Abstract
The gelation kinetics of an in situ gelable hydrogel formulated from oxidized dextran (Odex) and N-carboxyethyl chitosan (CEC) were investigated rheologically. Both Schiff base mediated chemical and physical crosslinking account for its rapid gelation (30–600 s) between 5–37 °C. The correlation between gelation kinetics and hydrogel properties with Odex/CEC concentration, their feed ratio and temperature were elucidated. The gelation time determined from crossing over of storage moduli (G′) and loss moduli (G″) was in good agreement with that of deduced from frequency sweeping tests according to Winter-Chambon power law. The power law exponents for a 2% (w/v) Odex/CEC solution (ratio 5:5) at gel point was 0.61, which was in excellent agreement with the value predicted from percolation theory (2/3). Temperature dependence of gelation time for the same hydrogel formulation was well described by Arrhenius plot with its apparent activation energy calculated at 51.9 kJ/mol.
Introduction
Hydrogel is a class of material composed of hydrophilic polymer networks with high water retention capacity; its physical properties such as, swelling, permeation, mechanical, surface and optical can be modulated. In the medical field, biocompatible hydrogels have broad potential applications, including tissue substitutes, surgical aids and drug delivery. 1
Natural polysaccharides are hydrophilic macromolecules suitable for fabrication of hydrogels targeting biomedical uses; in particular, chitosan and dextran have been widely explored in this arena. Chitosan, a crustacean or fungal derived polysaccharide composed of α-(1–4)-linked N-acetyl-D-glucosamine, is biocompatible and bioresorbable.2,3 It has been utilized in gene delivery,4 cell regulation and tissue regeneration.5 However, native chitosan is only soluble in diluted acid6 and this greatly limited its processibility and applications. Various derivatization techniques have been developed to enhance chitosan’s solubility;7–9 introducing acrylic acid into chitosan structure to form carboxyethyl chitosan (CEC) results in an ampholyte readily soluble in water.10 Dextran is a bacterial derived polysaccharide composed of an α-1,6 linked D-glucopyranose as well as some α-1,2-, α-1,3- or α-1,4-linked side chains.11,12 Being biodegradable in concert with its exceptional biocompatibility, dextran has been extensively used as a blood volume expander, in drug/protein delivery and for purification of biologics.13 The physicochemical properties of dextran could be modulated through modification of its hydroxyl groups.14–16 Oxidation is a facile technique to introduce multiple aldehyde functionalities enabling dextran to serve as a macromolecular crosslinker for polymers abundant in amino groups to formulate hydrogels.17,18
The viscoelastic properties of hydrogels correlate strongly with their microstructures and could provide useful information for modulating their performance characteristics.19 In general, the viscoelastic properties of material systems undergoing gelation could be monitored by rheometry with virtually no disturbance on their microstructures.20 Rheometry has been employed in characterizing viscous liquids formulated from chitosan,5,21–22 and physical gelation has been observed for chitosan solutions at high concentrations.7, 23 Moreover, the cure kinetics of chitosan chemically crosslinked with blocked diisocyinate were found to correlate with the rheology of the system.24 However, no systematic rheological study has been conducted to characterize the physical interactions and Schiff base mediated co-gelation process between macromolecules derived from chitosan and dextran.
In this investigation, rheological studies were performed to evaluate the viscoelastic profiles of crosslinking CEC with partially oxidized dextran (Odex) amid gelation. The effect of temperature, feed ratio of Odex and CEC, and polymer concentration on gelation were investigated. These results enable us to better understand the gelation kinetics and morphological evolution of the Odex/CEC system, which provides important insights for designing binary in situ gelable hydrogels for future biomedical applications such as endovascular emoblization of cerebral aneurysms and arteriovenous malformations.
Materials and Methods
Materials
Dextran (Leuconostoc mesentereoides, Mw = 76,000), chitosan (deacetylation degree 85%, Mw 750,000), sodium periodate, sodium hydroxide, acrylic acid, tert-butylcarbazate (TBC), and trinitrobenzenosulfonic acid (TNBS) were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were of reagent grade. Distilled deionized water was used.
Synthesis of N-Carboxyethyl Chitosan
CEC was synthesized by modifying a previously described method.10 Briefly, 1 g of chitosan was dissolved in 50 mL of water containing 1.88 mL of acrylic acid. The mixture was constantly stirred at 50 °C for 3 days. Thereafter, aqueous 10N NaOH was added to the reaction mixture to adjust the pH to 10–12 to convert the CEC formed into its sodium salt. This mixture was dialyzed (MWCO 8,000) extensively against water for 3 days and pure CEC was obtained by lyophilization.
Synthesis and Characterization of Oxidized Dextran
3.28 g NaIO4 (dissolved in 100 mL water) was added to 400 ml of dextran solution (1.25% w/v). This mixture was stirred at ambient temperature for 24 h, and an equimolar amount of diethylene glycol was added to quench the unreacted NaIO4. The Odex solution was dialyzed exhaustively (MWCO 3,500) for 3 days against water, and pure Odex was obtained by lyophilization (yield, 80%). The oxidation degree of dextran was determined using a modified method described by Maia et al.25 Briefly, 0.5 mL Odex (0.75% w/v) in tricholoracetic acid (1% w/v) was mixed with 0.5 ml TBC (0.03 M in 1% tricholoracetic acid) and incubated at ambient temperature for 24 h; then, 100 µL of this mixture was transferred to a vial containing 1 mL TNBS (4 mM in 0.1 M borate, pH = 8.0) solution and the reaction was conducted at ambient temperature for 1 h. The resulting solution was diluted with 0.5 N HCl and its absorbance was measured at 334 nm. Aqueous TBC solutions were used as standards for calibration. The molecular weights of Odex (0.5 mg/mL in water) were determined by HPLC (Waters 600e pump and controller, 715 Injector, 410 differential refractometer, USA) coupled to Waters Ultrahydrogel 2000, 1000, and 500; 300 mm×7.8 mm columns connected in series, using 0.1 M KNO3 as a mobile phase at a flow rate of 0.8 mL/min maintained at room temperature. Dextran standards (Fluka Chemie AG, Switzerland) in the molecular range of 12–80 kDa were used for calibration.
Preparation Odex/CEC Solutions and Hydrogels
Aqueous Odex and CEC solutions of 1%, 2% and 3% (w/v) in distilled water were prepared, respectively, and stored at 5 °C before use. To prepare hydrogels for linear viscoelastic region (LVR) characterizations, a 2% Odex solution was mixed with a 2% CEC aqueous solution in specific volume ratios (2:8, 5:5 and 8:2); these mixtures were used to determine the effect of feed ratio of Odex and CEC on the properties of hydrogels formed. To elucidate the influence of polymer concentration on hydrogel properties, 1%, 2%, and 3% Odex solutions were mixed with 1%, 2%, and 3% CEC solutions in a ratio of 5:5, respectively. The mixtures were gently stirred for 10 s to allow homogenous mixing followed by 12 h of incubation at 25 °C before use.
