Abstract
Myocilin is a 55–57 kDa secreted glycoprotein and member of the olfactomedin family, which is mutated in some forms of primary open-angle glaucoma. To assess the effects of elevated amounts of myocilin on aqueous humor outflow dynamics in an in vivo system, transgenic βB1-crystallin-MYOC mice have been developed that strongly overexpress myocilin in their eyes. The transgenic overexpression of myocilin results in an almost five-fold increase of secreted normal myocilin in the aqueous humor of βB1-crystallin-MYOC mice. In the present study, we wanted to use βB1-crystallin-MYOC as a tool to identify the response of ocular tissues to the presence of higher than normal amounts of myocilin, and to identify changes in gene expression that could help to shed light on the functional in vivo properties of myocilin. RNA was isolated from ocular tissues of βB1-crystallin-MYOC mice and wild-type littermates. Changes in gene expression were determined by hybridization of gene microarrays and confirmed by real-time RT-PCR and western blotting. The expression of genes that had been found to be differentially regulated in βB1-crystallin-MYOC mice was further analyzed in cultured human trabecular meshwork (HTM) cells treated with recombinant myocilin. Although βB1-crystallin-MYOC mice do not have an obvious phenotype, a statistically significant up- and downregulation of several distinct genes was found when compared to gene expression in wild-type littermates. Among the genes that were found to be differentially regulated were Wasl, Ceacam1, and Spon2, which are involved in cell adhesion and cell-matrix interactions. Differences in expression were also found for Six1 which encodes for a transcription factor, and for Pftk1 whose gene product is a cdc2-related protein kinase. The expression of these genes was also found to be regulated in vitro in HTM cells treated with recombinant myocilin. Substantially higher amounts in ocular tissues of βB1-crystallin-MYOC mice were found for connexin 46 and αB-crystallin. In addition, several genes that encode for olfactomedin proteins showed distinct changes in expression. Olfml3 was significantly downregulated, while Lphn1, Lphn2, and Lphn3 were significantly upregulated. Our findings support a role for myocilin in modulating cellular adhesion, and suggest functional processes that involve other proteins of the olfactomedin family.
Keywords: N-WASP, CEACAM1, mindin, αB-crystallin, trabecular meshwork
1. Introduction
Myocilin is a 55–57 kDa secreted glycoprotein and member of the olfactomedin family, which is characterized by a coiled-coil domain near the amino terminus and an olfactomedin domain near the carboxyl terminus (Tamm, 2002). Mutations in the MYOC gene that encodes for myocilin are causative for some forms of juvenile and adult-onset primary open-angle glaucoma (POAG) (Stone et al., 1997). The mechanisms that cause POAG in affected patients have not been finally clarified, but appear to involve dominant-negative effects that are associated with non-secretion of mutated myocilin (Jacobson et al., 2001; Liu and Vollrath, 2004; Shepard et al., 2007). Myocilin is highly expressed in several tissues of the anterior eye such as iris, ciliary body, cornea and sclera (Adam et al., 1997; Karali et al., 2000; Swiderski et al., 2000). An extremely high expression has been observed in the trabecular meshwork (Adam et al., 1997; Swiderski et al., 2000; Tamm et al., 1999; Tomarev et al., 2003). The available data on the functional properties of myocilin have been derived exclusively from in vitro studies and indicate a matricellular role of myocilin that modulates cellular adhesion (Peters et al., 2005; Shen et al., 2008; Wentz-Hunter et al., 2004). As mutant Myoc-deficient mice to not express an obvious phenotype (Kim et al., 2001), the relevance of a matricellular role for myocilin in vivo is unclear. Some experimental evidence supports the hypothesis that secreted myocilin plays a role in vivo in modulating the hydrodynamic outflow resistance in the trabecular meshwork, and that elevated amounts of myocilin could obstruct the outflow system. The expression of myocilin is induced by treatment with dexamethasone in cultured trabecular meshwork cells and in perfused anterior segment organ cultures in a time-dependant manner and comparable to the time course that is observed during the development of steroid-induced ocular hypertension and glaucoma (Nguyen et al., 1998). Furthermore, recombinant myocilin is very effective at blocking polycarbonate filters with a pore size similar to that of the trabecular meshwork (Goldwich et al., 2003), and myocilin in the aqueous humor (AH) is tightly bound to polycarbonate filters that become obstructed after perfusion with AH (Russell et al., 2001). Finally, the strongest support for an obstructive role of myocilin has been contributed by Fautsch and collegues who could show that perfusion of anterior segment organ cultures with human recombinant myocilin from an eukaryotic expression system causes a significant reduction in outflow facility (Fautsch et al., 2006). The effect required preincubation of myocilin with porcine aqueous humor indicating that recombinant myocilin appears to form a complex with other proteins in porcine aqueous humor that enables it to bind specifically within the trabecular meshwork. In contrast, a reduced secretion of myocilin in the trabecular meshwork correlates with an increase in outflow facility (Caballero et al., 2000), while perfusion with a C-terminal fragment of myocilin containing the entire olfactomedin domain has no effects on facility (Goldwich et al., 2003). To assess the effects of elevated amounts of myocilin on aqueous humor outflow dynamics in an in vivo system, we developed transgenic mice that strongly express myocilin in their lenses under control of the βB1-crystallin promoter (Zillig et al., 2005). The transgenic overexpression of myocilin from the lens results in an almost five-fold increase of secreted normal myocilin in the aqueous humor of βB1-crystallin-MYOC mice. Despite the high amounts of myocilin in their AH, intraocular pressure of βB1-crystallin-MYOC mice does not differ from that of control mice, a finding that argues against an obstructive role of myocilin for the mouse trabecular outflow system in vivo. Comparable data have been reported with another set of transgenic mice that have been genetically modified to overexpress myocilin (Gould et al., 2004). In the present study, we wanted to use βB1-crystallin-MYOC as a tool to identify the response of ocular tissues to the presence of higher than normal amounts of myocilin. The purpose of this study was to identify changes in gene expression that could help to shed light on the functional in vivo properties of myocilin. Our findings support a role for myocilin in modulating cellular adhesion, and suggest functional processes that involve other proteins of the olfactomedin family.
2. Material and methods
Transgenic mice and cell cultures
βB1-crystallin-MYOC mice were obtained and kept as described previously (Zillig et al., 2005). This mouse strain was generated in a FVB/N background with hereditary retinal degeneration (Gimenez and Montoliu, 2001). Since the purpose of this study was to analyze gene expression in βB1-crystallin-MYOC animals with a phenotypically normal retina, animals were bred in a mixed FVB/N × CD1 background. Cell cultures of human trabecular meshwork cells and optic nerve astrocytes were established and grown as described previously (Fuchshofer et al., 2005; Fuchshofer et al., 2007).
cDNA Microarray Analysis
Total RNA was isolated from ocular tissues (whole eyes without lens) of three week old βB1-crystallin-MYOC mice and wild-type littermates by using TRIzol (Invitrogen, Karlsruhe, Germany), according to the manufacturer’s instructions. The RNA concentration was determined by absorbance at 260 nm (Eppendorf BioPhotometer; Eppendorf, Hamburg, Germany). Doubled-stranded cDNA was synthesized from 5 μg purified total RNA with a kit (Superscript Double-Stranded cDNA Synthesis Kit, Invitrogen) and a T7-(dT)24 primer (Affymetrix, Santa Clara, CA). After the double-stranded cDNA was purified by phenol-chloroform extraction, in vitro transcription reactions were performed (Bioassay High Yield RNA Transcript Labeling Kit; Enzo Diagnostics, Farmingdale, NY), according to the manufacturer’s protocol. Biotin-labeled cRNA was purified (Qiagen, Valencia, CA) and quantified using a ND-1000 Nano-drop spectrophotometer (Nano-Drop Technologies, Wilmington, DE), before being fragmented to 35 to 200 base fragments in an alkaline buffer. Five Affymetrix Mouse 430 2.0 Arrays (Affymetrix) containing 34,000 well-characterized mouse genes were used. Washing, staining, and scanning were performed by using the Genechip Instrument System (Affymetrix) as recommended in the manufacturer’s technical manual. The arrays were scanned and data were analyzed on computer (Microarray Suite algorithm, ver. 5; Affymetrix). The absolute analysis results of each chip were scaled to the same target intensity value of 150 and could then be directly compared to one another. The absolute analysis calculates a variety of metrics using the probe array’s hybridization intensities measured by the scanner. The comparison analysis performs additional calculations on data from two separate probe array experiments to compare gene expression levels between two samples. The comparison analysis begins with the absolute analysis of one probe array experiment as the source of baseline data and a second probe array of the experimental condition as the source of data to be compared to the baseline.
