Summary
Integrin receptors for extracellular matrix (ECM) are critical determinants of biological processes. Regulation of integrin expression is one way for cells to respond to changes in the ECM, to integrate intracellular signals, and to obtain appropriate adhesion for cell motility, proliferation, and differentiation. Transcriptional and post-translational mechanisms for changing the integrin repertoire at the cell surface have recently been described. These mechanisms work through transcriptional regulation that alters the proportions of one integrin relative to another, referred to as integrin switching, or through localized regulation of integrin-ECM interactions, thus providing exquisite control over cell rearrangements during tissue morphogenesis and remodeling. These integrin regulatory pathways may also be important targets in such emerging fields as tissue engineering and regenerative medicine.
Introduction
Integrins are adhesion receptors that link the extracellular matrix to the cytoskeleton and have critical roles in early developmental events including fertilization, implantation, and early blastula formation [1]. These requirements continue over the course of embryonic development and throughout the formation and maintenance of adult tissues [2]. The requirements for integrin activity in development are conserved across multi-cellular species ranging from nematodes and fruit flies to mice and humans [3].
Integrins function as heterodimeric transmembrane receptors consisting of one α and one β subunit; humans have 18 α and 8 β subunits, which form 24 functional pairs [4]. In addition to their roles in adhesion, integrins are capable of bidirectional signaling across the plasma membrane. Adhesive and signaling events depend upon linkage of integrin pairs to a large and diverse collection of cytoplasmic interactors and downstream effectors including talin, focal adhesion kinase (FAK) and the Rho family of GTPases. Integrin-based signaling cascades, many times in cooperation with growth factor receptor signals, regulate and direct cell migration, cell division, and differentiation [5].
During tissue morphogenesis, there are changes in gene expression and cell shape, ECM remodeling, and cell rearrangements that are essential for formation of functionally distinct tissues and organs. This review contains several examples of divergent organogenic events that utilize differing integrin expression or activity patterns as a means to control cell adhesivity and thus differentiation.
An integrin switch mechanism to regulate cell migration and differentiation
Perhaps the first evidence for integrin switching as a regulator of cell fate decisions during morphogenesis was observed with primary muscle cells. Up-regulation of the α6 integrin receptor for laminin drove myoblast differentiation into multi-nucleated myotubes while increased expression of α5 fibronectin receptor maintained cells in the proliferative phase [6]. Changing the proportion of α5 relative to α6, through a process we refer to as an integrin switch, was a determining factor in the self-renewal versus differentiation decision. More recently, a similar integrin switch was reported during adipocyte differentiation. In this case, α5 expression by self-renewing preadipocytes decreased with a concurrent increase in α6 expression as differentiation progressed. Down-regulation of α5 integrin and signaling through Rac and Rho GTPases were required to stop proliferation and allow differentiation to commence [7].
Integrin switching also makes a major contribution to cell migration during development. Gonadogenesis in the nematode C. elegans relies on migration of two leader cells called the distal tip cells (DTC). These cells migrate away from the gonad primordium on the ventral surface of the nematode: one toward the anterior, the other toward the posterior. They each then turn toward and migrate to the dorsal surface where they turn yet again and migrate back toward the center of the nematode (Figure 1). As the DTCs migrate, they are followed by proliferating germ cells of the gonad resulting in two mirror image, U-shaped gonad arms [8].
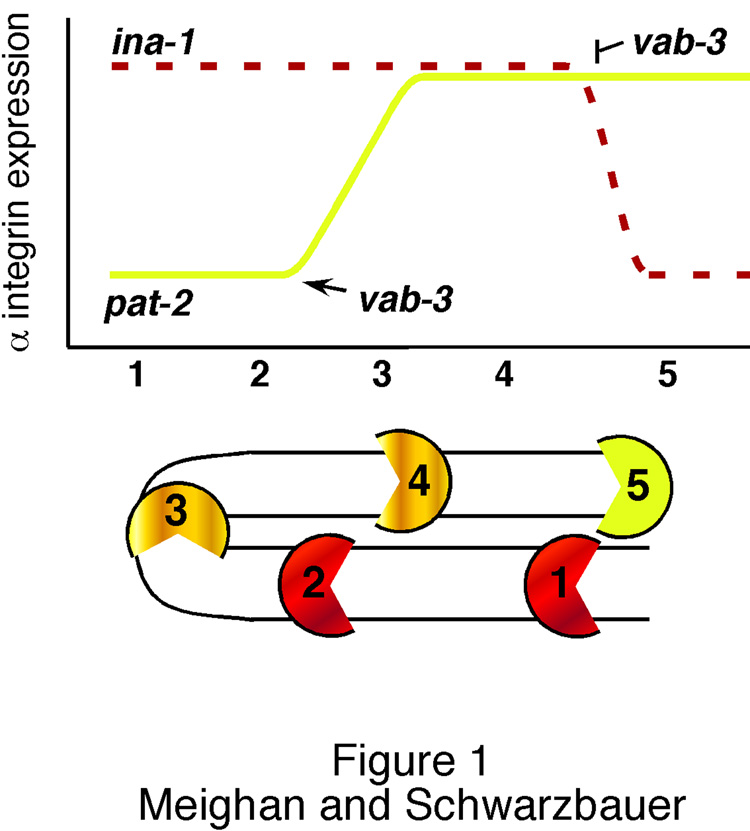
Figure 1. Integrin expression regulates C. elegans gonadogenesis.
