Abstract
Elevations in intrarenal angiotensin II (Ang II) cause reductions in renal function and sodium excretion that contribute to progressive hypertension and lead to renal and vascular injury. Augmentation of intrarenal Ang II occurs by several processes, leading to levels much greater than can be explained from the circulating levels. In Ang II-dependent hypertension, Ang II is internalized via an AT1 receptor mechanism, but there is also sustained intrarenal production of Ang II. Ang II exerts a positive feedback action on intrarenal angiotensinogen (AGT) mRNA and protein. The increased intrarenal AGT production is associated with increased intrarenal and intracellular Ang II contents and urinary AGT excretion rates. The increased urinary AGT indicates spillover of AGT into distal nephron segments supporting enhanced distal Ang II formation and sodium reabsorption. The augmentation of intrarenal Ang II provides the basis for sustained actions on renal function, sodium excretion, and maintenance of hypertension.
Introduction
There has been a paradigm shift in recent years from a focus primarily on the role of the systemic renin-angiotensin system (RAS) in the regulation of arterial pressure and in the pathophysiology of hypertension, to an emphasis on the changes in the components of the RAS at the tissue level in various organs. Emphasis in this article is on the renal RAS because of its unique significance in regulating sodium balance and thus long-term arterial pressure [1], and its role in the progression of renal diseases [2••]. Indeed, there is growing recognition that inappropriate activation of the intrarenal RAS limits the capability of the kidney to maintain sodium balance at normal arterial pressures and is an important cause of hypertension [1,3•,4,5]. In addition to sodium and fluid retention and progressive hypertension, there are critical long-term consequences of an inappropriately elevated RAS coexisting with hypertension, which lead to proliferative responses and vascular, glomerular, and tubular interstitial injury, and fibrosis [2••,3•,6,7•,8-11].
Regardless of the mechanism responsible for an over-active intrarenal RAS, the consequent impairment in sodium excretory capability contributes to the development of hypertension [1,4]. Varying degrees of reduced renal function have been found in many hypertensive patients associated with inappropriate activation of the RAS as reflected by their responsiveness to angiotensin-converting enzyme (ACE) inhibitors or angiotensin II (Ang II) receptor blockers, even when plasma renin levels are not elevated [12•,13-16]. The important role of the RAS is also supported by studies using various experimental models of hypertension having an overactive RAS. Specific examples include 2 kidney, 1 clip (2K1C) Gold-blatt hypertension [17,18,19•,20], Ang II-infused hypertension [4], the transgenic rat (TGR) (mRen2) model harboring an extra renin gene in its genome [21], the remnant kidney model produced by unilateral nephrectomy plus ligation of arterial branches in the remaining kidney [22,23], and several mouse models that overexpress renin or angiotensinogen (AGT) [24-26].
Intrarenal Angiotensin II Receptors
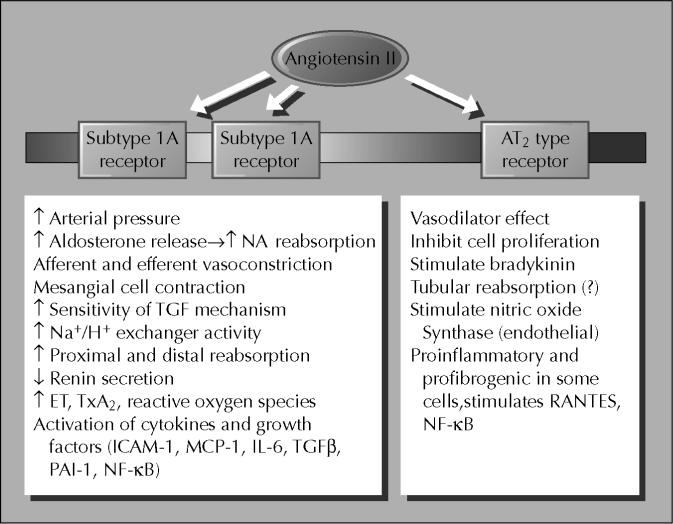
The AT1 receptor is the predominant Ang II receptor in the kidneys of adults and is widely distributed in essentially all regions and cell types, leading to a large number of directly mediated actions or activation by other paracrine systems [27]. AT1 receptor protein has been localized to vascular smooth muscle cells throughout the renal vasculature, including both afferent and efferent arterioles, mesangial cells and vasa recta, proximal tubule brush border and basolateral membranes, thick ascending limb epithelia, distal tubule cells, collecting duct cells, glomerular podocytes, and macula densa cells [27-34]. Importantly, there is extensive luminal localization of AT1 receptors in both proximal and distal nephron segments. In rodents, the AT1A is the predominant subtype, but AT1A and AT1B receptor mRNAs have been demonstrated in the glomerulus and in all nephron segments including the proximal tubule, distal tubule, thick ascending limb, and collecting ducts [30,34,35]. Some of the major renal actions reported for Ang II acting on AT1 and AT2 receptors are depicted in Figure 1. The role of AT2 receptors in mediating vascular injury remains controversial. Some investigators have demonstrated that AT2 receptors may contribute to pathobiology of vascular cells rather than being protective as is generally stated [2••,11].