Real time RT-PCR
Total RNA was isolated from ocular tissues (without lens) of βB1-crystallin-MYOC mice and wild-type littermates as described above. First strand cDNA synthesis was performed by using 1.0 μg of total RNA and the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Munich, Germany), as described by the manufacturer. Quantification by real time PCR was performed on a Rotor-Gene 3000 real time thermal analyzer (Corbett Life Science, Mortlake, Australia). The cDNA of Lamin A served as an endogenous control to normalize the differences in the amount of cDNA in each sample. PCR reaction was performed in a volume of 25 μl, consisting of 2.5 μl of 10× PCR buffer, 2.0 to 2.5 μl of MgCl2 (25 mM), 0.5 μl of dNTPs (10 mM each; Promega, Madison, WI), 0.5 μl of Hot Star Taq (5 U/μl, Qiagen), 0.5 μl of primer mix (20 μM each) and 2.5 μl of 1× SYBR Green I solution (Sigma Aldrich, Seelze, Germany). For Cryab and Gja3, the real time RT-PCR was done on a Lightcycler real time analyzer (Roche, Basel, Switzerland) using TaqMan reagents and the One-Step RT-PCR kit according to the manufacturer’s instructions (Applied BioSystems, Foster City, CA, USA). All samples that had to be compared for expression differences were run in the same assay as duplicate. Data were initially expressed as a threshold cycle and are expressed as fold increases in gene expression in βB1-crystallin-MYOC mice compared with the expression in wild-type littermates. For each experiment, the mean value in wild-type mice was set at 1. In experiments comparing gene expression between different ocular tissues, the mean value in the tissue with lowest expression was set at 1. After completion of PCR amplification, data were analyzed with Rotor-Gene Software version 6.0. For analyzing differentially expressed genes in individual tissues, the RNA from three week old transgenic (n = 3) and wild-type littermates (n = 4) was pooled and analyzed as duplicate. All other experiments were repeated at least four times and mean values and standard deviations were calculated. After amplification was complete, the PCR products were analyzed by agarose gel electrophoresis. PCR product sizes and sequences of primer pairs are listed in Table 1. All primer pairs crossed exon-intron boundaries. For each pair of primers, melting curves were ran to confirm that none of the primer pairs amplified additional bands or formed primer-dimers.
Table 1.
Primers used for PCR amplification in the present study.
| Target | Sequence | Position | Product Size |
|---|---|---|---|
| Ceacam1 | 5′-cattgctggcatcgtgatt-3′
5′-agatctcgctggtcacttcc-3′ |
755–862 | 108 |
| Six1 | 5′-accggaggcaaagagacc-3′
5′-ggagagagttgattctgcttgtt-3′ |
562–655 | 94 |
| Wasl | 5′-cgaactccgaagacaagca–3′
5′-cgaactccgaagacaagca-3 |
1129–1193 | 65 |
| Pftk1 | 5′-tcgaacagcaaacactgaca–3′
5′-ttcttcccagaatgatgacaac-3 |
1478–1551 | 74 |
| Spon2 | 5′-gcaactatcccacaagacacag–3′
5′-tgaggcgtgggtagtagaatg-3 |
843–927 | 85 |
| Gldn | 5′-aaggagcaattggcaagaga-3′
5′-ctcccttttcccctggatt-3′ |
551–619 | 69 |
| Olfml2A | 5′-ggcctcccagatgaatacg-3′
5′-ttgttctccaggctcaggtt-3′ |
548–607 | 60 |
| Olfml2B | 5′-gtggggcgagtggataaac-3′
5′-gctcattctccttggtgagg-3′ |
620–680 | 61 |
| Olfml3 | 5′-ctgctgctcctcttctttttg-3′
5′-ctactctgatcctggcattgg-3′ |
32–159 | 128 |
| Pancortin-1 | 5′-aataccacccggctttcg–3′
5′-tgtcctgggcagagctgta-3 |
371–479 | 109 |
| Pancortin-2 | 5′-gcctggagaccaagttcaag-3′
5′-agtcactggcgcattgaaa-3′ |
666–767 | 102 |
| Pancortin-3 | 5′-gcaccgaactcacccaag-3′
5′-tgtcctgggcagagctgta-3′ |
455–526 | 72 |
| Pancortin-4 | 5′-gcctggagaccaagttcaag-3′
5′-agtcactggcgcattgaaa-3′ |
713–814 | 102 |
| Olfm2 | 5′-aacaactattgctggaggtcaga-3′
5′-acgcacttgccatcaggt-3′ |
96–188 | 93 |
| Olfm3 | 5′-tgtgttccagagatgccaaa-3′
5′-tgaaaatctctctgagttctcaagtt-3′ |
319–427 | 109 |
| Olfm4 | 5′-gtgaggcctccaaaagtgac-3′
5′-aggatacttccagctgctcct-3′ |
676–760 | 85 |
| Lphn1 | 5′-cgccttcaaaatcatgtcac-3′
5′-caggaaaggcgtcagagc-3′ |
81–157 | 77 |
| Lphn2 | 5′-ccagggactcttcattttcatc-3′
5′-gcacttgccatactcttttcg-3′ |
1650–1719 | 70 |
| Lphn3 | 5′-tgaggataacagacccttcatca-3′
5′-aaggcccaggtcaatccta-3′ |
2355–2435 | 81 |
| Lamin A | 5′-agcaaagtgcgtgaggagtt-3′
5′-acaagtccccctccttcttg-3′ |
527–590 | 65 |
| LAMIN A | 5′-gacgaggatgaggatggaga-3′
5′-agcgcaggttgtactcagc-3′ |
1889–1959 | 71 |
| GAPDH | 5′-agccacatcgctcagaca-3′
5′-gcccaatacgaccaaatcc-3′ |
83–148 | 66 |
| GNB2L | 5′-gctactaccccgcagttcc-3′
5′-cagtttccacatgatgatggtc-3′ |
170–241 | 72 |
| CEACAM1 | 5′-cccatcatgctgaacgtaaa-3′
5′-gggccactactccaatcaca-3′ |
1338–1431 | 94 |
| SIX1 | 5′-gaaccggaggcaaagagac-3′
5′-ggagagagttggttctgcttg-3′ |
794–889 | 96 |
| WASL | 5′-ccagatacgacagggtatcca–3′
5′-gcaggtgttggtggtgtaga-3 |
1645–1713 | 69 |
| PFTK1 | 5′-acggttactttggctgcaat
5′-gttccgtgtagacatctttgtga-3 |
149–243 | 95 |
| SPON2 | 5′-ccttctcctcccccaactt–3′
5′-ttcagccgcgggtagtag-3 |
862–967 | 105 |
Sequences of primer pairs crossed exon-intron boundaries. Symbols of mouse genes are shown with first letter in capitals, symbols of human genes with all letters in capitals.
Western Blot Analysis
Ocular tissues (whole eyes without lens) were homogenized in RIPA (RadioImmuno Precipitation Assay) buffer (50mM Tris HCl pH 8, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) by a Power Gen 125 Homogenizer (Fisher Scientific, Schwerte, Germany). The protein concentration was quantified using the bicinchoninic acid (BCA) method, according to the manufacturer’s instructions (Pierce, Rockford, IL, USA). Proteins were separated by polyacrylamide gel electrophoresis using a 5% sodium dodecyl sulfate (SDS)-polyacrylamide stacking gel, and 10% or 12% SDS-polyacrylamide separating gels. All gels were run under reducing conditions. After electrophoresis, the proteins were transferred with semidry blotting (Peqlab Biotechnologie GMBH, Erlangen, Germany) on a polyvinylidene difluoride membrane (Roche, Mannheim, Germany). The membrane was blocked with Tris-buffered saline (TBS) containing 0.05% Tween 20 (TBST) and 0.5% bovine serum albumin (BSA) or 0.2% casein for 1h. Rabbit anti-mouse antibodies against Connexin 46 (diluted 1:100, Biomol GmbH, Hamburg, Germany) and αB-crystallin (diluted 1:4000 in 0.25% BSA or 0.1% Casein, Assay Designs, Ann Arbor, MI, USA) were then added over night at 4°C. After washing with TBST, horseradish peroxidase-conjugated chicken anti-rabbit antibodies or alkaline phosphatase-conjugated chicken anti-rabbit antibodies (diluted 1:2000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were added for 1h, respectively. Horseradish peroxidase-conjugated GAPDH-antibody (diluted 1:10000, Rockland, Gilbertsville, PA, USA) was used as loading control. Horseradish peroxidase and alkaline phosphatase were visualized by using chemiluminescence (Immobilon Western Chemiluminescent HRP Substrate, Millipore, Billerica, MA, USA; CDP-Star, Roche) and the LAS-3000 imaging system (Fujifilm). Exposure times ranged from 1 to 5 minutes.