Relative integrin expression in the DTCs is shown diagrammatically throughout gonadogenesis. DTC positions during migration are noted numerically, 1 – 5, with ina-1 expression in dashed red and pat-2 in yellow. Migration begins at position 1 with high levels of ina-1 expression maintained during ventral movement (2). As the DTC moves from the ventral to dorsal surface (position 2–3), pat-2 is up-regulated. Migration continues along the dorsal surface (4) and ends at position 5 with ina-1 down-regulation. The increase in pat-2 and decrease in ina-1 expression are both regulated by transcription factor vab-3 and are required for proper organ formation [10].
C. elegans nematodes have two α integrin subunits and one β subunit, creating two functional integrin pairs: ina-1/pat-3 and pat-2/pat-3. INA-1 is present throughout, and required for, proper DTC migration [9]. Knockdown of ina-1 expression by RNAi terminates migration early along the migratory route. Recently it has been shown that completion of DTC migration coincides with down-regulation of ina-1 expression [10]. Failure to down-regulate this integrin results in perpetual migration of the DTCs and continual expansion of the gonad arm. This down-regulation depends on the transcription factor VAB-3, an ortholog of vertebrate Pax6. Unexpectedly, VAB-3 also controls expression of the other α integrin, pat-2. During dorsal migration, this integrin is up-regulated by direct vab-3 activity on the pat-2 promoter. However, PAT-2 is not involved in DTC stopping. Instead, loss of PAT-2 causes misdirection of DTC migration yielding a gonad arm with aberrant shape [10]. Thus, during gonadogenesis, a single transcription factor is responsible for up-regulation of one integrin to dictate the directionality of migration and down-regulation of another to end motility.
Complementary expression patterns of two integrins are sufficient to determine organ size and shape, showing that simply modifying the adhesive repertoire of the cell can coordinate major developmental processes. This mechanism allows a migrating cell to change its developmental program through new interpretations of inputs without necessitating change in the ECM or surrounding cells. Determining the contribution of the ECM relative to integrin signaling in the DTCs is an important area for further study. In the case of muscle, however, distinct ECM components are required to initiate α integrin-specific signaling events to promote proliferation versus differentiation [6].
The migratory patterns of primordial germ cells (PGCs) also depend on alterations in adhesivity but these occur through a combination of selective integrin usage with changes in the ECM pathway. In mouse embryos, PGCs migrate from the developing hind gut to the genital ridges, the future site of the gonads [11]. The adhesive properties of PGCs have been shown to vary throughout the course of migration. Adhesion to fibronectin declined to its lowest point in the genital ridges while the ability to adhere to laminin was maintained upon reaching the genital ridges [12]. ECM protein distributions correlated with adhesive changes. In particular, PGCs accumulated along ribbons of laminin deposited on the migratory path (Figure 2A) and were surrounded by laminin within the genital ridges [12]. Expression patterns of integrin receptors for laminin coincided with migratory events. Integrin α6 was observed throughout migration while levels of α3 integrin mRNA were down-regulated as migration progressed [13], suggesting a role for transcriptional regulation of this integrin in ending PGC migration at the genital ridge. Genetic deletion of β1 integrin, the partner for α3, caused the majority of PGCs to fail to reach the genital ridges, but knockout of α3, α6, or αv did not reproduce the β1 phenotype [13]. Recent examination of connexin 43-null PGCs found loss of β1 integrin-mediated adhesion in the absence of this gap junction protein [14]. On the one hand, PGCs in vertebrates, like DTCs in nematodes, require proper regulation of integrin expression to migrate to their target sites. Unlike DTCs, however, are the findings that modulation of individual integrins cannot fully explain the process. PGCs also require changes in ECM protein deposition coupled with appropriate levels of cell-cell communication through gap junctions in order to maintain their directionality through varied extracellular environments.
