Abstract
Chronic infections play a significant role in the morbidity and mortality of patients with chronic airflow limitation. By stimulating airway inflammation, persistent infection has the potential to cause airway fibrosis. However, in patient this condition is most typically found in lungs damaged by other factors, such as smoking, abnormal secretions, or barotrauma. We report the characterization of Mycoplasma pulmonis infection-induced lung fibrosis in two immunocompetent rat strains with no preexisting lung disease. The fibrosis was predominantly in the airways, as demonstrated by the findings for infected animals of increased airway inflammation, airway fibrosis, and airway wall thickness, which correlated with the collagen content of the lungs. Also, the physiological alterations were the opposite of those found in interstitial fibrosis, with a positive correlation between lung compliance and collagen content. The airway fibrosis was noted earlier and to a greater extent in Lewis rats than in Fisher rats, and this result apparently was related to regulation of the inflammatory response. Airway wall thickness, airway inflammation, and airway fibrosis are commonly reported in tissue specimens from patients with chronic airway diseases and have been shown to correlate with airflow limitation in patients with chronic obstructive pulmonary disease. Thus, this model may be useful in furthering our understanding of the role of chronic infection and airway inflammation in airflow obstruction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesina A. M., Vallyathan V., McQuillen E. N., Weaver S. O., Craighead J. E. Bronchiolar inflammation and fibrosis associated with smoking. A morphologic cross-sectional population analysis. Am Rev Respir Dis. 1991 Jan;143(1):144–149. doi: 10.1164/ajrccm/143.1.144. [DOI] [PubMed] [Google Scholar]