Recombinant myocilin
The cDNA of human myocilin without sequences for the signal peptide (SP) was ligated into the expression plasmid pSecTag2/Hygro A (Invitrogen) using SfiI and ApaI resctriction sites that had been added to the 5′ and 3′ ends, respectively. After ligation, the endogenous SP had been replaced by the SP of the murine Igκ-chain and at the 3′ end, sequences of c-myc and 6×His epitopes had been added before the stop codon. The sequence of the resultant plasmid was verified by automated sequencing in both directions. HEK 293T cells were transfected with lipofectamin (Invitrogen) and grown for 2 days in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) plus 10 % (v/v) fetal bovine serum (FBS) at 37°C. Transfected cells were selected by adding hygromycin to a final concentration of 300 μg/ml. To obtain recombinant protein, the cells were kept in DMEM without FBS for 24 hours. The collected cell culture supernatant was then centrifuged at 15,000 g for 15 minutes and prefiltered through a 0.2 μm polyvinylidene difluoride (PVDF) filter (Millipore, Bedford, MA). The filtrate was diluted 1:1 (v/v) with binding buffer (20 mM Tris/HCl, 500 mM NaCl, 10 mM imidazole, 5 mM CaCl2, 0.5% Tween 20, pH 7.4) and passed through a 5 ml Ni-chelate column (Amersham Pharmacia Biotech, Freiburg, Germany) using a fast performance liquid chromatography (FPLC) system (Äktaprime, Amersham Pharmacia Biotech). After the binding step, the column was washed with 10 column volumes of washing buffer (20 mM Tris/HCl, 500 mM NaCl, 20 mM imidazole, 5 mM CaCl2, 0.5% Tween 20, pH 7.4). The bound proteins were then eluted with two column volumes of each elution buffer (20 mM Tris/HCl, 500 mM NaCl, 5 mM CaCl2, 0.5% Tween 20, pH 7.4) containing 100 mM imidazole, 150 mM imidazole or 200 mM imidazole, respectively. The collected fractions were tested by western Blot analysis using an antibody against myocilin (goat anti-myocilin N15, Santa Cruz, CA), and by silver staining (Heukeshoven and Dernick, 1985). Myocilin-containing fractions were pooled and dialyzed over night against 20 mM Tris/HCl, 100 mM NaCl, 5 mM CaCl2, 0.01% Tween 20, pH 7.4) using a dialysis membrane with a 12–14 kDa cut-off (Spectra/Por; Spectrum Laboratories, Rancho Dominguez, CA).
Cell Treatment
Human trabecular meshwork (HTM) cells were grown to confluence in a 6 well culture dish (Nunc, Roskilde, Danmark) using F10-HAM medium (Invitrogen) plus 10% (v/v) FBS without antibiotics at 37°C. The day before treatment, the cells were washed twice with F10-HAM medium without FBS and then kept in this medium. At the day of treatment, the medium was changed again and the cells were treated with 2.5 ng/ml, 5 ng/ml, 25 ng/ml, 50 ng/ml and 100 ng/ml of recombinant myocilin, respectively. After 24 hours, the RNA of the cells was isolated (TRIzol® Reagent; Invitrogen, Karlsruhe, Germany), according to the manufacturer’s instructions. First strand cDNA synthesis was performed by using 1.0 μg of total RNA and the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Munich, Germany), as described by the manufacturer. Quantification by real time RT-PCR was performed as described above. The cDNA of the LAMIN A, GAPDH, and GNB2L genes served as endogenous controls to normalize the differences in the amount of cDNA in each sample.
3. Results
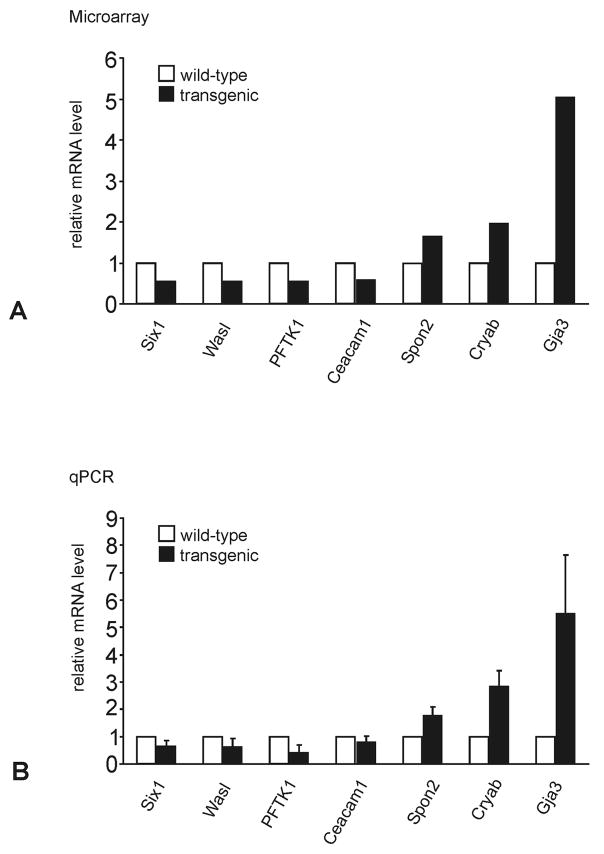
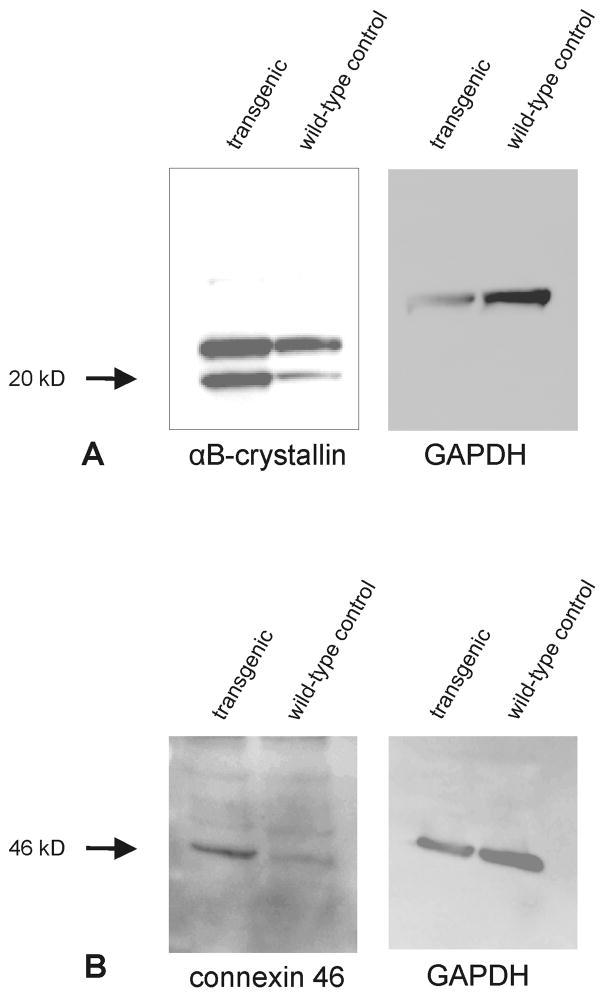
To identify genes that are modified in their expression pattern by the presence of higher than normal amounts of myocilin in the AH in vivo, hybridization of two DNA microarrays was performed with RNA from the eyes (whole eyes without lens) of two βB1-crystallin-MYOC transgenic animals. βB1-crystallin-MYOC animals have been genetically modified to ectopically express myocilin in their lenses (Zillig et al., 2005), and show an almost five-fold increase in the amounts of secreted myocilin in their AH. The lens was removed from the eye prior to RNA isolation in order to avoid a contribution of RNA from the site of ectopic expression. For controls, three microarrays were hybridized with whole eye RNA (without lens) from three wild-type littermates. Anticipating a considerable variability in gene expression between individual animals in a mixed FVB/N × CD1 background (CD1 is an outbred strain with a high degree of genetic variation), we sought to select stringent criteria to facilitate detection of changes in gene expression that had been selectively caused by higher than normal amounts of myocilin in the AH. Accordingly, only genes that fulfilled the following criteria were considered for subsequent confirmation by real time RT-PCR: An intensity level and detection call that qualified “presence” in all arrays, a p-value less than 0.05 using the Welch one-way Anova t-test, and a fold-change of at least 1.5 (wild-type relative to transgenic animals, or transgenic relative to wild-type animals). In addition, we required that the change in gene expression detected by microarray analysis could be confirmed either by real time RT-PCR or by western blot analysis in additional RNA or protein samples from at least two transgenic and two wild-type animals that had not been part of the microarray analysis. Following these criteria, four genes (Cryab, Spon2, Ceacam1, and Pftk1) were identified that were differentially expressed between βB1-crystallin-MYOC transgenic mice and wild-type littermates (Table 2). In a second candidate approach, we selected genes with p-value less than 0.05, and a fold-change of at least 1.5 that had not fulfilled the criterion of “presence” in all arrays. With this approach, three additional genes (Gja3, Wasl, Six1) were identified that could also be confirmed as differentially expressed between transgenic and wild-type animals by real time RT-PCR (Table 1). While the expression of Ceacam1, Pftk1, Wasl, and Six1 was found to be downregulated in the eyes of βB1-crystallin-MYOC mice, Cryab, Spon2 and Gja3 were upregulated (Table 2). The respective amounts of change in expression were remarkably similar when the data of the microarray analysis and the real time RT-PCR experiments were compared with each other (Fig. 1). 14 additional genes, for which microarray data had indicated a significant change in gene expression, could not be confirmed by subsequent real time RT-PCR and were identified as false positive (not shown). Cryab and Gja3 encode for αB-crystallin and connexin 46, respectively, two proteins that have been widely studied in the eye (Table 2). As both proteins are primarily expressed in the lens, we wanted to rule out the possibility that the observed changes in RNA expression were due to a contamination of our samples with lens RNA. To this end, we performed an additional western blot analysis with specific antibodies against αB-crystallin and connexin 46, and proteins from the eyes (without lens) of βB1-crystallin-MYOC animals and wild-type littermates. The results clearly indicate the presence of higher amounts of αB-crystallin and connexin 46 in the eyes of βB1-crystallin-MYOC animals (Fig. 2), and support the data obtained by RNA analysis.
Table 2.