Rheological Measurements
All rheological measurements were performed with a Physica MCR 301 rheometer (Anton Paar, Hertford Herts, UK). Isothermal and nonisothermal gelation studies were conducted with a Couette geometry (bob d = 26.687 mm, h = 39.994 mm, cup d = 28.930 mm). Liquid samples were transferred into a Couette cell immediately after mixing (time t = 0), and measurement started at t = 60–90 s. For time sweeping tests, storage moduli G′ and loss moduli G″ were monitored as a function of time at a 1 Hz frequency and a 2% stress strain under constant temperature (5, 10, 15, 25, and 37 °C). For temperature sweeping tests, evolution of G′ and G″ in the range of 1.5 °C – 80 °C were measured at a heating rate of 2 °C/min. Stress sweeping tests of hydrogels were conducted using parallel plates (d = 25 mm) at 37 °C. In frequency sweeping tests, hydrogels formulated from 2% Odex/2% CEC (ratio 5:5) were subjected to frequency scanning from 1 to 100 rad/s at 2% strain for 80 loops with 13.55 s under a constant temperature of 37 °C.
Results and Discussion
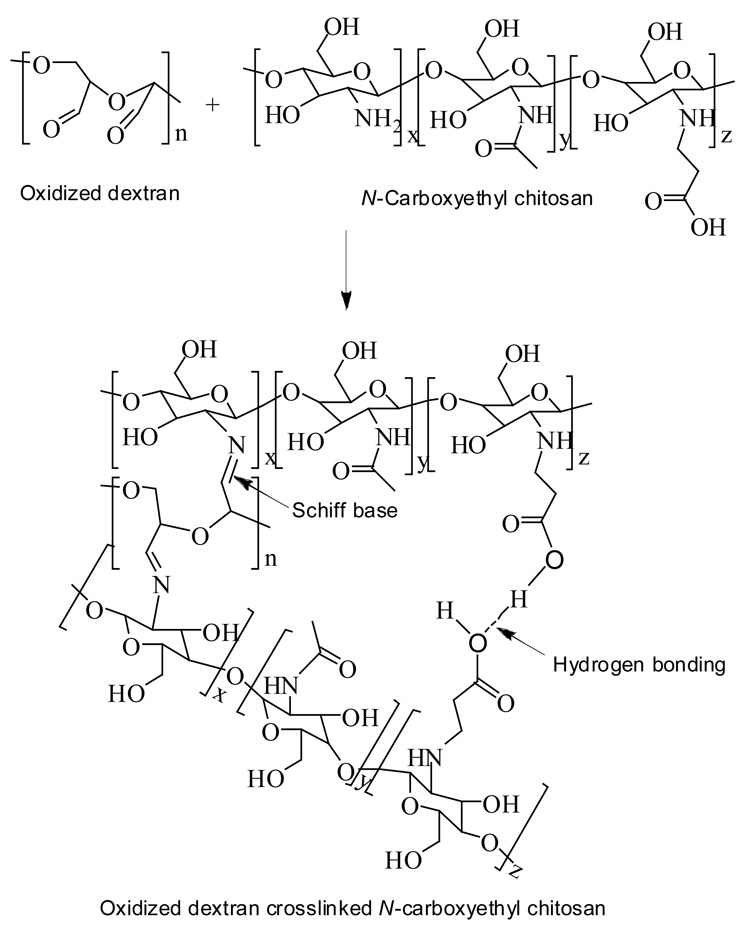
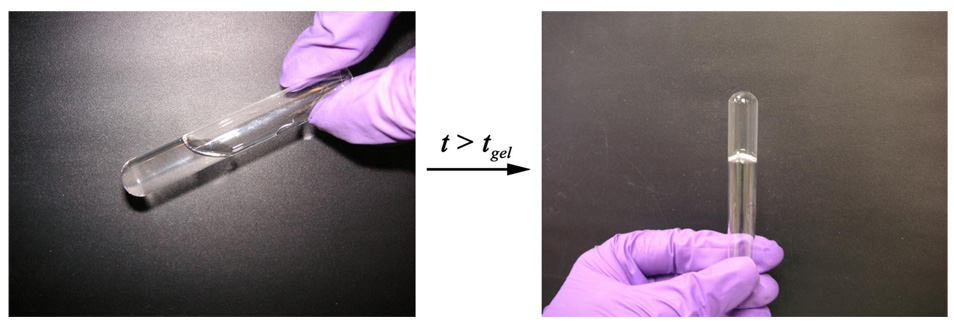
Grafting acrylic acid on chitosan to form CEC rendered it soluble in water at ambient temperature; the substitution degree of the chitosan used in this study was 45.5%.10 The oxidation degree of Odex obtained was 16.7% as determined by the TNBS assay, with an average molecular weight of 36,800 Da. Due to the co-existence of abundant amino, hydroxyl and carboxylic groups along the CEC molecular chains, in conjunction with the plentiful aldehyde and hydroxyl groups along the Odex molecular chains, Schiff base as well as hydrogen bond formation (shown in Scheme 1) were expected after blending CEC with Odex solutions.24 Depending on the polymer concentrations, upon stirring, the Odex/CEC gelled between 30–600 s at ambient temperature. Figure 1 depicted the appearance of the Odex/CEC system at time t < tgel and t > tgel (tgel is the time at gel point). The Odex/CEC hydrogels were optically clear initially; a brown-yellowish tint, characteristic of C=N chromophore formation, gradually emerged with the proceeding of chemical crosslinking. Moreover, this rapid gelation behavior also readily occurred at body temperature and physiological pH (results not shown), strongly suggesting that the Odex/CEC system could serve as an injectable in situ gelable vehicle for medical applications, such as endovascular embolization of cerebral aneurysm and arteriovenous malformation, and so forth. The gelation process and mechanical properties of hydrogels formed are likely affected by initial polymer concentrations, ratio of Odex/CEC, and temperature; importantly, these parameters are crucial for formulating biomaterials with definitive physicochemical properties for specific applications. In this study, we aimed to determine the interaction of these parameters on the gelation kinetics and gel properties of the Odex/CEC systems.
Scheme 1.
Schematic representation for the gelation process of Odex/CEC solutions.
Figure 1.
Visual aspect of the Odex/CEC system at time t < tgel and t > tgel. tgel is the gelation point.
