemic medication and adherence to medication regimens in persons with diabetes mellitus. Clin Ther 2007;29:177–180. [DOI] [PubMed] [Google Scholar]
- 80.Polonsky WH, Anderson BJ, Lohrer PA, Aponte JE, Jacobson AM, Cole CF. Insulin omission in women with IDDM. Diabetes Care 1994;17:1178–1185. [DOI] [PubMed] [Google Scholar]
- 81.Morris AD, Boyle DI, McMahon AD, Greene SA, MacDonald TM, Newton RW. Adherence to insulin treatment, glycaemic control, and ketoacidosis in insulin-dependent diabetes mellitus. The DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside Scotland. Medicines Monitoring Unit. Lancet 1997;350:1505–1510. [DOI] [PubMed] [Google Scholar]
- 82.Rhee MK, Slocum W, Ziemer DC, Culler SD, Cook CB, El-Kebbi IM, Gallina DL, Barnes C, Phillips LS. Patient adherence improves glycemic control. Diabetes Educ 2005;31:240–250. [DOI] [PubMed] [Google Scholar]
- 83.Fritsche A, Schweitzer MA, Häring HU; 4001 Study Group. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med 2003; 138:952–959. [DOI] [PubMed] [Google Scholar]
- 84.Tamás G, Marre M, Astorga R, Dedov I, Jacobsen J, Lindholm A; Insulin Aspart Study Group. Glycaemic control in type 1 diabetic patients using optimised insulin aspart or human insulin in a randomised multinational study. Diabetes Res Clin Pract 2001;54:105–114. [DOI] [PubMed] [Google Scholar]
- 85.Home PD, Lindholm A, Riis A; European Insulin Aspart Study Group. Insulin aspart vs. human insulin in the management of long-term blood glucose control in type 1 diabetes mellitus: a randomized controlled trial. Diabet Med 2000;17:762–770. [DOI] [PubMed] [Google Scholar]
- 86.Kotsanos JG, Vignati L, Huster W, Andrejasich C, Boggs MB, Jacobson AM, Marrero D, Mathias SD, Patrick D, Zalani S, Anderson J. Health-related quality-of-life results from multinational clinical trials of insulin lispro. Assessing benefits of a new diabetes therapy. Diabetes Care 1997;20:948–958. [DOI] [PubMed] [Google Scholar]
- 87.Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol 2003;2:33–47. [DOI] [PubMed] [Google Scholar]
- 88.Sallé A, Ryan M, Guilloteau G, Bouhanick B, Berrut G, Ritz P. ‘Glucose control-related’ and ‘non-glucose control-related’ effects of insulin on weight gain in newly insulin-treated type 2 diabetic patients. Br J Nutr 2005;94:931–937. [DOI] [PubMed] [Google Scholar]
- 89.Sallé A, Guilloteau G, Ryan M, Bouhanick B, Ritz P. Effect of insulin treatment on the body composition of type 2 diabetic patients. Diabet Med 2004;21:1298–1303. [DOI] [PubMed] [Google Scholar]
- 90.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA 1998;280:140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ridderstråle M, Gudbjörnsdottir S, Eliasson B, Nilsson PM, Cederholm J; Steering Committee of the Swedish National Diabetes Register (NDR). Obesity and cardiovascular risk factors in type 2 diabetes: results from the Swedish National Diabetes Register. J Intern Med 2006;259:314–322. [DOI] [PubMed] [Google Scholar]
- 92.Schaumberg DA, Glynn RJ, Jenkins AJ, Lyons TJ, Rifai N, Manson JE, Ridker PM, Nathan DM. Effect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the diabetes control and complications trial. Circulation 2005;111:2446–2453. [DOI] [PubMed] [Google Scholar]
- 93.Hermansen K, Davies M. Does insulin detemir have a role in reducing risk of insulin-associated weight gain? Diabetes Obes Metab 2007;9:209–217. [DOI] [PubMed] [Google Scholar]
- 94.Jacob AN, Salinas K, Adams-Huet B, Raskin P. Potential causes of weight gain in type 1 diabetes mellitus. Diabetes Obes Metab 2006;8:404–411. [DOI] [PubMed] [Google Scholar]