Genes that are differentially expressed in βB1-crystallin-MYOC mice
| Accession Number | p-Value | x-fold Change (Control/Trans-genic | Gene Symbol | Description/Function | Reference |
|---|---|---|---|---|---|
| NM_009189 | 0.0434 | 0.55 | Six1 | Transcription factor required for myogenesis and development of kidney, thymus, inner ear and craniofacial skeleton | (Laclef et al., 2003a; Laclef et al., 2003b; Xu et al., 2003) |
| NM_028459 | 0.0479 | 0.56 | Wasl | Wasl (N-WASP) regulates actin polymerization and promotes formation of actin-rich structures such as filopodia which play a role in cell adhesion and motility | (Kowalski et al., 2005; Martinez-Quiles et al., 2004; Martinez-Quiles et al., 2001; Misra et al., 2007) |
| NM_011074 | 0.046 | 0.56 | Pftk1 | Pftk1 (Pftaire1) is a cdc2-related protein kinase involved in cell cycle progression, proliferation, migration and motility | (Pang et al., 2007; Shu et al., 2007b) |
| NM_011926 | 0.0137 | 0.58 | Ceacam 1 | The carcinoembryonic-antigen-related cell-adhesion molecule (CEACAM) is involved in various intercellular-adhesion and intracellular signalling-mediated effects | (Gray-Owen and Blumberg, 2006; Kuespert et al., 2006) |
| NM_133903 | 0.0242 | 1.65 | Spon2 | Mindin (spondin2) is a member of the mindin-F-spondin family of secreted extracellular matrix proteins. It interacts with integrins and regulates Rho GTPase expression | (He et al., 2004; Jia et al., 2005; Li et al., 2006a) |
| NM_009964 | 0.0459 | 1.97 | Cryab | αB-crystallin is a molecular chaperone that interacts with the cytoskeleton and protects against apoptosis induced by oxidative stress | (Andley, 2007) |
| NM_016975 | 0.0332 | 5.04 | Gja3 | Gja3 encodes for the gap junction protein connexin 46 (Cx46; Cnx46; Gja-3), which is primarily expressed in the lens | (Gong et al., 1997; White, 2002) |
Fig. 1.
Differentially expressed genes in βB1-crystallin-MYOC mice as identified by microarray analysis (A) or quantitative real time RT-PCR (qPCR, B). B. For real time RT-PCR analysis, means ± standard deviation of four independent experiments run in duplicate are shown. The mean value obtained with RNA from wild-type animals was set at 1.
Fig. 2.
Western blot analysis for αB-crystallin (A) and connexin 46 (B) in ocular proteins (without lens) from βB1-crystallin-MYOC mice and wild-type littermates.
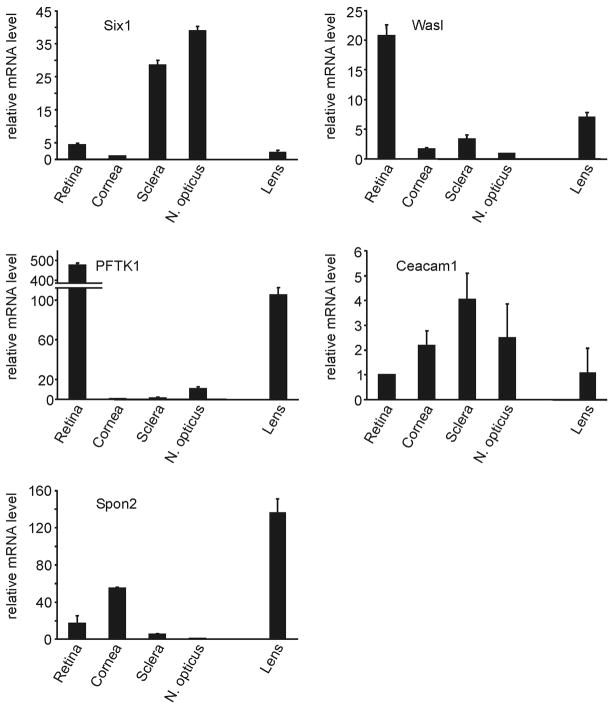
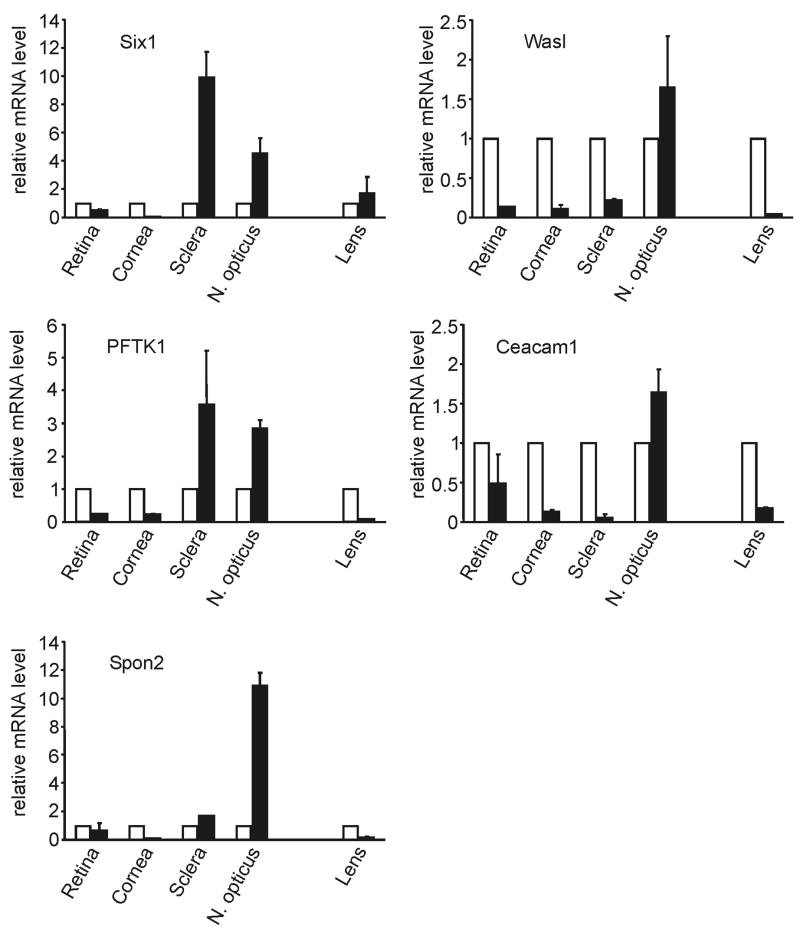
As no data are available on the localization and distribution of Ceacam1, Pftk1, WasI, Six1, and Spon2 in the adult mouse eye, we wanted to obtain information on their relative expression in different ocular tissues. RNA was isolated from retina, cornea, sclera, lens and optic nerve of wild-type mice, and examined by real time RT-PCR. RNA from lens and optic nerve had not contributed to the initial microarray experiments and their confirmation by real time RT-PCR. Six1 was found to be preferentially expressed in sclera and optic nerve, while the highest expression for Wasl and Pftk1 was seen in RNA from the retina (Fig. 3). In contrast, the expression of Ceacam1 was more evenly distributed (Fig. 3). The expression of Spon2 was considerably higher in RNA from lens and cornea, than in that from retina, sclera, and optic nerve (Fig. 3). The comparison of the relative amounts of Ceacam1, Pftk1, WasI, Six1, and Spon2 mRNA in different tissues of βB1-crystallin-MYOC mice with that of wild-type mice revealed substantial tissue-related differences. While the expression of Ceacam1, Pftk1, WasI, and Six1 was downregulated in most RNA samples taken from βB1-crystallin-MYOC mice, all four genes were upregulated in RNA from the optic nerve (Fig. 4). In addition, the expression of Pftk1 and Six1 was upregulated in RNA from the sclera of βB1-crystallin-MYOC (Fig. 4). The expression of Spon2 was downregulated in RNA from cornea and lenses of βB1-crystallin-MYOC mice, but substantially upregulated in RNA from the sclera and the optic nerve (Fig. 4). To obtain information, if the overexpression of myocilin in βB1-crystallin-MYOC mice did also lead to higher amounts of myocilin in the retina of the transgenic animals, we performed western blot analysis in proteins from transgenic and wild-type retinas. Applying the same antibodies that had been used to detect transgenic myocilin in the aqueous humor of βB1-crystallin-MYOC (Zillig et al., 2005), myocilin could not be detected, neither in the retina of transgenic nor in that of wild-type animals (not shown).
Fig. 3.
Real time RT-PCR analysis for the relative expression of Six1, Wasl, Pftk1, Caecam1, and Spon2 mRNA in different tissues of the mouse eye. For real time RT-PCR analysis, means ± standard deviation of four independent experiments run in duplicate are shown. The mean value obtained with RNA from the tissue with lowest expression was set at 1.
Fig. 4.
Real time RT-PCR analysis for the differential expression of Six1, Wasl, Pftk1, Caecam1, and Spon2 mRNA in different tissues of βB1-crystallin-MYOC mice and wild-type littermates. The RNA from three week old transgenic (n = 3) and wild-type littermates (n = 4) was pooled and analyzed as duplicate. The mean value obtained with RNA from wild-type animals (open bar) was set at 1.