- Beasley R., Roche W. R., Roberts J. A., Holgate S. T. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989 Mar;139(3):806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- Bedrossian C. W., Greenberg S. D., Singer D. B., Hansen J. J., Rosenberg H. S. The lung in cystic fibrosis. A quantitative study including prevalence of pathologic findings among different age groups. Hum Pathol. 1976 Mar;7(2):195–204. doi: 10.1016/s0046-8177(76)80023-8. [DOI] [PubMed] [Google Scholar]
- Bonikos D. S., Bensch K. G., NORTHWAY W. H., Jr, Edwards D. K. Bronchopulmonary dysplasia: the pulmonary pathologic sequel of necrotizing bronchiolitis and pulmonary fibrosis. Hum Pathol. 1976 Nov;7(6):643–666. doi: 10.1016/s0046-8177(76)80077-9. [DOI] [PubMed] [Google Scholar]
- Bosken C. H., Wiggs B. R., Paré P. D., Hogg J. C. Small airway dimensions in smokers with obstruction to airflow. Am Rev Respir Dis. 1990 Sep;142(3):563–570. doi: 10.1164/ajrccm/142.3.563. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Bitterman P. B., Rennard S. I., Hance A. J., Keogh B. A. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract (first of two parts). N Engl J Med. 1984 Jan 19;310(3):154–166. doi: 10.1056/NEJM198401193100304. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Bitterman P. B., Rennard S. I., Hance A. J., Keogh B. A. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract. N Engl J Med. 1984 Jan 26;310(4):235–244. doi: 10.1056/NEJM198401263100406. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Baum B. J., Bernardo J., Bradley K. H., Bruel S. D., Elson N. A., Fells G. A., Ferrans V. J., Gadek J. E. Cells, collagen and idiopathic pulmonary fibrosis. Lung. 1978;155(3):199–224. doi: 10.1007/BF02730694. [DOI] [PubMed] [Google Scholar]
- Davis J. K., Cassell G. H. Murine respiratory mycoplasmosis in LEW and F344 rats: strain differences in lesion severity. Vet Pathol. 1982 May;19(3):280–293. doi: 10.1177/030098588201900306. [DOI] [PubMed] [Google Scholar]