Isothermal Gelation Rheology
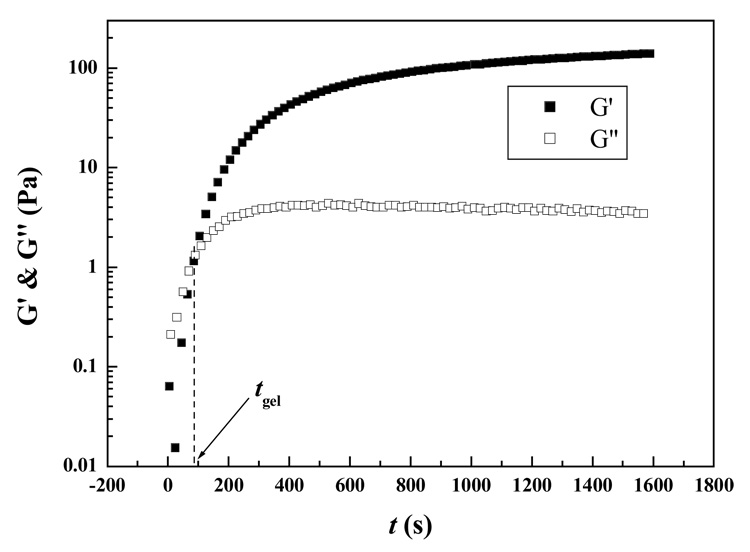
Isothermal time dependence of elastic storage moduli (G′) and viscous loss moduli (G″) for a 5:5 Odex/CEC (concentration: 2% for both) at 37 °C at an oscillation strain of 2% was depicted in Figure 2. Both G′ and G″ were very low at the beginning of the gelation process (t < tgel), with G′ lower than G″, corresponding to an Odex/CEC sol. Both moduli elevated rapidly as gelation proceeded; the buildup rate of G′ was much higher than that of G″ due to the formation of elastic hydrogel from crosslinking through Schiff base formation between aldehyde residues of Odex and amino residues of CEC. The differential on rates leads to a crossover of G′ and G″, which could be defined as the gel point (t = tgel), indicating transition of the Odex/CEC system from a liquid-phase dominated to a solid-phase dominated viscoelastic behavior, also suggesting three dimensional (3D) network formation. This type of material behavior is characteristic of hydrogels formed through physical or chemical crosslinking.26,27
Figure 2.
Time evolution of storage modulus (G′) and loss modulus (G″) with time (t) for 2% (w/v) Odex/CEC (ratio 5:5) at 37°C. The time where G′ and G″ crossover is denoted as tgel (gelation point).
The types of interaction enabling formation of 3D network structures contributing to mechanical properties include covalent bonding, crystallization, and molecular secondary forces such as hydrogen bonding, molecular entanglements, and hydrophobic interactions. Collectively, these interactions convert precursor sol to hydrogel through assembling 3D crosslinked networks. Depending on the mechanisms of network formation, the viscoelastic behaviors of gel systems vary greatly. At gel point, a 3D network has not fully developed since there are many non-interacting segments such as dangling ends, stray chains, and loops existing within the hydrogel system. These domains are relatively mobile within the developing hydrogel, and hence, less effective elastically.28 The viscous behavior of a gelling system, as manifested by G″, is attributable to these non-interacting segments. Proceeding of gelation results in establishment of more networks and consequently, G′ rapidly exceeds G″ (t > tgel), where solid-like behavior dominates the viscoelastic properties of the hydrogel formed. Finally, when the interaction between Odex and CEC approaches completion, both G′ and G″ level off, suggesting formation of a well-developed network in the hydrogel.
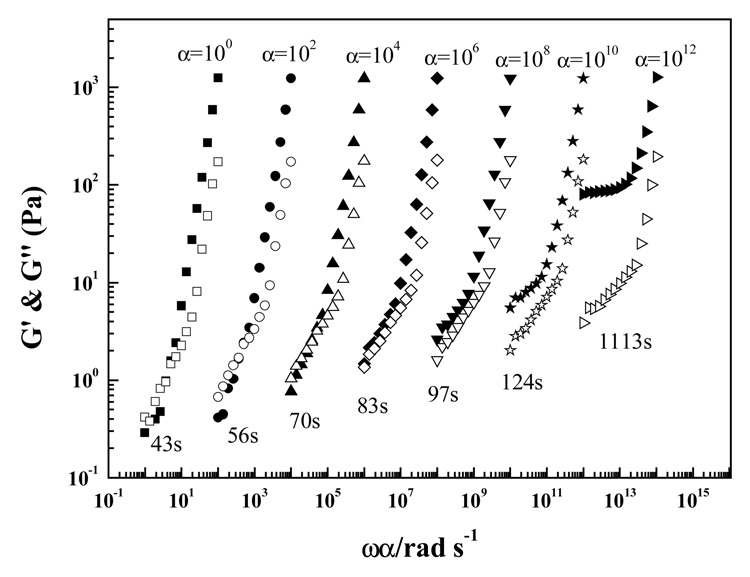
Figure 3 showed the frequency dependence of G′ and G″ at different time intervals for an Odex/CEC (2% concentration, in a ratio of 5:5) formulation at 37 °C under a shear strain of 2%. Due to unstable results of G′ and G″ at lower frequencies, the sweeping had to be performed in the range of 1–100 rad/s. Evidently, there were two regimes in the evolutions of G′ and G″ with angular frequency: 1–10 rad/s and 10–100 rad/s. With ω = 10 rad/s as the turning point, G′ in both 1–10 rad/s and 10–100 rad/s assumed a linear relationship with angular frequency. At t = 43 s, in the range of 1–10 rad/s G′ increased with frequency in a slope of approximately 1.8, indicating a classic liquid behavior.20 In addition, G′ and G″ increased, respectively, with both time and frequency in the range of 1–10 rad/s. At the extended time span of t = 1113 s, G′ became frequency independent, signifying formation of an elastic hydrogel. Similar behavior was also confirmed for G″ (i.e., increased rapidly with time and frequency) at the range of 1–10 rad/s. Compared to the value at 43 s, G″ at 1113 s became less frequency-dependent, which was different from the trend of G′.
Figure 3.
Frequency (ω) dependence of storage (G′, filled symbols) and loss modulus (G″, unfilled symbols) for a 2% Odex/CEC (ratio 5:5) solution at varying time loops during gelation at 37 °C. For clarity, data are shifted along the X-axis with shift factor α = 100, 102, 104, 106, 108, 1010, and 1012, respectively.