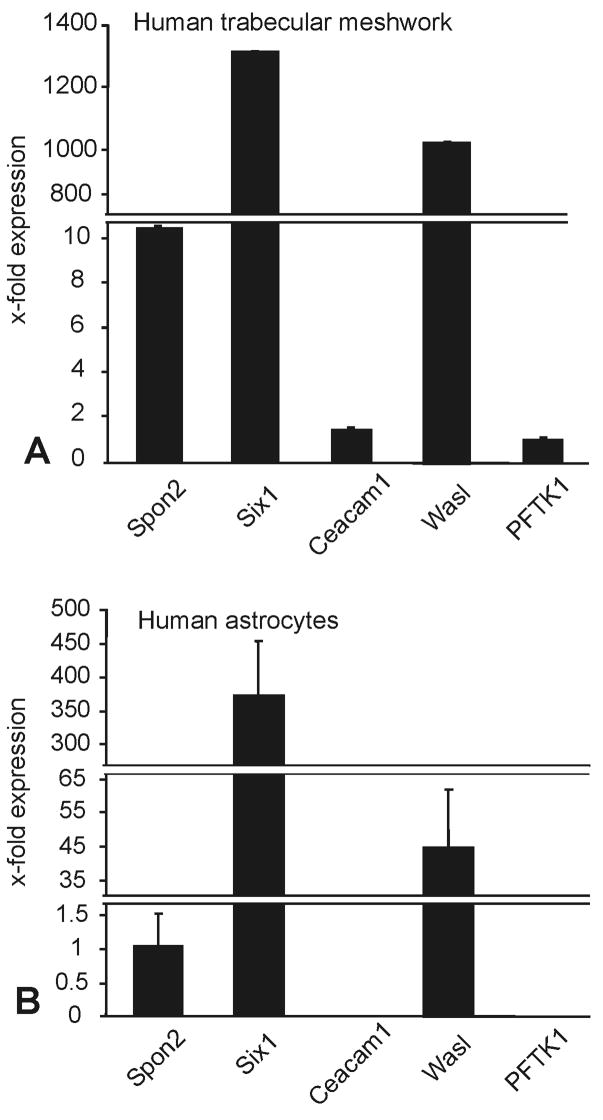
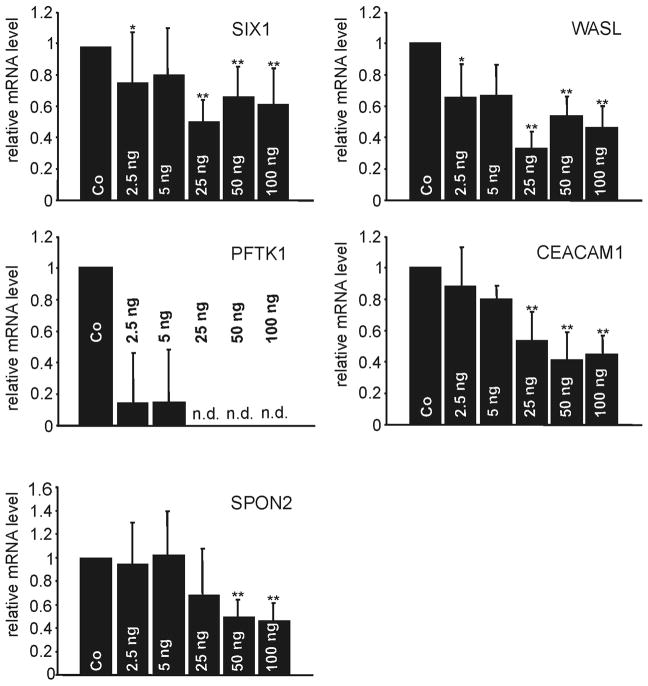
As myocilin accumulates in the chamber angle of βB1-crystallin-MYOC mice (Zillig et al., 2005), we were interested, if the genes that we had identified as differentially regulated in RNA from whole eyes are also expressed in trabecular meshwork cells. Because of its small size, mouse trabecular meshwork is difficult to isolate and to cleanly separate from adjacent tissues. We therefore isolated RNA from cultured human trabecular meshwork (HTM) cells and compared the expression of CEACAM1, PFTK1, WASL, SIX1, and SPON2 mRNA with that of cultured human optic nerve astrocytes. All five genes were found to be expressed in HTM cells. Within the limits of our method which does not allow for an accurate comparison, the expression of SIX1 and WASL appeared to be considerably higher than that of CEACAM1, PFTK1, and SPON2 (Fig. 5A). In RNA from human optic nerve astrocytes, real time RT-PCR experiments indicated the presence of higher mRNA amounts of SIX1 and WASL than of SPON2, while mRNA of CEACAM1 and PFTK1 was not detectable (Fig. 5B). In order to learn, if higher amounts of myocilin would cause similar changes in gene expression in cultured human trabecular meshwork in vitro, as in βB1-crystallin-MYOC mice in vivo, cells were treated for 24 hours with increasing concentrations (2.5 to 100 ng/ml) of recombinant human myocilin. Recombinant myocilin had been purified by chromatography, and western blot analysis and silver staining visualized the two characteristic bands of myocilin and its glycosylated form at 55 and 57 kDa, respectively (Fig. 6). By real time RT-PCR, a dose-dependant decrease in the expression of SIX1, WASL, CEACAM1, and PFTK1 was observed that was statistically significant (Fig. 7) and correlated with the results obtained in βB1-crystallin-MYOC mice. In contrast to the results obtained from RNA of the whole eye of βB1-crystallin-MYOC mice, but in correlation with those obtained from RNA of their cornea, the expression of SPON2 was found to be downregulated.
Fig. 5.
Real time RT-PCR analysis for the expression of SIX1, WASL, PFTK1, CAECAM1, and SPON2 mRNA in cultured human trabecular meshwork cells and human optic nerve astrocytes. For real time RT-PCR analysis, means ± standard deviation of four independent experiments run in duplicate are shown. The mean value obtained for the gene with lowest signal was set at 1.
Fig. 6.
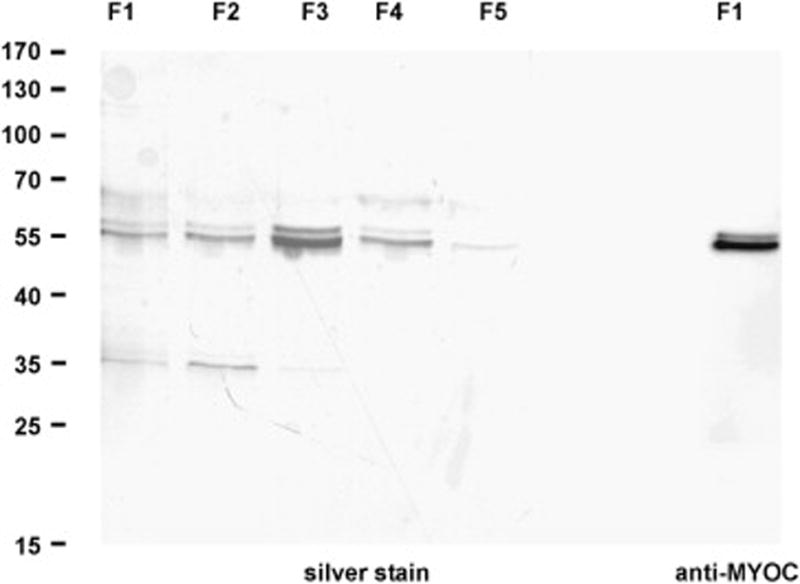
Silver stained SDS-PAGE and western blot with antibodies against myocilin of myocilin containing fractions (F1 to F5) after Ni-chelate chromatography. Proteins from fraction 3 (F3) were used for treatment of HTM cells.
Fig. 7.
Real time RT-PCR analysis for the expression of SIX1, WASL, PFTK1, CAECAM1, and SPON2 mRNA in cultured human trabecular meshwork cells after treatment with different amounts of recombinant myocilin (2.5ng, 5 ng, 25 ng, 50 ng, 100 ng) per ml cell culture medium. For real time RT-PCR analysis, means ± standard deviation of two independent experiments run in duplicate are shown. The mean value obtained for untreated control cells was set at 1. Asterisks mark statistical significant differences between treated cells and controls (p < 0.05 for * and p <0.02 for **). N.d.: not detectable.
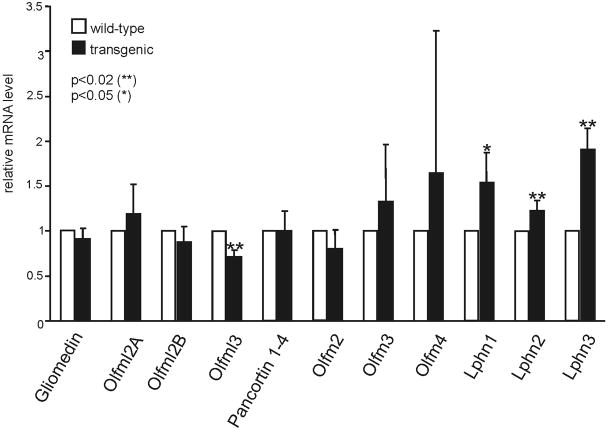
Myocilin has been shown to interact with olfactomedin 3 (optimedin), another member of the olfactomedin family, which is expressed in eye and brain (Torrado et al., 2002). The interaction between myocilin and optimedin involves their olfactomedin domains indicating that myocilin might also be able to interact with other members of this family. As no gene encoding for a protein of the olfactomedin family had shown a significant change in its expression in our microarray data, we wondered if false negative data could be present, comparable to the false positive ones that we had identified by real time RT-PCR. We therefore obtained RNA from the eyes (without lens) of four additional βB1-crystallin-MYOC mice and four wild-type littermates, and performed real time RT-PCR with primer pairs specific for a broad panel of olfactomedin genes (Fig. 7). Most of the genes encoding for secreted members of this family showed no change in expression, with the exception of Olfml3, which showed a 0.72-fold downregulation that was statistically significant (p< 0.05). Olfml3 encodes for olfactomedin-like 3, an olfactomedin protein that, similar to myocilin, contains a signal peptide, an N-terminal coiled-coil domain, and a C-terminal olfactomedin domain (Ikeya et al., 2005). In addition, a significant increase in expression was observed for Lphn1 (1.54-fold, p< 0.05), Lphn2 (1.23-fold, p< 0.02), and Lphn3 (1.91-fold, p< 0.02), the three genes that encode for latrophilin 1–3, a group of transmembrane receptors with an extracellular olfactomedin domain (Ushkaryov et al., 2008). In addition, we also investigated the expression of Flot1, and Sparcl1 as they encode for proteins (flotillin 1 and hevin) that have been shown to interact with myocilin (Joe et al., 2005; Li et al., 2006b). Neither the amounts of mRNA for Flot1 nor that for Sparcl1 showed a change that was statistically significant.