- Davis J. K., Simecka J. W., Williamson J. S., Ross S. E., Juliana M. M., Thorp R. B., Cassell G. H. Nonspecific lymphocyte responses in F344 and LEW rats: susceptibility to murine respiratory mycoplasmosis and examination of cellular basis for strain differences. Infect Immun. 1985 Jul;49(1):152–158. doi: 10.1128/iai.49.1.152-158.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Thorp R. B., Maddox P. A., Brown M. B., Cassell G. H. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982 May;36(2):720–729. doi: 10.1128/iai.36.2.720-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterly J. R., Oppenheimer E. H. Observations in cystic fibrosis of the pancreas. 3. Pulmonary lesions. Johns Hopkins Med J. 1968 Feb;122(2):94–101. [PubMed] [Google Scholar]
- Finucane K. E., Colebatch H. J. Elastic behavior of the lung in patients with airway obstruction. J Appl Physiol. 1969 Mar;26(3):330–338. doi: 10.1152/jappl.1969.26.3.330. [DOI] [PubMed] [Google Scholar]
- Freitag L., Firusian N., Stamatis G., Greschuchna D. The role of bronchoscopy in pulmonary complications due to mustard gas inhalation. Chest. 1991 Nov;100(5):1436–1441. doi: 10.1378/chest.100.5.1436. [DOI] [PubMed] [Google Scholar]
- Friedman P. J., Harwood I. R., Ellenbogen P. H. Pulmonary cystic fibrosis in the adult: early and late radiologic findings with pathologic correlations. AJR Am J Roentgenol. 1981 Jun;136(6):1131–1144. doi: 10.2214/ajr.136.6.1131. [DOI] [PubMed] [Google Scholar]
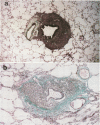
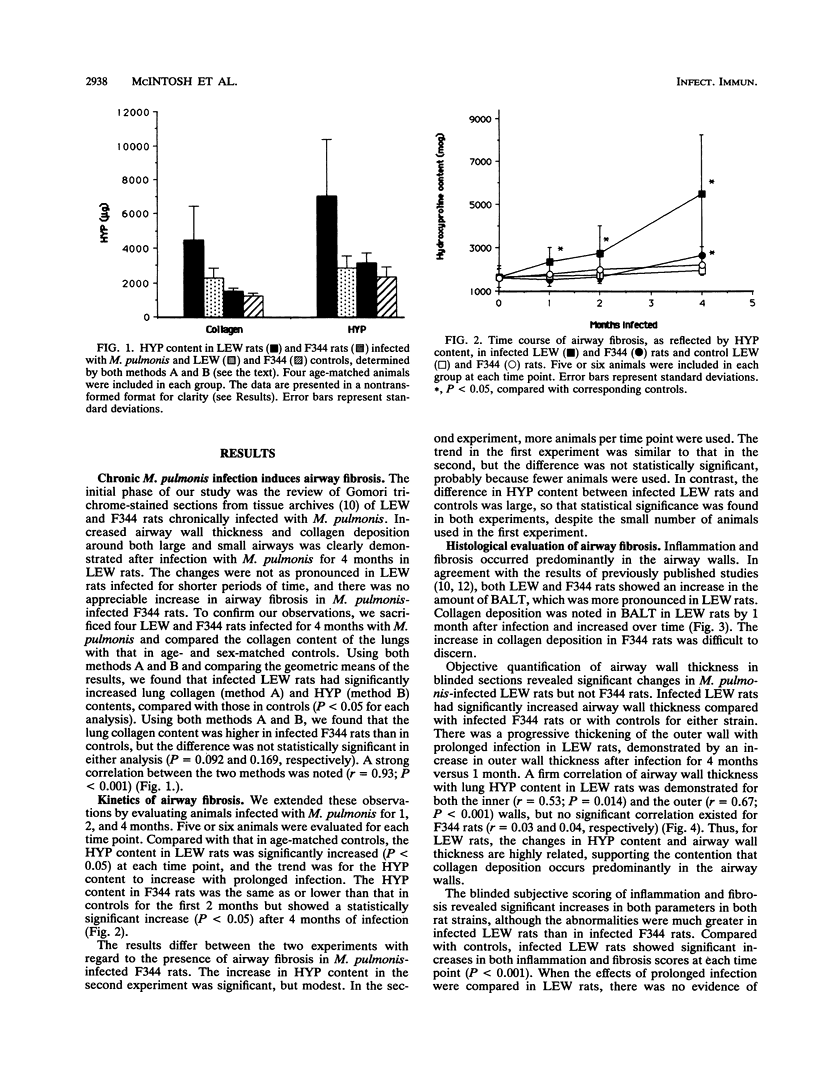
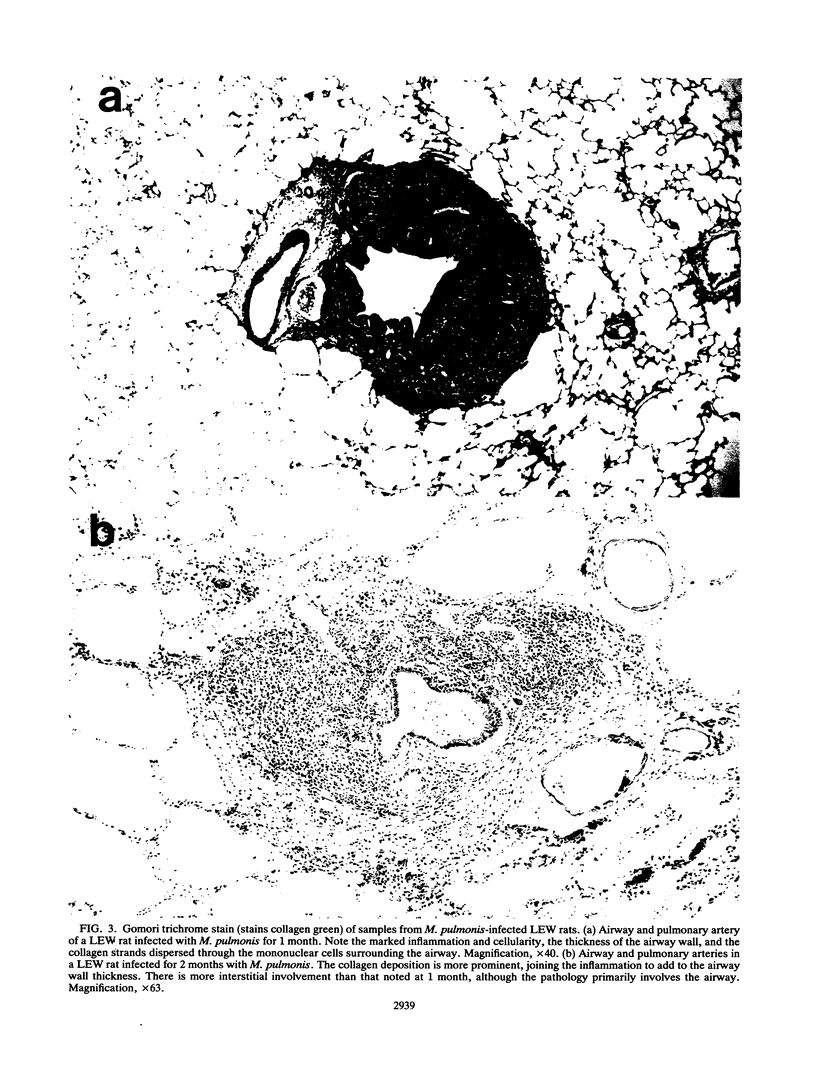
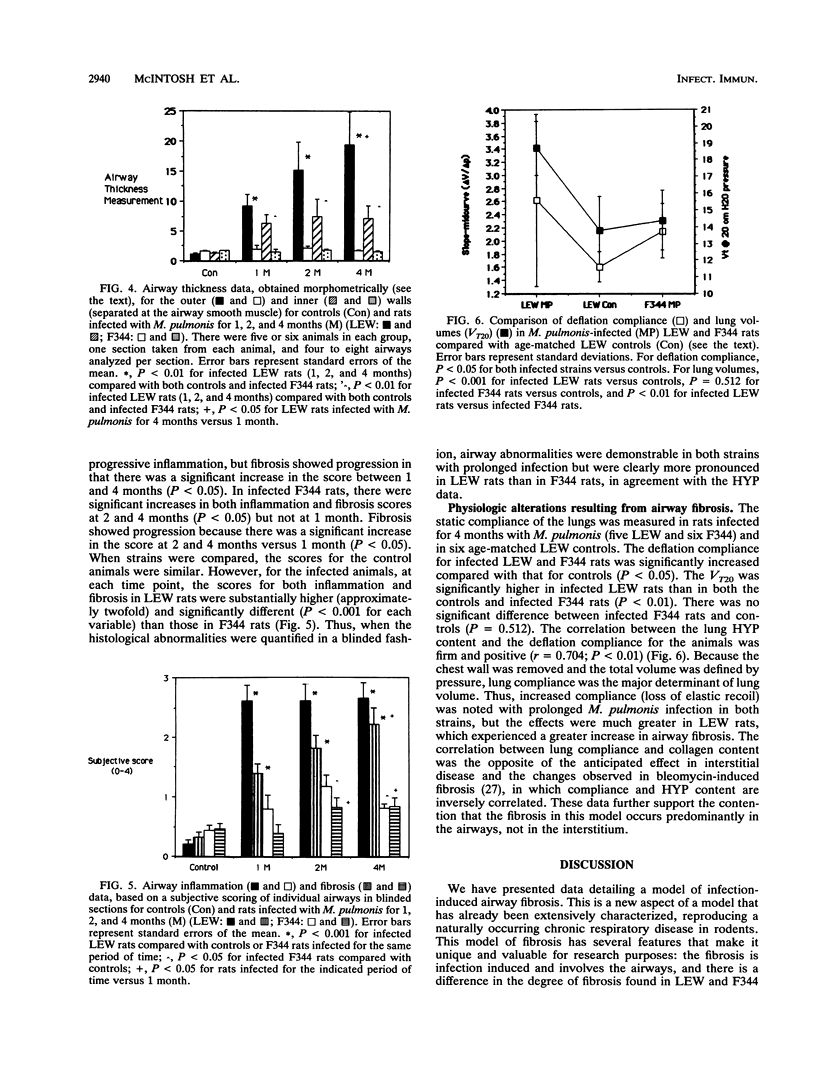
- GOMORI G. A rapid one-step trichrome stain. Am J Clin Pathol. 1950 Jul;20(7):661–664. doi: 10.1093/ajcp/20.7_ts.661. [DOI] [PubMed] [Google Scholar]
- Gold W. M., Kaufman H. S., Nadel J. A. Elastic recoil of the lungs in chronic asthmatic patients before and after therapy. J Appl Physiol. 1967 Oct;23(4):433–438. doi: 10.1152/jappl.1967.23.4.433. [DOI] [PubMed] [Google Scholar]