- 95.Mäkimattila S, Nikkilä K, Yki-Järvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia 1999;42:406–412. [DOI] [PubMed] [Google Scholar]
- 96.Larger E. Weight gain and insulin treatment. Diabetes Metab 2005;31:4S51–4S56. [DOI] [PubMed] [Google Scholar]
- 97.Yki-Järvinen H, Dressler A, Ziemen M; HOE 901/300s Study Group. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care 2000;23:1130–1136. [DOI] [PubMed] [Google Scholar]
- 98.Raslová K, Bogoev M, Raz I, Leth G, Gall MA, Hâncu N. Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Res Clin Pract. 2004;66:193–201. [DOI] [PubMed] [Google Scholar]
- 99.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29:1269–1274. [DOI] [PubMed] [Google Scholar]
- 100.Rosenstock J, Schwartz SL, Clark CM Jr, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001;24:631–636. [DOI] [PubMed] [Google Scholar]
- 101.Raskin P, Klaff L, Bergenstal R, Hallé JP, Donley D, Mecca T. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care 2000;23:1666–1671. [DOI] [PubMed] [Google Scholar]
- 102.Dornhorst A, Lüddeke HJ, Koenen C, Meriläinen M, King A, Robinson A, Sreenan S; PREDICTIVE Study Group. Transferring to insulin detemir from NPH insulin or insulin glargine in type 2 diabetes patients on basal-only therapy with oral antidiabetic drugs improves glycaemic control and reduces weight gain and risk of hypoglycaemia: 14-week follow-up data from PREDICTIVE. Diabetes Obes Metab 2008;10:75–81. [DOI] [PubMed] [Google Scholar]
- 103.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008;371:1073–1084. [DOI] [PubMed] [Google Scholar]
- 104.Barnett AH, Burger J, Johns D, Brodows R, Kendall DM, Roberts A, Trautmann ME. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007;29:2333–2348. [DOI] [PubMed] [Google Scholar]
- 105.Schreiber SA, Russmann A. Long-term efficacy of insulin glargine therapy with an educational programme in type 1 diabetes patients in clinical practice. Curr Med Res Opin 2007;23:3131–3136. [DOI] [PubMed] [Google Scholar]
- 106.Siegmund T, Weber S, Blankenfeld H, Oeffner A, Schumm-Draeger PM. Comparison of insulin glargine versus NPH insulin in people with type 2 diabetes mellitus under outpatient-clinic conditions for 18 months using a basal-bolus regimen with a rapid-acting insulin analogue as mealtime insulin. Exp Clin Endocrinol Diabetes 2007;115:349–353. [DOI] [PubMed] [Google Scholar]
- 107.Davies M, Evans R, Storms F, Gomis R, Khunti K. Initiation of insulin glargine in suboptimally controlled patients with type 2 diabetes: sub-analysis of the AT.LANTUS trial comparing treatment outcomes in subjects from primary and secondary care in the UK. Diabetes Obes Metab 2007; 9:706–713. [DOI] [PubMed] [Google Scholar]
- 108.Pieber TR, Treichel HC, Hompesch B, Philotheou A, Mordhorst L, Gall MA, Robertson LI. Comparison of insulin detemir and insulin glargine in subjects with type 1 diabetes using intensive insulin therapy. Diabet Med 2007; 24:635–642. [DOI] [PubMed] [Google Scholar]
- 109.Schreiber SA, Haak T. Insulin glargine benefits patients with type 2 diabetes inadequately controlled on oral antidiabetic treatment: an observational study of everyday practice in 12,216 patients. Diabetes Obes Metab 2007;9:31–38. [DOI] [PubMed] [Google Scholar]
- 110.Kann PH, Wascher T, Zackova V, Moeller J, Medding J, Szocs A, Mokan M, Mrevlje F, Regulski M. Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin Aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes 2006; 114:527–532. [DOI] [PubMed] [Google Scholar]
- 111.Gerstein HC, Yale JF, Harris SB, Issa M, Stewart JA, Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with Type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet Med 2006; 23:736–742. [DOI] [PubMed] [Google Scholar]