4. Discussion
We have identified a distinct small number of genes that are differentially expressed in βB1-crystallin-MYOC mice with ectopic, lens-derived overexpression of myocilin, a scenario which results in almost 5-fold more myocilin in the AH as compared to wild-type littermates (Zillig et al., 2005). In the absence of an obvious phenotype of βB1-crystallin-MYOC mice, we conclude that the genes which were identified as differentially expressed are directly associated with the functional properties of myocilin. This assumption is supported by additional in vitro data that show changes in the expression of the same genes in HTM cells treated with recombinant myocilin. It is interesting to note that three of the genes (Wasl, Caecam, and Spon2) are involved in cellular processes associated with cell adhesion. Wasl encodes for N-WASP (neural Wiskott-Aldrich syndrome protein), an actin-binding protein that is abundant in the brain, but is also present in several other tissues (Miki et al., 1998). N-WASP and related proteins of the WASP and WAVE family contribute to the rapid reorganization of cortical actin filaments, which are induced during cell shape changes and in response to extracellular stimuli (Takenawa and Miki, 2001; Takenawa and Suetsugu, 2007). N-WASP is also involved in cell substratum adhesion through interaction with the focal adhesion kinase (Wu et al., 2004). N-WASP deficient mice die in early embryonic life (Lommel et al., 2001; Snapper et al., 2001). Mouse embryonic cells that are derived from N-WASP deficient mice show reduced adhesion and spreading on fibronectin as substrate, and an increase in motility (Misra et al., 2007). Ceacam1 encodes for a member of the carcinoembyronic antigen (CEA)-related family of cell adhesion molecules (CAMs). CEACAM1 is a transmembrane receptor with an extracellular part that comprises a variable number of immunoglobulin constant-region-type-2-like (IgC2-like) domains, and a cytoplasmic tail. The expression of CEACAM1 has been observed at multiple sites in epithelial and endothelial cells, as well as in cells of the immune system (Gray-Owen and Blumberg, 2006). A major function of CEACAM1 appears to be the mediation of homophilic intercellular binding through interaction between its extracellular domains. The cytoplasmic tail of CEACAM1 binds to actin and tropomyosin (Schumann et al., 2001), and influences the localization of cytokeratin filaments and desmosomes in epithelial cells (Sundberg et al., 2004). Spon2 finally encodes for mindin (M-spondin, spondin2), a protein that is widely expressed (Feinstein et al., 1999) and belongs to the subgroup of matricellular thrombospondin type 1 repeat (TSR) molecules (Adams and Tucker, 2000; Feinstein and Klar, 2004). The available data on the functional properties of mindin are limited, but indicate roles in promoting neurite outgrowth during development (Feinstein et al., 1999), and in regulating Rho GTPase expression in cells of the immune system via mindin-integrin interactions (Li et al., 2006a).
Taken together, a decrease in the expression of Wasl and Ceacam1 that occurs simultaneously with changes in the expression of Spon2 might likely reflect a scenario with diminished cellular adhesion. Such a scenario correlates markedly well with the available in vitro data on the cell biological functions of myocilin. Fibroblasts attach, but fail to spread on recombinant eukaryotic myocilin as substrate (Peters et al., 2005). In addition, focal adhesion formation and the incorporation of paxillin into focal adhesions are significantly inhibited. Using recombinant myocilin from a bacterial source as substrate, a loss of actin stress fibers and focal adhesions has been observed in HTM cells, and adhesion on fibronectin as substrate has been found to be compromised (Wentz-Hunter et al., 2004). Comparable data have been reported for HTM cells that have been modified to secrete larger amounts of myocilin (Shen et al., 2008). The available data appear to indicate that myocilin acts as matricellular protein which modifies the number of contacts between cells and extracellular matrix of trabecular meshwork cells, or of other cells that are in contact with and respond to myocilin. In general, matricellular proteins are secreted proteins that influence cell function by modulating cell–matrix interactions (Bornstein, 2001; Sage and Bornstein, 1991). Prominent matricellular proteins are thrombospondin-1 and SPARC, which both inhibit spreading of fibroblasts under culture conditions (Murphy-Ullrich and Hook, 1989; Sage et al., 1989), and are constitutively expressed (albeit at much lower amounts than myocilin) in the trabecular meshwork (Flügel-Koch et al., 2004; Rhee et al., 2003). To further characterize a possible matricellular function of myocilin, it appears to be worthwhile to study its interaction with or relation to N-WASP, CEACAM1, or mindin, and such studies are underway in our laboratory.
The only transcription factor that could be identified as differentially regulated in βB1-crystallin-MYOC mice is Six1, a member of the Six homeoproteins which are characterized by a Six domain, and a Six-type homeodomain. Observations in Six1 deficient mice, which die at birth, indicate an important role of Six1 for development of thymus, kidney, skeletal muscle and craniofacial structures (Laclef et al., 2003a; Laclef et al., 2003b; Xu et al., 2003). Our observation of a strong Six1 expression in sclera and trabecular meshwork of adult mice correlates with findings that Six1 is expressed in cranial neural crest cells during embryonic development (Laclef et al., 2003b). Sclera and trabecular meshwork belong to those tissues in the mammalian eye that derive from the cranial neural crest (Cvekl and Tamm, 2004). It is tempting to speculate that Six1 is involved in the transcriptional regulation of genes that contribute to cellular adhesion in neural crest–derived tissues of the adult eye.
The upregulation of αB-crystallin expression and the accumulation of the protein in ocular tissues of βB1-crystallin-MYOC mice correlates markedly well with the observation that myocilin and αB-crystallin are both found at higher concentrations in the trabecular meshwork of eyes from patients with POAG (Lütjen-Drecoll et al., 1998). αB-crystallin is a structural protein in the lens and member of the small heat-shock family of proteins (Andley, 2007), which is also found in other tissues in- and outside the eye including the trabecular meshwork (Tamm et al., 1996).
αB-crystalllin is induced in response to heat shock and oxidative stress (Tamm et al., 1996), and is known to protect cells from apoptotic cell death following oxidative stress (Alge et al., 2002). It has been hypothesized that the presence of higher amounts of myocilin and αB-crystallin in eyes with POAG reflects a stress response of both proteins (Lütjen-Drecoll et al., 1998). Our findings of higher amounts of αB-crystallin under conditions of a constitutively high expression of myocilin offers the alternative explanation that αB-crystallin is upregulated not in response to stress, but rather in response to higher amounts of myocilin. αB-crystallin might be involved in a matricellular role of myocilin, as recent studies indicate a potential role for α-crystallins in actin dynamics during cell migration (Maddala and Rao, 2005).
Among the differentially expressed genes, Pftk1 appears to be the only one that is found to be expressed with high preference in the retina. Pftk1 (Pftaire1) is a cdc2-related protein kinase involved in cell cycle progression, proliferation, migration and motility (Pang et al., 2007; Shu et al., 2007a). The fact that higher amounts of myocilin lead to changes in gene expression in the retina suggests that myocilin might also have a direct or indirect function for retinal cells in the normal eye. Indeed the presence of myocilin was shown in the vitreous body (Karali et al., 2000), and in retinal neurons of various species (Ahmed et al., 2001; Karali et al., 2000; Knaupp et al., 2004; Ohlmann et al., 2003; Takahashi et al., 1998), although its retinal expression is considerably lower than in the anterior eye (Swiderski et al., 2000). βB1-crystallin-MYOC mice synthesize myocilin in lens fibers, and ectopic myocilin might not only enter AH, but also the vitreous body and finally the retina. Still, the fact that we could not detect transgenic myocilin in the retina by western blot analysis indicates that the amounts (if any) of transgenic myocilin in the posterior eye are very small. Changes in retinal gene expression in βB1-crystallin-MYOC might not be the result of a direct interaction of myocilin with retinal cells, but indirectly caused by changes in gene expression in the anterior eye.