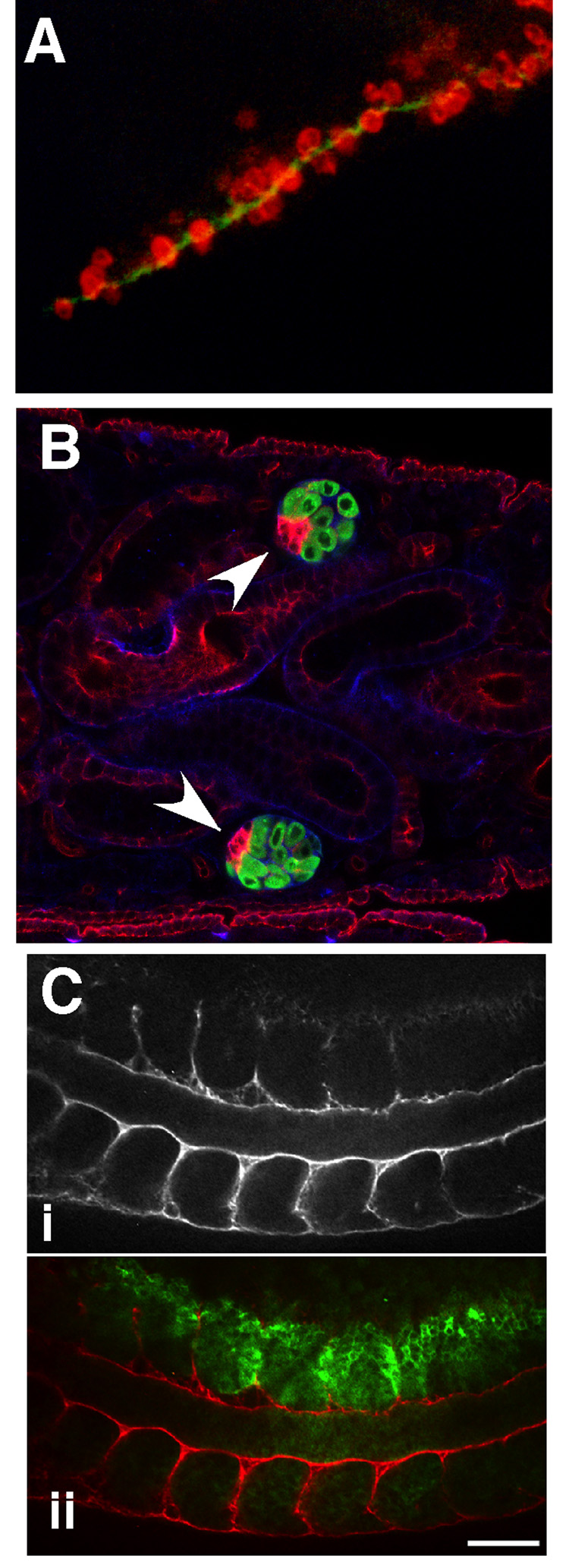
Figure 2. Integrins and ECM in tissue organization.
Three examples of adhesion-dependent cell organization during development are shown. (A) Migrating PGCs (red) accumulate along a ribbon of laminin (green) in the E10.5 mouse embryo (Reproduced from [12]; © 1997 The Rockefeller University Press). (B) In the male Drosophila embryo, hub cells (red, arrowheads) localize to the anterior edge of the male gonad (green) (Reproduced from [17], © 2007 Nature Publishing Group). (C) Localized knockdown by injection of α5 integrin morpholinos in a Xenopus embryo (marked by green in ii) results in discontinuous deposition of fibronectin at somite boundaries (anti-fibronectin staining in i and ii (red)) (Reproduced from [19], © 2007 Wiley-Liss, Inc., reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.).