According to Winter-Chambon criterion, the gel point of a system is defined as the instant of time when the moduli scale in:
| (1) |
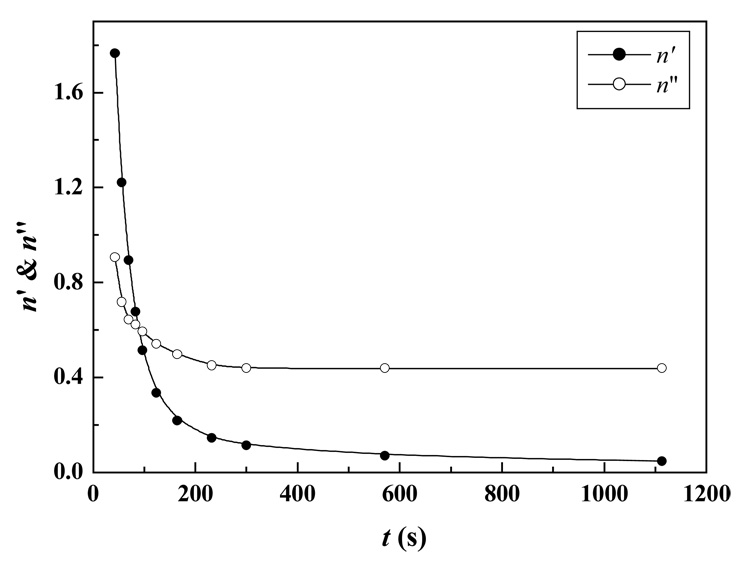
where n is the relaxation exponent closely related the gelation system’s microstructural parameters.29,30 Here we selected the data in the range of 1–10 rad/s for fitting because both G′ and G″ at low frequency were of greater importance for the present method in gel point determination. Theoretically, longer experimental time was necessary to obtain satisfactory data for G′ and G″ at low frequency; however, swift gelation of Odex/CEC sample (less than 2 min) dictated that the lowest frequency for reasonably measuring both G′ and G″ was higher than 1 rad/s. Moreover, G′ and G″ of all curves were of similar value at the high frequency region (ω at 10 – 100 rad/s), indicating that the gelation proceeding only slightly affected G′ and G″ at this region. Thus, it was reasonable to fit the data in the range of 1 to 10 rad/s (ω) in this study. From the G′ and G″ data shown in Figure 3, variations of G′ and G″ in the shear frequency range of 1 to 10 rad/s were in accordance with the power law shown in Eq 1. It was apparent that the exponents of n′ and n″ were gelation time dependent. Figure 4 depicted the time dependence of n′ and n″ for a formulation of Odex/CEC (2%, in a ratio of 5:5) solutions at 37 °C. Both n′ and n″ decreased exponentially with increase of gelation time. The two plots intersected at t ≈ 88 s (the gel point) with n′ and n″ = 0.61, which was in strong agreement with the value predicted from the percolation theory (n ~ 2/3).31 The value of n′ and n″ at the gel point obtained was slightly higher than that of the gel point at n′ and n″ = 0.58 obtained by Otaigbe et al while investigating the rheokinetics of thermal induced gelation of a 40 wt% waterborne polyurethane dispersions at 70 °C.20
Figure 4.
Time (t) dependence of exponents n′ and n″ obtained from fitting the frequency dependence data of G′ and G″ for a 2 % Odex/CEC (ratio 5:5) solutions at 37°C according to eq 1.
Effect of temperature
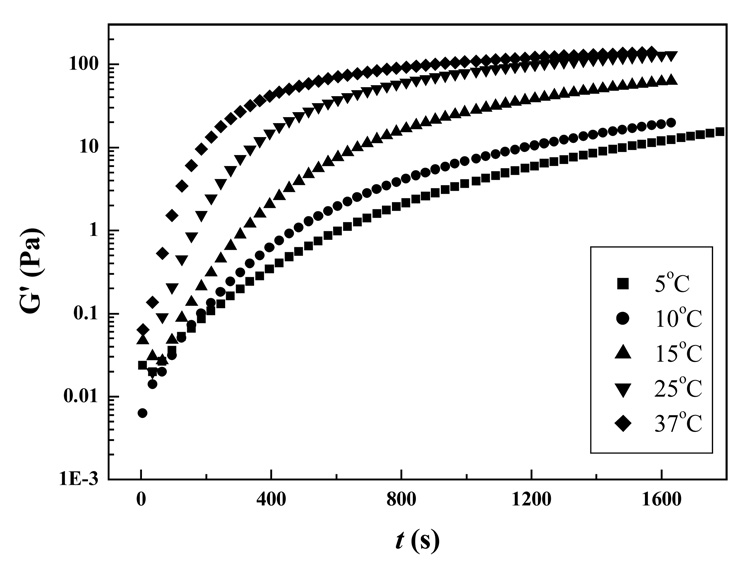
The time required for gelation of Odex/CEC solution was temperature dependent and Figure 5 depicted this effect in the range of 5–37 °C on a typical Odex/CEC formulation (concentration 2%, 5:5 ratio). In general, G′ showed a rapid buildup regime followed by a plateau regime; this fast buildup of G′ was attributable to 3D crosslink networks formation. Elevation in temperature accelerated molecular motion and thus, increased the chance of inter-or intra-molecular interactions, such as association or reaction leading to significant acceleration of gelation. Likewise, it took considerably longer for G′ to reach its equilibrium state in the lower temperature range. It should be noted that the plateaued G′ at 25 °C was comparable to that of at 37 °C, suggesting that given time, G′ s at 5, 10, and 15 °C could reach the same final value as their counterpart at 37 °C when development of 3D network reached completion. Existence of an energy barrier was hypothesized in the Odex/CEC solution to explain the temperature dependent gelation phenomenon. The energy barrier, apparent activation energy (Ea), was calculated according to Arrhenius equation,32
| (2) |
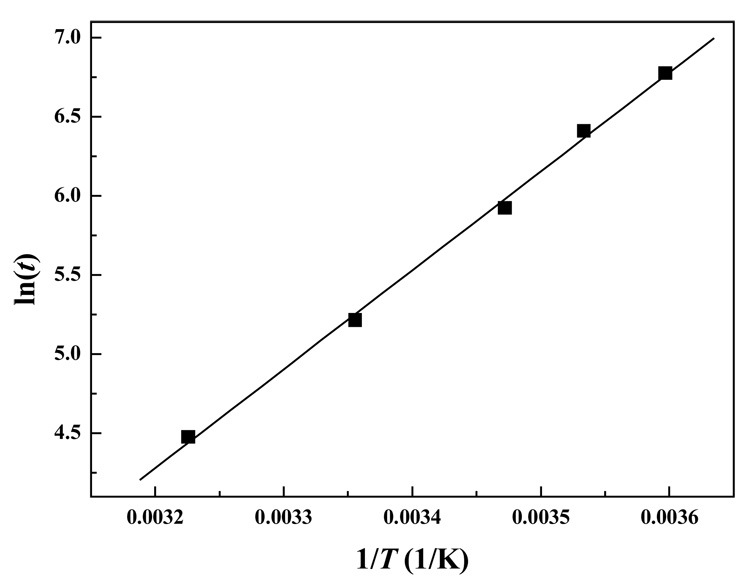
where tgel is the gelation time determined, T is temperature in Kelvin, R is the gas constant 8.314 J·mol−1·K−1, and K is a constant. Figure 6 depicted the semi-logarithmic plot of gel time as a function of inverse reaction temperature. The linear relationship suggested that the underlying gelation mechanism was not affected by change of reaction temperature. As determined from the slope of the linear plot, the apparent activation energy of the Odex/CEC reaction was 51.9 ± 2 kJ/mol, which was in the range of the chitosan-scheroglucan reaction (43.3 kJ/mol),33 chitosan-glutaraldehyde reaction (63.1 kJ/mol),34 and N-acetylchitosan (68 kJ/mol);35 but considerably lower than that of oxidized schizophyllan-gelatin reaction (100.4 kJ/mol)36 and chitosan/blocked-diisocyanate reaction (103 kJ/mol).24 The gelation time of Odex/CEC (concentration 2%, 5:5 ratio) at any given temperature could be predicted with the aid of Ea.