- Groeneveld K., van Alphen L., Eijk P. P., Visschers G., Jansen H. M., Zanen H. C. Endogenous and exogenous reinfections by Haemophilus influenzae in patients with chronic obstructive pulmonary disease: the effect of antibiotic treatment on persistence. J Infect Dis. 1990 Mar;161(3):512–517. doi: 10.1093/infdis/161.3.512. [DOI] [PubMed] [Google Scholar]
- Gross N. J. Radiation pneumonitis in mice. Some effects of corticosteroids on mortality and pulmonary physiology. J Clin Invest. 1980 Sep;66(3):504–510. doi: 10.1172/JCI109881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg J. C., James A. L., Pare P. D. Evidence for inflammation in asthma. Am Rev Respir Dis. 1991 Mar;143(3 Pt 2):S39–S42. doi: 10.1164/ajrccm/143.3_Pt_2.S39. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Astry C. L., Warr G. A. Alveolitis induced by influenza virus. Am Rev Respir Dis. 1983 Oct;128(4):730–739. doi: 10.1164/arrd.1983.128.4.730. [DOI] [PubMed] [Google Scholar]
- Kim W. D., Eidelman D. H., Izquierdo J. L., Ghezzo H., Saetta M. P., Cosio M. G. Centrilobular and panlobular emphysema in smokers. Two distinct morphologic and functional entities. Am Rev Respir Dis. 1991 Dec;144(6):1385–1390. doi: 10.1164/ajrccm/144.6.1385. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- Löwenberg A., Orie N. G., Sluiter H. J., de Vries K. Bronchial hyperreactivity and bronchial obstruction in respiratory viral infection. An attempt to evaluate the relationship. Respiration. 1986;49(1):1–9. doi: 10.1159/000194852. [DOI] [PubMed] [Google Scholar]
- Macklem P. T. Factors determining bronchial smooth muscle shortening. Am Rev Respir Dis. 1991 Mar;143(3 Pt 2):S47–S48. doi: 10.1164/ajrccm/143.3_Pt_2.S47. [DOI] [PubMed] [Google Scholar]
- Mullen J. B., Wright J. L., Wiggs B. R., Pare P. D., Hogg J. C. Reassessment of inflammation of airways in chronic bronchitis. Br Med J (Clin Res Ed) 1985 Nov 2;291(6504):1235–1239. doi: 10.1136/bmj.291.6504.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche W. R., Beasley R., Williams J. H., Holgate S. T. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989 Mar 11;1(8637):520–524. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- Sado Y., Naito I., Akita M., Okigaki T. Strain specific responses of inbred rats on the severity of experimental autoimmune glomerulonephritis. J Clin Lab Immunol. 1986 Apr;19(4):193–199. [PubMed] [Google Scholar]