Figure 1.
Angiotensin II receptor subtypes and multiple renal actions. ET—endothelin; ICAM-1—intracellular adhesion molecule-1; IL-6—interleukin-6; MCP-1—monocyte chemoattractant protein-1; NF—nuclear factor; PAI-1—platelet activator inhibitor-1; TGF-β—transforming growth factor-β; TxA2–thromboxane A2.
The regulation of intrarenal Ang II receptors in hypertensive models is complex since vascular and tubular receptors respond differently during high Ang II states [27]. In general, high Ang II levels associated with a low-salt diet decrease glomerular AT1 receptor expression but increase tubular AT1 receptor levels [36]. Studies in 2K1C Goldblatt hypertensive rats demonstrated that glomerular AT1 receptors were decreased by 2 weeks after clipping, but vascular receptors were not decreased until 16 weeks [37]. However, glomerular AT1 receptor density was not increased in the 1K1C model, although vascular AT1 receptor density was increased [38].
In the Ang II-infused model of hypertension, total kidney AT1 mRNA levels and receptor protein were not significantly altered by 2 weeks of Ang II infusion sufficient to cause marked hypertension [39]. However, Wang et al. [29] reported that AT1A receptor protein was reduced in ischemic and contralateral kidneys of 2K1C Goldblatt and 2 kidney, 1 wrap hypertensive models and in kidneys of Ang II-infused rats. AT2 receptors were downregulated only in ischemic kidneys. In the TGR (mRen2) harboring the mouse renin gene, Zhuo et al. [40] found increased AT1 receptor binding in vascular smooth muscle of afferent and efferent arterioles, juxtaglomerular apparatus, glomerular mesangial cells, proximal tubular cells, and renomedullary interstitial cells. It was suggested that upregulation of AT1 receptors in multiple renal cells may contribute to the pathogenesis of hypertension in these rats. Harrison-Bernard et al. [41] extended the analysis in Ang II-infused rats with in vitro autoradiography and showed differential responses with significant decreases in glomeruli and inner stripe but not in proximal tubules. Furthermore, ACE binding was significantly increased in proximal tubules of Ang II-infused rats. Thus, the data suggest that the vascular and glomerular AT1 receptors are downregulated, but the proximal tubular receptors are either upregulated or not significantly altered in Ang II-dependent hypertension.
Intrarenal Levels of Angiotensin II
In response to variations in dietary sodium intake, total renal renin, Ang I, and Ang II levels respond in a coordinated manner [27,42-44]. In addition to intrarenal conversion of Ang II from systemically delivered Ang I, intrarenal Ang II is also derived from intrarenally formed Ang I originating from either circulating or locally formed AGT. Indeed, van Kats et al. [45•] found that under normal conditions most of the intrarenal Ang II and Ang I was derived from local production. They infused labeled Ang I and Ang II systemically into pigs and compared the tissue contents of labeled and endogenous peptides produced from locally generated Ang I. They also reported that renal tissue Ang II was not significantly reduced by treatment with an ACE inhibitor, suggesting the presence of alternative Ang II-forming pathways. However, studies in rats by Komine et al. [46] showed that chronic treatment with an ACE inhibitor, an AT1 receptor blocker, and the combination of an ACE inhibitor as well as an AT1 receptor blocker lowered renal tissue Ang II contents in rats maintained on a low-salt diet to stimulate the RAS. They found that the combination lowered renal Ang II content to a greater extent than either treatment alone.