Figure 5.
Storage modulus (G′) as a function of time (t) for 2% Odex/CEC (ratio 5:5) at different temperature.
Figure 6.
Arrhenius plot for a 2% Odex/CEC (ratio 5:5) reaction. The line represents a linear fit for the data points.
Effect of polymer concentration
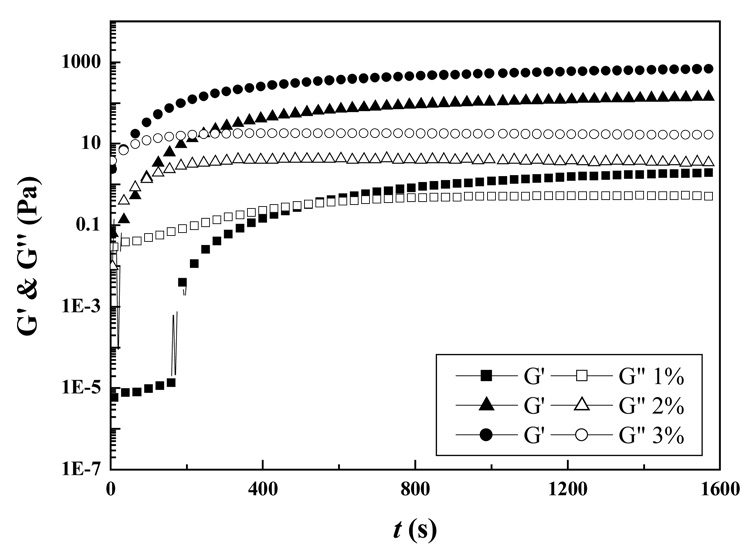
The relationship between gelation time and Odex/CEC (ratio 5:5) concentration at 37 °C was depicted in Figure 7. For 1% Odex/CEC solution, the gelation time appeared to decrease with an increase in the polymer concentration; with three apparently distinct regimes for the evolution of G′. First, a transient plateau was observed at t < 200 s (regime I), where G′ was considerably smaller than G″ (building up at a moderate rate), suggesting the occurrence of molecular interactions between Odex and CEC. Second, G′ increased abruptly between t ≥ 200 s and t < 900 s (regime II), and G′ exceeded G″ attributable to formation of elastic 3D networks; it should be noted that the gel point was situated in this regime. The higher the Odex/CEC concentration, the shorter the gelation time. Finally, G′ and G″ both leveled off when approaching completion of gelation (regime III). Nonetheless, only the latter two regimes were observed for both 2% and 3% Odex/CEC solutions. At t < 300 s (regime II), both G′ and G″ increased rapidly with time, and G′ surpassed G″; subsequently, both G′ and G″ leveled off (regime III). The value of G′ in regime III increased with an increase in polymer concentration from 1% to 3%. A high Odex/CEC concentration increased the probability of encounter of functional groups on polymer chains in confined space, and thus increased the chances of reaction among these groups. Chemical crosslinkings started to develop while physical crosslinkings occurred simultaneously. Dependence of physical crosslinking on polymer concentration was mostly due to secondary forces such as hydrogen bonding and chain entanglements that could further reinforce the network architecture formed by chemical crosslinking. A higher degree of physical crosslinking would reduce the average molecular weight between crosslinking point and thus, a rise of G′ at low temperatures.37 For 2% and 3% solutions, the concentrations were sufficient to enable extensive chemical crosslinking as well as hydrogen bonding among polymer chains and thus, rapid enhancement of G′; the slow increasing regime G′ (i.e., regime I) could not be detected within the time range of the experiments performed.
Figure 7.
Influence of polymer concentration on the gelation behavior of Odex/CEC (ratio 5:5) at 37 °C.
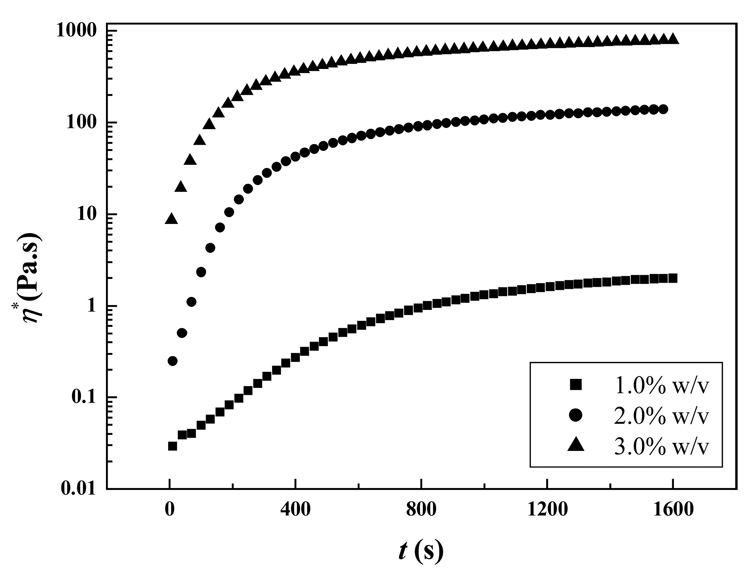
Amongst all material viscoelasic functions, complex viscosity (η*) is very sensitive to structural changes during gelation and fractal polymer gel formation.31 Figure 8 depicted the time dependence of η* of Odex/CEC solutions of 1%, 2%, and 3% (w/v) at ω = 1 Hz under 37 °C. Similar to the behavior of G′ with time and polymer concentration, η* increased with gelation time in conjunction with long time-spans for leveling-off (i.e., approaching equilibrium value). Within the time range of 0–1600 s, the peak η* was boosted from approximately 100 to 103 Pa·s when the corresponding polymer concentration increased from 1% to 3%, indicating the formation of a more viscous hydrogel at higher polymer concentration.