- 112.Rosenstock J, Sugimoto D, Strange P, Stewart JA, Soltes-Rak E, Dailey G. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patients. Diabetes Care 2006;29:554–559. [DOI] [PubMed] [Google Scholar]
- 113.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569. [DOI] [PubMed] [Google Scholar]
- 114.Raskin P, Allen E, Hollander P, Lewin A, Gabbay RA, Hu P, Bode B, Garber A; INITIATE Study Group. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care 2005;28:260–265. [DOI] [PubMed] [Google Scholar]
- 115.Russell-Jones D, Simpson R, Hylleberg B, Draeger E, Bolinder J. Effects of QD insulin detemir or neutral protamine Hagedorn on blood glucose control in patients with type 1 diabetes mellitus using a basal-bolus regimen. Clin Ther 2004;26:724–736. [DOI] [PubMed] [Google Scholar]
- 116.Raslová K, Tamer SC, Clauson P, Karl D. Insulin detemir results in less weight gain than NPH insulin when used in basal-bolus therapy for type 2 diabetes mellitus, and this advantage increases with baseline body mass index. Clin Drug Investig 2007;27:279–285. [DOI] [PubMed] [Google Scholar]
- 117.Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086. [DOI] [PubMed] [Google Scholar]
- 118.Hordern SV, Wright JE, Umpleby AM, Shojaee-Moradie F, Amiss J, Russell-Jones DL. Comparison of the effects on glucose and lipid metabolism of equipotent doses of insulin detemir and NPH insulin with a 16-h euglycaemic clamp. Diabetologia 2005;48:420–426. [DOI] [PubMed] [Google Scholar]
- 119.Vinik AI, Zhang Q. Adding insulin glargine versus rosiglitazone: health-related quality-of-life impact in type 2 diabetes. Diabetes Care 2007;30:795–800. [DOI] [PubMed] [Google Scholar]
- 120.Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients’ fears and hopes about insulin therapy. The basis of patient reluctance. Diabetes Care 1997;20:292–298. [DOI] [PubMed] [Google Scholar]
- 121.Grey M, Boland EA, Tamborlane WV. Use of lispro insulin and quality of life in adolescents on intensive therapy. Diabetes Educ 1999;25:934–941. [DOI] [PubMed] [Google Scholar]
- 122.Urakami T, Kawamura T, Sugihara S, Miyamoto S, Amemiya S, Sasaki N, Matsuura N; Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes. A questionnaire survey on the use of quick-acting insulin analog in Japanese children and adolescents with type 1 diabetes. Pediatr Int 2004;46:285–290. [DOI] [PubMed] [Google Scholar]
- 123.Caro JJ, Ward AJ, O’Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care 2002;25:476–481. [DOI] [PubMed] [Google Scholar]
- 124.Valentine WJ, Palmer AJ, Erny-Albrecht KM, Ray JA, Cobden D, Foos V, Lurati FM, Roze S. Cost-effectiveness of basal insulin from a US health system perspective: comparative analyses of detemir, glargine, and NPH. Adv Ther 2006;23:191–207. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Q, Menditto L. Incremental cost savings 6 months following initiation of insulin glargine in a Medicaid fee-for-service sample. Am J Ther 2005;12:337–343. [DOI] [PubMed] [Google Scholar]
- 126.Chen K, Chang EY, Summers KH, Obenchain RL, Yu-Isenberg KS, Sun P. Comparison of costs and utilization between users of insulin lispro versus users of regular insulin in a managed care setting. J Manag Care Pharm 2005;11:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hall JA, Summers KH, Obenchain RL. Cost and utilization comparisons among propensity score-matched insulin lispro and regular insulin users. J Manag Care Pharm 2003; 9:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]