The effects of higher amounts of myocilin on gene expression have also been studied in a microarray analysis by Borrás and coworkers, who overexpressed myocilin by adenoviral-mediated gene transfer in cultured HTM cells (Borras et al., 2006). None of the genes that were found to be differentially regulated by Borrás et al. were found to be regulated in our study and vice versa. It is of interest to note that the response of HTM cells to higher amounts of myocilin in their immediate surroundings differs from that observed in HTM cells that have been genetically engineered to synthesize higher amounts of myocilin under control of a strong viral promoter.
Myocilin is a member of the olfactomedin family, and it is of interest to note that we could find mRNA for a considerable large number of other family members in the mouse eye. Among the secreted members of the family only olfactomedin-like 3 was found to be changed in its mRNA expression. Olfactomedin-like 3 is expressed in lens and brain during development (Ikeya et al., 2005). To our knowledge, the functional properties of olfactomedin-like 3 have not been studied. Structurally, it shows distinct similarities with myocilin, as it also contains a signal peptide, an N-terminal coiled-coil domain, and a C-terminal olfactomedin domain (Ikeya et al., 2005). The observed downregulation in the expression of olfactomedin-like 3 might indicate a compensatory mechanism induced by high amounts of myocilin, and point to some functional properties that are shared by both proteins. Of interest is the significant increase in expression of Lphn1, Lphn2, and Lphn3, the three genes that encode for latrophilin 1–3, a group of transmembrane receptors with an extracellular olfactomedin domain (Ushkaryov et al., 2008). Latrophilins which bind α-latrotoxin from black widow spider venom are G-protein-coupled receptors (GPCR) that belong to the larger group of “adhesion GPCRs” which have in common that their extracellular domain contain motifs indicating possible binding to ECM molecules (Fredriksson and Schioth, 2005; Ushkaryov et al., 2008). Since myocilin and olfactomedin 3 (optimedin) have been shown to interact via their olfactomedin domains (Torrado et al., 2002), it is tempting to speculate that myocilin might be able to bind to the olfactomedin domain of latrophilin receptors, which are upregulated as response to the presence of higher amounts of the ligand. Studies are underway in our laboratories to test this hypothesis.
Fig. 8.
Real time RT-PCR analysis for the differential expression of the mRNA of various olfactomedin proteins in RNA from βB1-crystallin-MYOC mice and wild-type littermates. Means ± standard deviation of four independent experiments run in duplicate are shown. The mean value obtained with RNA from wild-type animals was set at 1. Asterisks mark statistical significant differences between βB1-crystallin-MYOC mice and wild-type littermates (p < 0.05 for * and p <0.02 for **).
Acknowledgments
The authors thank Angelika Pach and Tina Schiereis for excellent technical help. This study was supported by grants from the Deutsche Forschungsgemeinschaft (TA 115/15-1, FU 738/1-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam MF, Belmouden A, Binisti P, Brézin AP, Valtot F, Béchetoille A, Dascotte JC, Copin B, Gomez L, Chaventre A, Bach JF, Garchon HJ. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Gen. 1997;12:2091–2097. doi: 10.1093/hmg/6.12.2091. [DOI] [PubMed] [Google Scholar]
- Adams JC, Tucker RP. The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn. 2000;218:280–99. doi: 10.1002/(SICI)1097-0177(200006)218:2<280::AID-DVDY4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ahmed F, Torrado M, Johnson E, Morrison J, Tomarev SI. Changes in mRNA levels of the Myoc/Tigr gene in the rat eye after experimental elevation of intraocular pressure or optic nerve transection. Invest Ophthalmol Vis Sci. 2001;42:3165–3172. [PubMed] [Google Scholar]
- Alge CS, Priglinger SG, Neubauer AS, Kampik A, Zillig M, Bloemendal H, Welge-Lussen U. Retinal pigment epithelium is protected against apoptosis by alphaB-crystallin. Invest Ophthalmol Vis Sci. 2002;43:3575–82. [PubMed] [Google Scholar]
- Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107:929–34. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borras T, Bryant PA, Chisolm SS. First look at the effect of overexpression of TIGR/MYOC on the transcriptome of the human trabecular meshwork. Exp Eye Res. 2006;82:1002–10. doi: 10.1016/j.exer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Caballero M, Rowlette LL, Borrás T. Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin domain. Biochim Biophys Acta. 2000;1502:447–460. doi: 10.1016/s0925-4439(00)00068-5. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–86. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fautsch MP, Bahler CK, Vrabel AM, Howell KG, Loewen N, Teo WL, Poeschla EM, Johnson DH. Perfusion of his-tagged eukaryotic myocilin increases outflow resistance in human anterior segments in the presence of aqueous humor. Invest Ophthalmol Vis Sci. 2006;47:213–21. doi: 10.1167/iovs.05-0334. [DOI] [PubMed] [Google Scholar]
- Feinstein Y, Borrell V, Garcia C, Burstyn-Cohen T, Tzarfaty V, Frumkin A, Nose A, Okamoto H, Higashijima S, Soriano E, Klar A. F-spondin and mindin: two structurally and functionally related genes expressed in the hippocampus that promote outgrowth of embryonic hippocampal neurons. Development. 1999;126:3637–48. doi: 10.1242/dev.126.16.3637. [DOI] [PubMed] [Google Scholar]
- Feinstein Y, Klar A. The neuronal class 2 TSR proteins F-spondin and Mindin: a small family with divergent biological activities. Int J Biochem Cell Biol. 2004;36:975–80. doi: 10.1016/j.biocel.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Flügel-Koch C, Ohlmann A, Fuchshofer R, Welge-Lüssen U, Tamm ER. Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone in vitro. Exp Eye Res. 2004;79:649–63. doi: 10.1016/j.exer.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Schioth HB. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol Pharmacol. 2005;67:1414–25. doi: 10.1124/mol.104.009001. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Birke M, Welge-Lüssen U, Kook D, Lütjen-Drecoll E. Transforming growth factor-beta 2 modulated extracellular matrix component expression in cultured human optic nerve head astrocytes. Invest Ophthalmol Vis Sci. 2005;46:568–78. doi: 10.1167/iovs.04-0649. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Yu AH, Welge-Lüssen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:715–26. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- Gimenez E, Montoliu L. A simple polymerase chain reaction assay for genotyping the retinal degeneration mutation (Pdeb(rd1)) in FVB/N-derived transgenic mice. Lab Anim. 2001;35:153–6. doi: 10.1258/0023677011911525. [DOI] [PubMed] [Google Scholar]
- Goldwich A, Ethier CR, Chan DW, Tamm ER. Perfusion with the olfactomedin domain of myocilin does not affect outflow facility. Invest Ophthalmol Vis Sci. 2003;44:1953–61. doi: 10.1167/iovs.02-0863. [DOI] [PubMed] [Google Scholar]
- Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–43. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- Gould DB, Miceli-Libby L, Savinova OV, Torrado M, Tomarev SI, Smith RS, John SW. Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Mol Cell Biol. 2004;24:9019–25. doi: 10.1128/MCB.24.20.9019-9025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–46. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- He YW, Li H, Zhang J, Hsu CL, Lin E, Zhang N, Guo J, Forbush KA, Bevan MJ. The extracellular matrix protein mindin is a pattern-recognition molecule for microbial pathogens. Nat Immunol. 2004;5:88–97. doi: 10.1038/ni1021. [DOI] [PubMed] [Google Scholar]
- Heukeshoven J, Dernick R. Simplified method for silver staining of proteins in polyacrylamide gels and the mechanism of silver staining. Electrophoresis. 1985;6:103–112. [Google Scholar]
- Ikeya M, Kawada M, Nakazawa Y, Sakuragi M, Sasai N, Ueno M, Kiyonari H, Nakao K, Sasai Y. Gene disruption/knock-in analysis of mONT3: vector construction by employing both in vivo and in vitro recombinations. Int J Dev Biol. 2005;49:807–23. doi: 10.1387/ijdb.051975mi. [DOI] [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- Jia W, Li H, He YW. The extracellular matrix protein mindin serves as an integrin ligand and is critical for inflammatory cell recruitment. Blood. 2005;106:3854–9. doi: 10.1182/blood-2005-04-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe MK, Sohn S, Choi YR, Park H, Kee C. Identification of flotillin-1 as a protein interacting with myocilin: implications for the pathogenesis of primary open-angle glaucoma. Biochem Biophys Res Commun. 2005;336:1201–6. doi: 10.1016/j.bbrc.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Karali A, Russell P, Stefani FH, Tamm ER. Localization of myocilin/trabecular meshwork inducible glucocorticoid response protein in the human eye. Invest Ophthalmol Vis Sci. 2000;41:729–740. [PubMed] [Google Scholar]
- Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS, Tomarev SI, John SW, Johnson RL. Targeted disruption of the myocilin gene (Myoc) suggests that human glaucoma-causing mutations are gain of function. Mol Cell Biol. 2001;21:7707–7713. doi: 10.1128/MCB.21.22.7707-7713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaupp C, Flügel-Koch C, Goldwich A, Ohlmann A, Tamm ER. The expression of myocilin during murine eye development. Graefes Arch Clin Exp Ophthalmol. 2004;242:339–45. doi: 10.1007/s00417-003-0851-1. [DOI] [PubMed] [Google Scholar]