- Sobonya R. E., Taussig L. M. Quantitative aspects of lung pathology in cystic fibrosis. Am Rev Respir Dis. 1986 Aug;134(2):290–295. doi: 10.1164/arrd.1986.134.2.290. [DOI] [PubMed] [Google Scholar]
- Sternberg E. M., Hill J. M., Chrousos G. P., Kamilaris T., Listwak S. J., Gold P. W., Wilder R. L. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker J. T. Pathologic features of long-standing "healed" bronchopulmonary dysplasia: a study of 28 3- to 40-month-old infants. Hum Pathol. 1986 Sep;17(9):943–961. doi: 10.1016/s0046-8177(86)80646-3. [DOI] [PubMed] [Google Scholar]
- Södervik H., Syrjälä H., Räisänen S. Interstitial pneumonia and sepsis caused by Yersinia enterocolitica serotype 3. Scand J Infect Dis. 1986;18(3):241–243. doi: 10.3109/00365548609032333. [DOI] [PubMed] [Google Scholar]
- Wilder R. L., Calandra G. B., Garvin A. J., Wright K. D., Hansen C. T. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1982 Sep;25(9):1064–1072. doi: 10.1002/art.1780250906. [DOI] [PubMed] [Google Scholar]
- Williams R. M., Moore M. J. Linkage of susceptibility to experimental allergic encephalomyelitis to the major histocompatibility locus in the rat. J Exp Med. 1973 Oct 1;138(4):775–783. doi: 10.1084/jem.138.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmott R. W., Tyson S. L., Matthew D. J. Cystic fibrosis survival rates. The influences of allergy and Pseudomonas aeruginosa. Am J Dis Child. 1985 Jul;139(7):669–671. doi: 10.1001/archpedi.1985.02140090031019. [DOI] [PubMed] [Google Scholar]
- Yoneda K. Subendothelial electron-dense deposits in pulmonary alveolar wall in pulmonary actinomycosis. Am Rev Respir Dis. 1981 Jun;123(6):686–686. doi: 10.1164/arrd.1981.123.6.686. [DOI] [PubMed] [Google Scholar]