Although under normal physiologic conditions plasma and kidney renin activities change in concert with plasma and kidney Ang I and Ang II levels [42,43], these relationships can be disrupted in some forms of hypertension [5]. This has been shown in 2K1C Goldblatt hypertension, Ang II-induced hypertension, and TGR (mRen2) hypertensive models [5,19•]. The net intrarenal Ang II content is due to formation of intrarenal Ang II as well as sequestration of Ang II from the circulation via an AT1 receptor–mediated process [5,47,48•]. This is particularly apparent when there are sustained elevations in circulating Ang II, which cause progressive accumulation of intrarenal Ang II levels even under conditions of marked suppression of renin formation and release such as occurs in the nonclipped kidney of 2K1C Goldblatt hypertensive rats [18], Ang II-infused hypertensive rats [49•], and TGR (mRen2) [50]. The remnant kidney model of hypertension has elevated renin and intrarenal Ang II levels primarily in the peri-infarct borders [22]. It is important to emphasize that much of the increase in intrarenal Ang II that occurs during chronic Ang II infusion is due to an AT1 receptor– dependent process since it can be prevented by concomitant treatment with AT1 receptor blockers, suggesting receptor-mediated endocytosis [47,49•]. As recently shown by Ingert et al. [48•], uptake of Ang II via AT1 receptors also contributes to the increase in renal Ang II levels that occur with low-salt intake.
A recent study by Tokuyama et al. [19•] provides further support to the important role of locally formed intrarenal Ang II in hypertension models. Using a dog 2K1C Goldblatt hypertensive model, they demonstrated significant increases in intrarenal Ang II in both the clipped and nonclipped kidneys. However, ACE activity was increased only in the nonclipped kidney, but not in the clipped kidney. Interestingly, the elevated Ang II in the clipped kidney was suppressed by a chymase inhibitor, suggesting a role for chymase in Ang II formation in the clipped kidney. This study demonstrated that Ang II levels are elevated in both clipped and nonclipped kidneys, however, the mechanisms for Ang II generation may differ. These findings were extended in a recent paper by Sadjadi et al. [20] who found that intrarenal Ang II levels were increased in both the clipped and unclipped kidneys of 2K1C Goldblatt hypertensive rats, not only during the development phase at 1 week, but also up to 12 weeks following unilateral constriction. Furthermore, adrenal Ang II content was also increased during chronic renal vascular hypertension. These increases in nonclipped kidney and adrenals persisted even in the absence of significant increases in plasma Ang II. In addition, renal AT1 receptor density was maintained in both clipped and unclipped kidneys during renal vascular hypertension, providing a potential mechanism for the persistence of hypertension despite a lack of increased plasma Ang II concentration.
Interstitial and Tubular Angiotensin II
Intrarenal Ang II is not distributed in a homogenous manner but is compartmentalized in a regional and segmental manner [44]. Earlier studies indicated that medullary Ang II levels are higher than the cortical levels in normal rats and increase further in Ang II-infused hypertensive rats [27]. The combination of high Ang II levels in the medulla coupled with the high density of Ang II receptors suggest that Ang II exerts a major role in regulating hemodynamics and tubular function in the medulla [30,41]. The higher Ang II levels in the medulla suggest that there may be specialized Ang II-forming pathways or accumulation mechanisms in medullary tissues that are subject to local regulation. However, Ingert et al. [43,48•] failed to confirm that medullary Ang II contents are higher than cortical Ang II contents. These authors found that Ang I and Ang II levels in cortex and medulla are equivalent and respond in a similar manner to alterations in dietary salt intake.