- Kowalski JR, Egile C, Gil S, Snapper SB, Li R, Thomas SM. Cortactin regulates cell migration through activation of N-WASP. J Cell Sci. 2005;118:79–87. doi: 10.1242/jcs.01586. [DOI] [PubMed] [Google Scholar]
- Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006;18:565–71. doi: 10.1016/j.ceb.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laclef C, Hamard G, Demignon J, Souil E, Houbron C, Maire P. Altered myogenesis in Six1-deficient mice. Development. 2003a;130:2239–52. doi: 10.1242/dev.00440. [DOI] [PubMed] [Google Scholar]
- Laclef C, Souil E, Demignon J, Maire P. Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech Dev. 2003b;120:669–79. doi: 10.1016/s0925-4773(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Li H, Oliver T, Jia W, He YW. Efficient dendritic cell priming of T lymphocytes depends on the extracellular matrix protein mindin. EMBO J. 2006a;25:4097–107. doi: 10.1038/sj.emboj.7601289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Aroca-Aguilar JD, Ghosh S, Sanchez-Sanchez F, Escribano J, Coca-Prados M. Interaction of myocilin with the C-terminal region of hevin. Biochem Biophys Res Commun. 2006b;339:797–804. doi: 10.1016/j.bbrc.2005.11.082. [DOI] [PubMed] [Google Scholar]
- Liu Y, Vollrath D. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum Mol Genet. 2004;13:1193–204. doi: 10.1093/hmg/ddh128. [DOI] [PubMed] [Google Scholar]
- Lommel S, Benesch S, Rottner K, Franz T, Wehland J, Kuhn R. Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep. 2001;2:850–7. doi: 10.1093/embo-reports/kve197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, May CA, Polansky JR, Johnson DH, Bloemendal H, Nguyen TD. Localization of the stress proteins αB-crystallin and trabecular meshwork inducible glucocorticoid response protein in normal and glaucomatous trabecular meshwork. Invest Ophthalmol Vis Sci. 1998;39:517–525. [PubMed] [Google Scholar]
- Maddala R, Rao VP. alpha-Crystallin localizes to the leading edges of migrating lens epithelial cells. Exp Cell Res. 2005;306:203–15. doi: 10.1016/j.yexcr.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol. 2004;24:5269–80. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Quiles N, Rohatgi R, Anton IM, Medina M, Saville SP, Miki H, Yamaguchi H, Takenawa T, Hartwig JH, Geha RS, Ramesh N. WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nat Cell Biol. 2001;3:484–91. doi: 10.1038/35074551. [DOI] [PubMed] [Google Scholar]
- Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–41. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A, Lim RP, Wu Z, Thanabalu T. N-WASP plays a critical role in fibroblast adhesion and spreading. Biochem Biophys Res Commun. 2007;364:908–12. doi: 10.1016/j.bbrc.2007.10.086. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Hook M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989;109:1309–19. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- Ohlmann A, Goldwich A, Flügel-Koch C, Fuchs AV, Schwager K, Tamm ER. Secreted glycoprotein myocilin is a component of the myelin sheath in peripheral nerves. Glia. 2003;43:128–40. doi: 10.1002/glia.10233. [DOI] [PubMed] [Google Scholar]
- Pang EY, Bai AH, To KF, Sy SM, Wong NL, Lai PB, Squire JA, Wong N. Identification of PFTAIRE protein kinase 1, a novel cell division cycle-2 related gene, in the motile phenotype of hepatocellular carcinoma cells. Hepatology. 2007;46:436–45. doi: 10.1002/hep.21691. [DOI] [PubMed] [Google Scholar]
- Peters DM, Herbert K, Biddick B, Peterson JA. Myocilin binding to Hep II domain of fibronectin inhibits cell spreading and incorporation of paxillin into focal adhesions. Exp Cell Res. 2005;303:218–28. doi: 10.1016/j.yexcr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Rhee DJ, Fariss RN, Brekken R, Sage EH, Russell P. The matricellular protein SPARC is expressed in human trabecular meshwork. Exp Eye Res. 2003;77:601–7. doi: 10.1016/s0014-4835(03)00190-8. [DOI] [PubMed] [Google Scholar]
- Russell P, Tamm ER, Grehn FJ, Picht G, Johnson M. The presence and properties of myocilin in the aqueous humor. Invest Ophthalmol Vis Sci. 2001;42:983–986. [PubMed] [Google Scholar]
- Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991;266:14831–4. [PubMed] [Google Scholar]
- Sage H, Vernon RB, Funk SE, Everitt EA, Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol. 1989;109:341–56. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann D, Chen CJ, Kaplan B, Shively JE. Carcinoembryonic antigen cell adhesion molecule 1 directly associates with cytoskeleton proteins actin and tropomyosin. J Biol Chem. 2001;276:47421–33. doi: 10.1074/jbc.M109110200. [DOI] [PubMed] [Google Scholar]
- Shen X, Koga T, Park BC, SundarRaj N, Yue BY. Rho GTPase and cAMP/protein kinase A signaling mediates myocilin-induced alterations in cultured human trabecular meshwork cells. J Biol Chem. 2008;283:603–12. doi: 10.1074/jbc.M708250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard AR, Jacobson N, Millar JC, Pang IH, Steely HT, Searby CC, Sheffield VC, Stone EM, Clark AF. Glaucoma-causing myocilin mutants require the Peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum Mol Genet. 2007;16:609–17. doi: 10.1093/hmg/ddm001. [DOI] [PubMed] [Google Scholar]
- Shu F, Lv S, Qin Y, Ma X, Wang X, Peng X, Luo Y, Xu BE, Sun X, Wu J. Functional characterization of human PFTK1 as a cyclin-dependent kinase. Proc Natl Acad Sci U S A. 2007a;104:9248–53. doi: 10.1073/pnas.0703327104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu F, Lv S, Qin Y, Ma X, Wang X, Peng X, Luo Y, Xu BE, Sun X, Wu J. Functional characterization of human PFTK1 as a cyclin-dependent kinase. Proc Natl Acad Sci U S A. 2007b;104:9248–53. doi: 10.1073/pnas.0703327104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper SB, Takeshima F, Anton I, Liu CH, Thomas SM, Nguyen D, Dudley D, Fraser H, Purich D, Lopez-Ilasaca M, Klein C, Davidson L, Bronson R, Mulligan RC, Southwick F, Geha R, Goldberg MB, Rosen FS, Hartwig JH, Alt FW. N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat Cell Biol. 2001;3:897–904. doi: 10.1038/ncb1001-897. [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WLM, Nguyen TD, Polansky JR, Sunden SLF, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Sundberg U, Beauchemin N, Obrink B. The cytoplasmic domain of CEACAM1-L controls its lateral localization and the organization of desmosomes in polarized epithelial cells. J Cell Sci. 2004;117:1091–104. doi: 10.1242/jcs.00944. [DOI] [PubMed] [Google Scholar]
- Swiderski RE, Ross JL, Fingert JH, Clark AF, Alward WL, Stone EM, Sheffield VC. Localization of MYOC transcripts in human eye and optic nerve by in situ hybridization. Invest Ophthalmol Vis Sci. 2000;41:3420–3428. [PubMed] [Google Scholar]
- Takahashi H, Noda S, Imamura Y, Nagasawa A, Kubota R, Mashima Y, Kudoh J, Oguchi Y, Shimizu N. Mouse myocilin (Myoc) gene expression in ocular tissues. Biochem Biophys Res Commun. 1998;248:104–109. doi: 10.1006/bbrc.1998.8917. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci. 2001;114:1801–9. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Tamm E, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of Myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:2577–2582. [PubMed] [Google Scholar]
- Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21:395–428. doi: 10.1016/s1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Russell P, Johnson DH, Piatigorsky J. Human and monkey trabecular meshwork accumulate alpha B-crystallin in response to heat shock and oxidative stress. Invest Ophthalmol Vis Sci. 1996;37:2402–13. [PubMed] [Google Scholar]
- Tomarev SI, Wistow G, Raymond V, Dubois S, Malyukova I. Gene expression profile of the human trabecular meshwork: NEIBank sequence tag analysis. Invest Ophthalmol Vis Sci. 2003;44:2588–96. doi: 10.1167/iovs.02-1099. [DOI] [PubMed] [Google Scholar]
- Torrado M, Trivedi R, Zinovieva R, Karavanova I, Tomarev SI. Optimedin: a novel olfactomedin-related protein that interacts with myocilin. Hum Mol Genet. 2002;11:1291–1301. doi: 10.1093/hmg/11.11.1291. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Rohou A, Sugita S. alpha-Latrotoxin and its receptors. Handb Exp Pharmacol. 2008:171–206. doi: 10.1007/978-3-540-74805-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentz-Hunter K, Kubota R, Shen X, Yue BY. Extracellular myocilin affects activity of human trabecular meshwork cells. J Cell Physiol. 2004;200:45–52. doi: 10.1002/jcp.10478. [DOI] [PubMed] [Google Scholar]
- White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–20. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- Wu X, Suetsugu S, Cooper LA, Takenawa T, Guan JL. Focal adhesion kinase regulation of N-WASP subcellular localization and function. J Biol Chem. 2004;279:9565–76. doi: 10.1074/jbc.M310739200. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130:3085–94. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig M, Wurm A, Grehn FJ, Russell P, Tamm ER. Overexpression and properties of wild-type and Tyr437His mutated myocilin in the eyes of transgenic mice. Invest Ophthalmol Vis Sci. 2005;46:223–34. doi: 10.1167/iovs.04-0988. [DOI] [PubMed] [Google Scholar]