These examples show how transcriptional regulation of integrin or ECM gene expression directs cell migration and differentiation. A recent study provides new insights into a specific mechanism for control of differential integrin transcription. Experiments with corneal epithelial cells in a wound healing model have shown that the transcription factor Sp1 directly binds and up-regulates the α5 integrin promoter in a process that is stimulated by fibronectin [15]. Sp1 and Sp3 also regulate expression of α6 integrin, but in contrast to the effects of fibronectin, corneal cell growth on laminin, an α6 ligand, repressed expression from an α6 promoter construct [16]. Nuclear levels of Sp1/Sp3 were dramatically reduced in cells on laminin, and western blots revealed smaller protein products suggesting transcription factor degradation. The effects of ECM on Sp1/Sp3 suggest an interesting model for temporal regulation of complementary integrins. Upon corneal cell wounding, Sp1/Sp3 activate α5 expression to coincide with increased fibronectin deposition. α6 is also up-regulated by these factors, perhaps to participate in deposition of newly-synthesized laminin. Once a laminin-rich basement membrane is in place, adhesion to laminin leads to Sp1/Sp3 turnover returning cells to their pre-injury state. This mechanism relies on regulation of transcription factor levels to control integrin expression patterns and has the advantage of providing a way for cells to respond rapidly by concerted control of a set of co-regulated genes.
Integrins as determinants of tissue boundaries
During morphogenesis, neighboring cells make divergent cell fate choices. For example, germ cells maintain an undifferentiated state while surrounded by cellular activity in the adjacent somatic tissue. In Drosophila males, germline stem cells maintain mitotic divisions by contact with a somatic region of the testis called the hub. Null mutations in the βPS integrin gene result in the mis-positioning of the hub from the edge of the gonad to its center and a shift of germ cells into their differentiated fate as sperm [17]. Integrins are not required for contact of the germ cells with the hub region but are, instead, necessary for proper hub positioning at the edge of the gonad (Figure 2B). This location is critical for control of germline cell divisions. Loss of the hub also occurs with knockdown of expression of talin, an integrin binding protein [17]. The requirement for integrins in hub cells appears related to their role in basement membrane assembly, which is severely limited in the absence of βPS integrins. Thus, development and maintenance of Drosophila somatic-germline tissue organization depends on localized integrin-ECM interactions.
Somite boundary formation also requires region-specific expression of integrins. Integrin α5 activity is present very early after fertilization. α5 expression is later lost in many tissues but is maintained in the somites, putting α5 in a position to participate in somite morphogenesis [18]. Localized knockdown of α5 by morpholino injection into frog embryos delayed somite rotation, disrupted boundary formation, and resulted in abnormal myotome boundaries at the injection sites (Figure 2C) [19]. This shows that maintenance of α5 in somite tissue is required for proper development and suggests that down-regulation of α5 in the surrounding tissue is also critical for cell separation and boundary formation. In both the somitic cells and the hub cells, expression of α5 integrin was a determining factor in cell positioning within the tissue and thus proper tissue morphogenesis. These examples highlight the importance of local rather than global alterations in integrin function for providing precision to developmental events.
Variations in expression of integrins based on cell location, cell type, and time occurs throughout development of the cerebral cortex [20]. Blocking or knockout experiments have identified distinct roles for different integrins. For example, αv is required for proper vascular organization in the brain [21]. Antibody blocking of αv or α3 integrins reduced neuronal migration rates and glial to neuronal adhesion in vitro, and caused disorganization of the cerebral cortex in vivo [22]. Removal of α6 activity by genetic knockout resulted in defective organization of the cerebral cortex and retina [23,24]. To decipher cell-specific roles for these integrins, the β1 integrin gene was targeted in radial glia and neurons. Surprisingly, neuronal migration into cortical layers still occurred in mice lacking β1 in neurons [25]. However, the absence of β1 in both neurons and radial glia severely affected the formation of cortical layers. While the precise role of the various β1-containing integrin heterodimers during corticogenesis is still an area of active investigation, these results suggest that integrin-mediated radial glial adhesion plays a critical role in organizing the cortical layers.
Local changes in integrin expression also contribute to stem cell differentiation. Integrin-dependent localization of stem cells to adhesive environments favorable for self-renewal has been shown in the haematopoietic stem cell niche [26], human epidermal stem cells [27], and neural stem cells [28]. Along with adult stem cells, embryonic stem (ES) cells also appear reliant on integrins for the choice between self-renewal and differentiation. Comparison of ECM substrates showed that type I or type IV collagen, gelatin, or poly-D-lysine encouraged self-renewal of mouse ES cells whereas laminin or fibronectin induced differentiation [29]. Expression of markers for self-renewal was maintained by blocking ES cell adhesion to fibronectin or laminin with anti-β1 integrin antibodies whereas over-expression of a collagen-binding integrin shifted the cells toward differentiation. Changes in FAK and ERK signaling pathways suggest that integrins and ECM modulate signals that are required for mouse ES cell self-renewal. From this study it seems clear that cell adhesion is essential for maintaining self-renewing ES cells in the stem cell niche but that some pliability in the specific integrin-ECM interactions may be acceptable. Determining the factors that drive integrin expression on pluripotent stem cells could provide a novel means to control their differentiation.