Within the cortex, there is distribution of Ang II in the interstitial fluid, tubular fluid, and the intracellular compartments. The interstitial as well as the intratubular compartments contribute to the disproportionately high total Ang II levels. Studies using microdialysis probes implanted in the renal cortex demonstrated that Ang II concentrations in interstitial fluid are much higher than the plasma concentrations, with recent results suggesting values in the range of 3 to 5 pmol/mL [51-54]. Importantly, Nishiyama et al. [53,54] was not able to show substantive suppression of renal interstitial fluid Ang II levels with administration of ACE inhibitors administered either directly into the renal artery or via the microdialysis probe. These studies have suggested that much of the Ang II in the renal interstitial compartment is formed through non–ACE-dependent pathways or by ACE that is not easily accessed by the exogenously administered ACE inhibitors. Increases in renal interstitial fluid Ang II levels have been reported for two models of hypertension. Siragy and Carey [52] found that renal interstitial Ang II is also increased in the wrapped kidney of rats with Grollman hypertension. Nishiyama et al. [55] reported that renal interstitial fluid Ang II concentrations are also increased in rats infused with Ang II for 2 weeks. Because the renal interstitial values are so much higher than can be explained on the basis of equilibration with the plasma concentrations, the data suggest local regulation of Ang II formation in the renal interstitial compartment and an enhancement of interstitial Ang II production in Ang II-dependent hypertension.
Micropuncture studies have shown that proximal tubule fluid concentrations of Ang I and Ang II are also much greater than the plasma concentrations [56]. The finding that fluid samples collected from perfused segments had Ang II concentrations similar to those measured in nonperfused tubules indicates that the proximal tubule secretes Ang II or a precursor into the proximal tubule fluid. Importantly, when proximal tubule fluid was incubated with excess renin, the resultant Ang I generated indicated large amounts of substrate in proximal tubular fluid in the range of 300 pmol/mL. These results indicate that there is abundant AGT in the proximal tubule fluid, suggesting secretion by the proximal tubule cells [5]. In addition to AGT, proximal tubule cells also have renin mRNA that is stimulated by a low-sodium diet that may thus act on AGT to generate Ang I [57]. At present, it is not clear how much of the Ang I and II found in the proximal tubule fluid is formed intracellularly or formed intraluminally from AGT secreted into the tubule.
Measurements of tubular fluid Ang II concentrations in anesthetized rats have not revealed significant differences among control rats and several hypertensive models [18,50,58]. Considering that kidneys of the hypertensive rats are markedly renin depleted and exposed to elevated arterial pressures, the maintenance of high proximal tubular Ang II concentrations reflects an inappropriate maintenance of intrarenal Ang II formation levels. Nevertheless, the results so far have not demonstrated further elevations in proximal tubule Ang II concentrations above the levels found in normal anesthetized rats. In normal rats, volume expansion failed to suppress proximal tubule Ang II concentrations, but increased levels were documented following reductions in renal perfusion pressure [59].
The Ang II concentrations in tubular fluid from the other segments of the nephron remain unknown. Several studies support an important role for Ang II in regulating reabsorptive function in distal nephron and collecting duct segments, as well as in proximal tubule segments, which activate the Ang II receptors on the luminal borders [56,60]. Recently, a direct action of Ang II on the luminal amiloride-sensitive sodium channel was reported [61•]. These data indicate that when luminal distal nephron Ang II concentrations are augmented, they could contribute directly to the regulation of distal tubule and collecting duct sodium reabsorption.
Intracellular Angiotensin II in Hypertension
As indicated earlier, some of the Ang II that binds to receptors is internalized via AT1 receptor–mediated endocytosis [47,48•,62]. Recent studies by Zhuo et al. [49•] provided direct evidence of endosomal accumulation of Ang II in intermicrovillar clefts and endosomes of Ang II-infused hypertensive rats. It was also shown that AT1 receptor blockade with candesartan prevented the ensodomal accumulation, even though plasma Ang II increased further, demonstrating the importance of AT1 receptor–mediated uptake. The presence of Ang II in renal endosomes indicates that some of the internalized Ang II remains intact and contributes to the total Ang II content measured in tissue homogenates [5,42,49•,62,63,64••]. As shown for proximal tubule cells, endocytosis of the Ang II–AT1 receptor complex seems to be required for the full expression of functional responses coupled to the activation of signal transduction pathways [65,66]. In Ang II-dependent hypertension, a higher fraction of the total kidney Ang II is internalized into intracellular endosomes (light endosomes as well as intramicrovillar clefts) via an AT1 receptor–mediated process [49•]. The demonstration that AT1 receptor blockade prevents the augmentation of intrarenal Ang II that occurs during chronic infusions of Ang II suggests a progressive AT1 receptor–mediated accumulation of Ang II into an intracellular compartment, and suggests that some of the internalized Ang II is protected from degradation [48•,49•]. This process can also occur during acute infusions of Ang II [62]. van Kats et al. [62] infused labeled Ang II and showed a six- to sevenfold increase in intrarenal Ang II, which was prevented by an AT1 receptor antagonist.