Figure 8.
Evolution of complex viscosity (η*) with time (t) at 37 °C during the gelation process for Odex/CEC solutions of different concentrations: 1%, 2%, and 3%, respectively.
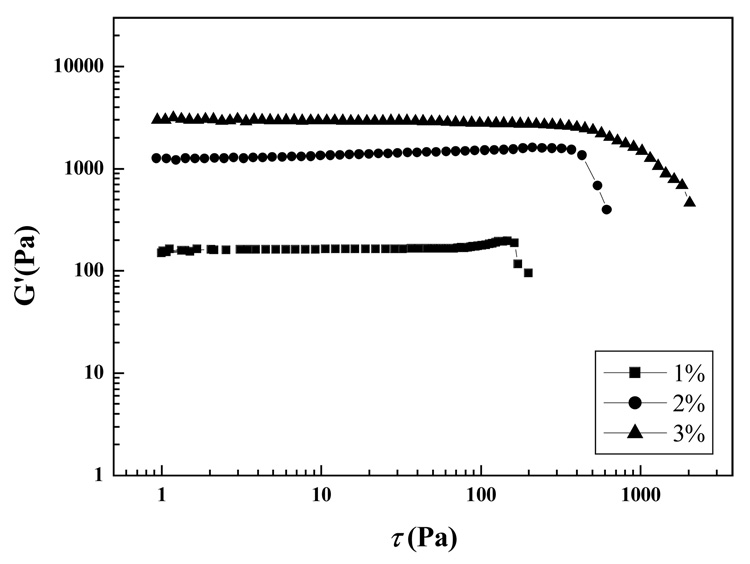
Figure 9 depicted the oscillation shear stress profiles of 1%, 2%, and 3% Odex/CEC (ratio 5:5) hydrogels cured for 12 h. The G′ of hydrogel increased with an increase in the polymer concentration from 1% to 3%., which was predicted by the results shown in Figure 7. Maximum G′ (plateau) of the gelation systems were enhanced with increase in polymer concentration. G′ of the three systems shown in Figure 9 were, respectively, higher than their counterparts shown in Figure 7, indicating the reactions of the latter systems did not reach completion. The LVR, where G′ was independent of applied shear stress, elevated with an increase in polymer concentration (accordingly, the crosslinking density). Breakdown of hydrogel structures, denoted by a rapid decline of G′ , marked the end of LVR at the critical shear stress.38 Compared to 1% (160 Pa) and 2% Odex/CEC hydrogels (370 Pa), 3% Odex/CEC hydrogel exhibited the highest critical shear stress of 580 Pa.
Figure 9.
Dependence of storage modulus (G′) on the shear stress (τ) for the Odex/CEC hydrogels of different concentrations at 37 °C.
Effect of Odex/CEC ratio
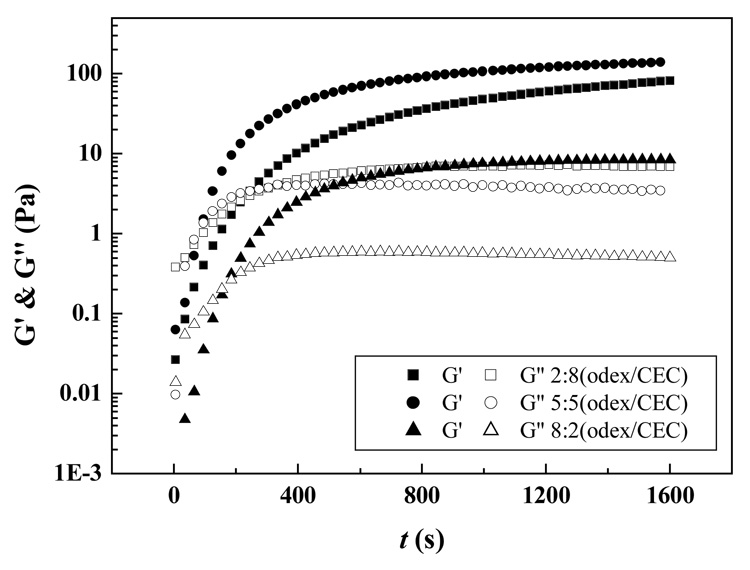
Chemical crosslinking efficiency is closely related to the relative amount of aldehyde groups on Odex versus amino groups on CEC; thus, the ratio of Odex to CEC in the solution is another controlling parameter for gelation. Figure 10 depicted the time dependence of G′ and G″ for the Odex/CEC at various ratios. Gelation rate was in the sequence of 5:5 (Odex:CEC) > 8:2 (Odex:CEC) > 2:8 (Odex:CEC), while plateau G′ was in the sequence of 5:5 (Odex:CEC) > 2:8 (Odex:CEC) > 8:2 (Odex:CEC). Both the gelation rate and mechanical properties of the gel network formed echoed the crosslinking density in the gelation systems. For a 5:5 (Odex/CEC) solution, the calculated [CHO]/[NH2] ratio was approximately 1:1, indicating among the three Odex/CEC ratios studied, the highest crosslinking efficiency was achieved at 5:5 (Odex/CEC), yielding a hydrogel with the shortest gelation time and highest storage modulus. Consequently, either CHO groups or NH2 groups were in relatively excess in the other two formulations. It should be noted that the viscosity of a 2% CEC solution was substantially higher than that of a 2% Odex solution (data not shown). Since there were either approximately 60% excess of CHO groups or NH2 groups, Odex:CEC (2:8) containing a higher proportion of the more viscous CEC solution, possessed a greater plateau G′ as compared to its counterpart formulated from Odex:CEC (8:2). Compared with the latter, the higher viscosity of Odex:CEC (2:8), limited the mobility of functional group bearing polymer chains within the solution, hindering their interaction and consequently contributing to the differential storage modulus.
Figure 10.
Time dependence of storage modulus (G′) and loss modulus (G″) for Odex/CEC solutions at different ratios at 37 °C.