Post-translational regulation of integrins
A cleaved variant of α6 integrin, α6p, in a heterodimeric complex with β1 or β4 subunits has previously been identified on the surface of human cancer cells [30]. Cleavage of α6 integrin now also appears to have a role in post-gastrulation development of Xenopus laevis. The α6p levels increased along with intact α6 after stage 17. Surprisingly, by the tadpole stage, it was the predominant band in western blots [31]. Although the protease responsible for cleavage was not identified and α6p activity was not measured, these suggestive data raise the possibility that integrin cleavage mechanisms may contribute to developmental processes. Moreover, they indicate that RT-PCR and antibody staining may not always provide a full picture of integrins in cells or tissues. Mechanistically, sustained presence of cleaved integrin heterodimers on the cell surface may disconnect extracellular attachment from cytoskeletal organization and cell architecture and allow cells to remain functional while detached [32].
Conclusions
Modulation of integrin expression patterns is a critical aspect of organogenesis. Recent studies highlighted here identify diverse mechanisms of integrin regulation: integrin switching in gonadogenesis and adipogenesis, localized expression in the stem cell niche and somites, and the potential for post-translational processing. These examples document critical developmental roles for integrins in cell motility, cell differentiation, and tissue architecture. As the extracellular environment undergoes many changes during development, modulation of integrin levels on the cell surface provides a direct way to regulate cell reactions to these changes. Defining the pathways that impact transcriptional regulation of integrins could provide a means to alter cell reactions to the microenvironment. This information would not only provide insights into morphogenesis and remodeling but would also provide targets for use in tissue engineering, regenerative medicine, and therapeutics.
Acknowledgments
The authors acknowledge support from the National Institutes of Health (R01 GM059383 and NIGMS Cell Migration Consortium U54 GM064346). We also thank Drs. Christopher Wylie, Guy Tanentzapf, and Katherine Kragtorp for generously providing their images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tarone G, Hirsch E, Brancaccio M, De Acetis M, Barberis L, Balzac F, Retta SF, Botta C, Altruda F, Silengo L. Integrin function and regulation in development. Int J Dev Biol. 2000;44:725–731. [PubMed] [Google Scholar]
- 2.De Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16:389–395. doi: 10.1016/s0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- 3.Bokel C, Brown NH. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev. Cell. 2002;3:311–321. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- 4.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 5.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 6.Sastry SK, Lakonishok M, Thomas DA, Muschler J, Horwitz AF. Integrin alpha subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J. Cell Biol. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, DeYoung SM, Zhang M, Cheng A, Saltiel AR. Changes in integrin expression during adipocyte differentiation. Cell Metab. 2005;2:165–177. doi: 10.1016/j.cmet.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- 9.Baum PD, Garriga G. Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron. 1997;19:51–62. doi: 10.1016/s0896-6273(00)80347-5. [DOI] [PubMed] [Google Scholar]
- 10.Meighan CM, Schwarzbauer JE. Control of C. elegans hermaphrodite gonad size and shape by vab-3/Pax6-mediated regulation of integrin receptors. Genes Dev. 2007;21:1615–1620. doi: 10.1101/gad.1534807.**The complementary functions of two integrin heterodimers show that major developmental processes can be coordinated simply by modifying the adhesion receptor repertoire of the cell.
- 11.Molyneaux K, Wylie C. Primordial germ cell migration. Int. J. Dev. Biol. 2004;48:537–544. doi: 10.1387/ijdb.041833km. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Castro MI, Anderson R, Heasman J, Wylie C. Interactions between germ cells and extracellular matrix glycoproteins during migration and gonad assembly in the mouse embryo. J. Cell Biol. 1997;138:471–480. doi: 10.1083/jcb.138.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson R, Fassler R, Georges-Labouesse E, Hynes RO, Bader BL, Kreidberg JA, Schaible K, Heasman J, Wylie C. Mouse primordial germ cells lacking beta1 integrins enter the germline but fail to migrate normally to the gonads. Development. 1999;126:1655–1664. doi: 10.1242/dev.126.8.1655. [DOI] [PubMed] [Google Scholar]
- 14.Francis RJB, Lo CW. Primordial germ cell deficiency in the connexin 43 knockout mouse arises from apoptosis associated with abnormal p53 activation. Development. 2006;133:3451–3460. doi: 10.1242/dev.02506.*Connexin 43 targeting highlights a role for gap junction communication in regulating integrin-mediated cell migration of primordial germ cells.