There are several possible functions of the internalized Ang II. Ang II could be recycled and secreted in order to exert further actions by binding to Ang II receptors on the cell membranes. Ang II may also act on cytosolic receptors to stimulate IP3 as has been described for vascular smooth muscle cells [67]. A particularly intriguing hypothesis is that Ang II migrates to the nucleus to exert genomic effects [64••]. Nuclear binding sites for Ang II in renal cells were recently reported by Licea et al. [68]. The nuclear receptors were primarily of the AT1 subtype since they were displaced by losartan as well as saralasin. Nuclear Ang II receptor density was not altered in Ang II-infused hypertension. Chen et al. [64••] transfected Chinese hamster ovary cells with an AT1A receptor fused with green fluorescent protein (GFP), which allowed visualization of trafficking of the internalized ligand-receptor complex. Ang II increased colocalization of GFP fluorescence with nuclear markers, suggesting migration of the receptor complex to the nucleus [64••]. Because Ang II exerts a positive stimulation on AGT mRNA and protein production, it is possible that the intracellular Ang II may have genomic actions to regulate AGT or renin mRNA expression in proximal tubule cells [5].
Intrarenal Angiotensinogen Production
Most of the intrarenal AGT mRNA and protein have been localized to proximal tubule cells indicating that much of the intratubular Ang II could be derived from locally formed and secreted AGT [56,69-71]. As has been found for liver cells, renal AGT mRNA levels and protein are also stimulated by Ang II, so there is a paradoxical positive amplification mechanism by which local production of substrate is enhanced by its own product, thus helping to maintain or even increase further the production of Ang II [69,70,72]. The AGT produced in proximal tubule cells appears to be secreted directly into the tubular lumen in addition to producing its metabolites intracellularly and secreting them into the tubule lumen [56,71,73•,74-76]. Proximal tubule AGT concentrations in anesthetized rats have been reported in the range of 300 nmol/L, which greatly exceed the free Ang I and Ang II tubular fluid concentrations [77]. Because of its size, it seems unlikely that much of the plasma AGT filters across the glomerular membrane, further supporting the concept that proximal tubule cells secrete AGT directly into the tubule [71,73•, 78,79]. Recent studies infusing human AGT into hypertensive rats indicated that circulating AGT was not detectable in the urine [80]. Thus, most of the AGT in the urine is of renal origin. Formation of Ang I and II in the tubular lumen subsequent to AGT secretion is possible because some renin is filtered. There is also a low-level constitutive renin expression and secretion in proximal tubule cells [57,74,81]. Once Ang I is formed, conversion readily occurs because there are abundant amounts of ACE associated with the proximal tubule brush border. ACE has also been measured in proximal and distal tubular fluid as well as in urine [82]. At present, however, there are no data indicating how much of the peptides are formed intracellularly and how much are formed in the tubule lumen.
The findings that renal interstitial fluid and intratubular concentrations of Ang II are much higher than can be explained by the plasma levels support the concept that these may be due to Ang II formed as a consequence of Ang II-stimulated AGT production. In vivo and in vitro studies have shown that Ang II stimulates AGT mRNA levels in rats and in a murine proximal tubule cell line [70,72]. Kobori et al. [69] demonstrated that there were significant increases in intrarenal AGT protein as well as AGT mRNA levels in response to 2 weeks of Ang II infusion. This positive feedback system may be responsible for sustained or enhanced generation of AGT leading to continued intrarenal production of Ang II under conditions of elevated circulating concentrations of Ang II. It is also likely that the increased intrarenal AGT levels lead to increased secretion of AGT into the tubular fluid [73•,75,83]. Indeed, a portion of the enhanced renal AGT that is secreted into the tubular fluid progresses to the distal nephron segments and is ultimately excreted in the urine [80,83]. This may occur not only for Ang II-induced hypertension, but also for other forms of renal injury associated with stimulation of the RAS.