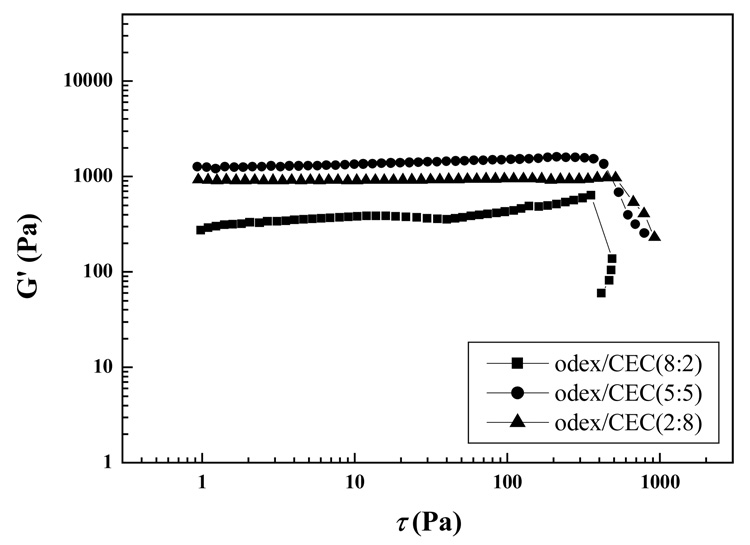
Dependence of G′ of Odex/CEC hydrogels in different ratios on the shear stress was depicted in Figure 11. With the highest crosslinking density, the Odex/CEC hydrogel with a ratio of 5:5 had the highest G′, however, higher crosslinking density could result in brittleness of the hydrogel as well. Although the Odex/CEC hydrogel in a ratio of 5:5 possessed a higher G′, its fracture shear stress was moderately lower when compared with the Odex/CEC hydrogel in a ratio of 2:8. Since Odex/CEC hydrogel in a ratio of 8:2 had excessive Odex, and thus, a low viscosity, its G′ was considerably lower than those of the other two formulations (5:5 and 2:8).
Figure 11.
Dependence of storage modulus (G′) on the shear stress (τ) for Odex/CEC hydrogels at different ratios at 37 °C.
Nonisothermal gelation
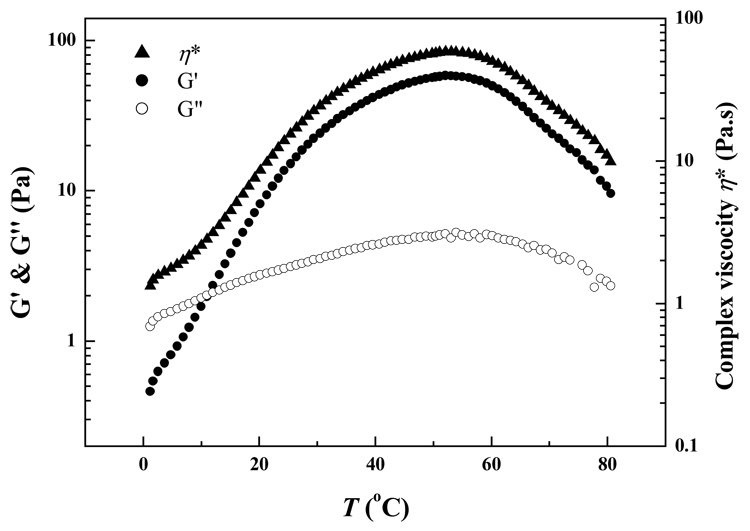
The gelation behaviors of Odex/CEC systems at different energy states were investigated. A 2% Odex/CEC solution in a ratio of 5:5 was subjected to temperature ramping. An initial temperature of 1 °C for thermal scanning was chosen due to its proximity to the refrigeration temperature. The temperature ranged from 1 °C to 80 °C and a heating rate of 2 °C/min was utilized. As shown in Figure 12, G′, G″ and η* displayed parabolic profiles with elevation of temperature. It should be noted that no gelation occurred between CEC and unmodified dextran (results not shown), implicating chemical crosslinking played a key role in the gelation of Odex/CEC in addition to physical crosslinking. At the temperature range of 1–10 °C, G′, G″ and η* increased steadily with temperature, where polymer chain branching caused by chemical crosslinking in conjunction with physical association such as chain entanglements and hydrogen bonding (see Scheme 1), dominated the elastic modulus increase in the Odex/CEC system. Beyond 10 °C with increase of temperature up to 55 °C, rapid development of 3D chemical crosslink network resulted in considerable increase of both G′ and η*; whereas, G″ increased in a moderate rate, leading to a crossover thereby signifying the beginning of gelation. Theoretically, hydrogel network should be entropic;39 however, beyond 55 °C, all three functions declined, particularly, G′ and η*. Moreover, hydrogen bonding in the systems could be incrementally disrupted when the temperature approached 60 °C, leading to a partial breakdown of the 3D network. Nonetheless, the chemical crosslinks remained largely intact, and the system still showed an elasticity dominated rheological behavior, where the hydrogel network was enthalpic in nature.39 Lastly, the decrease in G′, G″ and η* could be due to rapid gelation at a high temperature such that synereses occurred.36
Figure 12.
Dynamic shear viscosity (η*), dynamic storage modulus (G′) and loss modulus (G″) as a function of temperature (T) at a heating rate of 2 °C/min and a shear frequency of 1 rad/s.
Conclusions
The rheological properties of a series of Odex/CEC solutions during gelation were studied. Oscillatory time, temperature and frequency sweeps were performed to evaluate the effect of initial polymer concentrations, polymer ratios and temperatures on gelation kinetics. The viscoelastic material functions G′, G″ and η* of the Odex/CEC solutions showed significant change near the gel points. The gel point could either be estimated from crossing over of G′(t) and G″(t), or by frequency sweeping according to Winter-Chambon power law (i.e. G′(ω) ~ G″(ω) ~ ωn ). The power law exponents at the gel point n′ = n″=0.61, was in excellent agreement with the value predicted by percolation theory (n ~ 2/3). An increase in polymer concentration resulted in a corresponding increase of interacting functional groups per finite volume of solution, leading to decrease of gelation time. Temperature was another key factor influencing gelation kinetics; during isothermal gelation, Odex/CEC solution exhibited great reduction in gelation time with elevation of temperature. In addition, the results of non-isothermal kinetic study showed that both Schiff base formation and physical interactions such as hydrogen bonding contributed to the viscoelastic properties of the hydrogel.
Both chitosan and dextran’s biocompatibility and biodegradability in concert with the favorable gelation properties of the Odex/CEC solution suggest that this system has great potential for biomedical applications. Its rapid gelation time can be modulated by varying either the concentration or the ratio of the components rendering it quite suitable for designing in situ injectable systems. A significant implication is that the mechanical properties of the hydrogel formed could be tailored to closely match the mechanical properties of the biological tissues of interest. Moreover, at ambient temperature, this binary system is very easy to prepare and it is also completely devoid of small molecule crosslinker and cumbersome manipulations such as temperature control, photo-initiation, incubation, etc. Lastly, for site-specific controlled release drug delivery, the abundance of functional groups on both Odex and CEC could act as binding sites to provide a facile mechanism for modulating drug release kinetics. These investigations are underway.