- 15.Gingras ME, Larouche K, Larouche N, Leclerc S, Salesse C, Guerin SL. Regulation of the integrin subunit alpha5 gene promoter by the transcription factors Sp1/Sp3 is influenced by the cell density in rabbit corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2003;44:3742–3755. doi: 10.1167/iovs.03-0191. [DOI] [PubMed] [Google Scholar]
- 16.Gaudreault M, Vigneault F, Leclerc S, Guerin SL. Laminin reduces expression of the human alpha6 integrin subunit gene by altering the level of the transcription factors Sp1 and Sp3. Invest. Ophthalmol. Vis. Sci. 2007;48:3490–3505. doi: 10.1167/iovs.07-0016.*Transcription factor cleavage to regulate integrin expression could allow concerted control of other genes required in a common developmental pathway.
- 17.Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat. Cell Biol. 2007;9:1413–1418. doi: 10.1038/ncb1660.**Microscopic analysis of mutant Drosophila embryos shows that integrins are required in the Drosophila gonad for proper positioning of the hub cells and maintenance of the stem cell niche.
- 18.Joos TO, Whittaker CA, Meng F, DeSimone DW, Gnau V, Hausen P. Integrin alpha 5 during early development of Xenopus laevis. Mech. Dev. 1995;50:187–199. doi: 10.1016/0925-4773(94)00335-k. [DOI] [PubMed] [Google Scholar]
- 19.Kragtorp KA, Miller JR. Integrin alpha5 is required for somite rotation and boundary formation in Xenopus. Dev. Dyn. 2007;236:2713–2720. doi: 10.1002/dvdy.21280.*Localized knockdown of α5 integrin identifies a critical role for integrin-fibronectin interactions during formation of somite boundaries.
- 20.Schmid RS, Anton ES. Role of integrins in the development of the cerebral cortex. Cereb. Cortex. 2003;13:219–224. doi: 10.1093/cercor/13.3.219. [DOI] [PubMed] [Google Scholar]
- 21.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 22.Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- 23.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 24.Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr. Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 25.Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Muller U. Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J. Neurosci. 2007;27:13854–13865. doi: 10.1523/JNEUROSCI.4494-07.2007.*Cell-specific integrin knockouts in the developing cerebral cortex are a promising step in elucidating specific integrin functions during corticogenesis.
- 26.Williams DA, Rios M, Stephens C, Patel VP. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature. 1991;352:438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- 27.Zhu AJ, Haase I, Watt FM. Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc. Natl. Acad. Sci. U S A. 1999;96:6728–6733. doi: 10.1073/pnas.96.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–3444. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi Y, Furue MK, Okamoto T, Ohnuma K, Myoishi Y, Fukuhara Y, Abe T, Sato JD, Hata R, Asashima M. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells. 2007;25:3005–3015. doi: 10.1634/stemcells.2007-0103. [DOI] [PubMed] [Google Scholar]
- 30.Davis TL, Rabinovitz I, Futscher BW, Schnolzer M, Burger F, Liu Y, Kulesz-Martin M, Cress AE. Identification of a novel structural variant of the alpha 6 integrin. J. Biol. Chem. 2001;276:26099–26106. doi: 10.1074/jbc.M102811200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demetriou MC, Stylianou P, Andreou M, Yiannikouri O, Tsaprailis G, Cress AE, Skourides P. Spatially and temporally regulated alpha6 integrin cleavage during Xenopus laevis development. Biochem. Biophys. Res. Commun. 2008;366:779–785. doi: 10.1016/j.bbrc.2007.12.040.**Provides evidence that a cleaved integrin may have a role during vertebrate development and morphogenesis.
- 32.Demetriou MC, Cress AE. Integrin clipping: a novel adhesion switch? J. Cell. Biochem. 2004;91:26–35. doi: 10.1002/jcb.10675. [DOI] [PMC free article] [PubMed] [Google Scholar]