Acknowledgement
Funding of this study by the National Institutes of Health (DK068401, WC) is gratefully acknowledged. The authors would like to thank Mr. Christopher Judd of Engelhard, Inc. for his assistance in HPLC analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Crescenzi V, Francescangeli A, Taglienti A, Capitani D, Mannina L. Biomacromolecules. 2003;4:1045–1054. doi: 10.1021/bm0340669. [DOI] [PubMed] [Google Scholar]
- 2.Noble L, Gray AI, Sadiq L, Uchegbu IF. Int. J. Pharm. 1999;192:173–182. doi: 10.1016/s0378-5173(99)00306-3. [DOI] [PubMed] [Google Scholar]
- 3.Ravi MNVK. Reactive & Functional Polymers. 2000;46:1–27. [Google Scholar]
- 4.Lee KY, Kwon IC, Kim YH, Jo WH, Jeong SY. J. Controlled Release. 1998;51:213–220. doi: 10.1016/s0168-3659(97)00173-9. [DOI] [PubMed] [Google Scholar]
- 5.Montembaulta A, Vitonb C, Domard A. Biomaterials. 2005;26:1633–1643. doi: 10.1016/j.biomaterials.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Welsh ER, Schauer CL, Qadri SB, Price RR. Biomacromolecules. 2002;3:1370–1374. doi: 10.1021/bm025625z. [DOI] [PubMed] [Google Scholar]
- 7.Felix L, Hernandez J, Arguelles-Monal WM, Goycoolea FM. Biomacromolecules. 2005;6:2408–2415. doi: 10.1021/bm0501297. [DOI] [PubMed] [Google Scholar]
- 8.Yalpani M, Hall LD. Macromolecules. 1984;17:272–281. [Google Scholar]
- 9.Qu X, Wirsen A, Albertsson AC. J. Appl. Polym. Sci. 1999;74:3193–3202. [Google Scholar]
- 10.Jiang H, Wang Y, Huang Q, Li Y, Xu C, Zhu K, Chen W. Macromol. Biosci. 2005;5:1226–1233. doi: 10.1002/mabi.200500126. [DOI] [PubMed] [Google Scholar]
- 11.Cana HK, Denizlia BK, Gunera A, Rzaev ZMO. Carbohydr. Polym. 2005;59:51–56. [Google Scholar]
- 12.Nowakowska M, Zapotoczny S, Sterzel M, Kot E. Biomacromolecules. 2004;5:1009–1014. doi: 10.1021/bm034506w. [DOI] [PubMed] [Google Scholar]
- 13.Mehvar R. J. Controlled Release. 2000;69:1. doi: 10.1016/s0168-3659(00)00302-3. [DOI] [PubMed] [Google Scholar]
- 14.Kumashiro Y, Huh KM, Ooya T, Yui N. Biomacromolecules. 2004;5:1009–1014. doi: 10.1021/bm015527y. [DOI] [PubMed] [Google Scholar]
- 15.Ferdous A, Akaike T, Maruyama A. Biomacromolecules. 2000;1:186–193. doi: 10.1021/bm9900141. [DOI] [PubMed] [Google Scholar]
- 16.Trudela J, Massia SP. Biomaterials. 2002;23:3299–3307. doi: 10.1016/s0142-9612(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 17.Ferdous A, Akaike T, Maruyama A. Macromolecules. 2004;37:3239–3248. [Google Scholar]
- 18.Draye J, Delaey B, Voorde AV, Bulcke AVD, Bogdanov B, Schacht E. Biomaterials. 1998;19:99–107. doi: 10.1016/s0142-9612(97)00164-6. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh K, Shu XZ, Mou R, Lombardi J, Prestwich GD, Rafailovich MH, Clark RAF. Biomacromolecules. 2005;6:2857–2865. doi: 10.1021/bm050361c. [DOI] [PubMed] [Google Scholar]
- 20.Madbouly SA, Otaigbe JU. Macromolecules. 2005;38:10178–10184. [Google Scholar]
- 21.Rwei SP, Chen TY, Cheng YY. J. Biomater. Sci., Polym. Ed. 2005;16:1433–1445. doi: 10.1163/156856205774472290. [DOI] [PubMed] [Google Scholar]
- 22.Rinaudo M, Auzely R, Vallin C, Mullagaliev I. Biomacromolecules. 2005;6:2396–2407. doi: 10.1021/bm0580025. [DOI] [PubMed] [Google Scholar]
- 23.Boucard N, Viton C, Domard A. Biomacromolecules. 2005;6:3227–3237. doi: 10.1021/bm050653d. [DOI] [PubMed] [Google Scholar]
- 24.Lin-Gibson S, Walls HJ, Kennedy SB, Welsh ER. Carbohydr. Polym. 2003;54:193–199. [Google Scholar]
- 25.Maia J, Ferreirab L, Carvalhoc R, Ramosd MA, Gil MH. Polymer. 2005;46:9604–9614. [Google Scholar]
- 26.Sahiner N, Singh M, Kee DD, John VT, McPherson GL. Polymer. 2006;47:1124–1131. [Google Scholar]
- 27.Chambon F, Winter HH. J Rheol. 1987;31:683–697. [Google Scholar]
- 28.Taylor C, Allen A, Dettmar PW, Pearson JP. Biomacromolecules. 2003;4:922–927. doi: 10.1021/bm025767t. [DOI] [PubMed] [Google Scholar]
- 29.Winter HH. Encyclopedia of Polymer Science and Engineering. New York: John Wiley & Sons; 1989. [Google Scholar]
- 30.Winter HH, Chambon F. J. Rheol. 1986;30:367–382. [Google Scholar]
- 31.Takenaka M, Kobayashi T, Hashimoto T, Takahashi M. Phys. Review E. 2002;65 doi: 10.1103/PhysRevE.65.041401. 041401. [DOI] [PubMed] [Google Scholar]
- 32.Madbouly SA, Otaigbe JU. Macromolecules. 2006;39:4144–4151. [Google Scholar]
- 33.Guo B, Elgsaeter A, Stokke BT. Polym. Gels Networks. 1998;6:113–135. [Google Scholar]
- 34.Roberts GAF, Taylor KE. Chitosan Gels. 3. Makromol. Chem.—Macromol. Chem. and Phys. 1989;190:951–960. [Google Scholar]
- 35.Moore GK, Roberts GAF. Inter. J. Biol. Macromol. 1980;2:78–80. [Google Scholar]
- 36.Fang Y, Takahashi R, Nishinari K. Biomacromolecules. 2005;6:3202–3208. doi: 10.1021/bm0505383. [DOI] [PubMed] [Google Scholar]
- 37.Bulcke AIVD, Bogdanov B, Rooze ND, Schacht EH, Cornelissen M, Berghmans H. Biomacromolecules. 2000;1:31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 38.Rudraraju VS, Wyandt CM. Inter. J. Pharm. 2005;292:63–73. doi: 10.1016/j.ijpharm.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Nishinari K, Watase M, Ogino K. Macromol. Chem. Phys. 1984;185:2663. [Google Scholar]