Intact AGT in urine indicates its presence throughout the nephron and, to the extent that renin and ACE are available along the nephron, substrate availability supports continued Ang I generation and Ang II conversion in distal segments [73•,74,79,84]. Using immunoblotting, Rohrwasser et al. [73•] found that renin was secreted by microdissected arcades of connecting tubule cells, indicating that renin is probably secreted into the distal tubular fluid. When coupled with the findings of AGT in urine, it is likely that some of the proximally formed AGT that is secreted into the tubular fluid flows into the distal nephron allowing intraluminal Ang II formation to continue throughout the length of the nephron with the residual AGT appearing in the urine [73•,79]. Ding et al. [79] demonstrated in mice harboring the gene for human AGT fused to the kidney androgen-protein promoter, that human AGT was localized primarily to proximal tubule cells. They found abundant human AGT in the urine but only slight traces in the systemic circulation. This finding suggests that most of the AGT formed in proximal tubule cells is destined for secretion into the lumen. Rohrwasser et al. [73•] demonstrated luminal localization of AGT in proximal tubular cells in vivo, and showed in monolayer proximal tubule cell cultures that most of the AGT was detected near the apical membrane. They also reported that AGT was detected at low nanomolar concentrations in urine from mice and human volunteers.
Kobori et al. [83] evaluated the changes in urinary AGT excretion rates in Ang II-infused rats maintained on high salt in order to suppress basal levels, and observed an approximately fourfold increase in urinary AGT excretion rates. AGT was measured using both Western blot analysis as well as by radioimmunoassay determination of generated Ang I after incubation with excess renin, thus demonstrating that urinary AGT contained intact active AGT. They extended these results further to show that chronic Ang II infusions to normal rats significantly increased urinary excretion rate of AGT in a time- and dose-dependent manner, which was associated with elevations in kidney Ang II levels [80]. As shown in Figure 2, urinary excretion rate of AGT was closely correlated with kidney Ang II content. It was also shown that urinary AGT was correlated with systolic arterial pressure, but not with plasma Ang II concentration. To determine if the increase in urinary AGT excretion was simply a nonspecific consequence of the proteinuria and hypertension, further studies were done in rats made hypertensive with deoxycorticosterone acetate plus a high-salt diet. Although urinary protein excretion in volume-dependent hypertensive rats was increased to the same or greater extent, urinary AGT was significantly lower in volume-dependent hypertensive rats than in Ang II-dependent hypertensive rats, and was not greater than in control rats. This study also demonstrated that there was a significant relationship between urinary AGT and kidney Ang II content in rats given different doses of Ang II to achieve different levels of hypertension. These results provide further evidence that urinary AGT may be a useful index of intrarenal Ang II activity [75,80,83]
Figure 2.

Regression analysis between kidney angiotensin II (Ang II) contents and urinary excretion of angiotensinogen (AGT). Kidney Ang II levels are highly correlated (r = 0.76) with urinary excretion rates of AGT. Data were obtained from Sprague-Dawley rats and combined from two publications [80,83], with Ang II-infused hypertensive or sham-operated normotensive animals fed a normal or high-salt diet.
Conclusions
The data provided in this paper and in other recent reviews support the critical role of the intrarenal RAS in the pathophysiology of hypertension. They also serve as the foundation for the hypothesis that in Ang-dependent hypertension, there is increased AGT secretion by the proximal tubule cells, leading to spillover of intact AGT into distal nephron segments [3••,75]. This hypothesis is depicted in Figure 3. Because renin and ACE have also been demonstrated in distal nephron segments, the necessary factors for enhanced and sustained distal tubular formation of Ang II are available even when there is suppression of plasma renin levels and juxtaglomerular apparatus renin content. As noted earlier, the increased distal nephron Ang II activity would activate luminal AT1 receptors and stimulate distal nephron sodium reabsorption. The distal nephron effect would be synergistic with the actions of Ang II to enhance proximal reabsorption rate, as well as with the actions of aldosterone to increase the distal nephron sodium reabsorption. However, more data on the regulation of distal tubular renin expression, especially in hypertensive conditions, are needed [74]. This functional analysis is consistent with and provides further support to the genetic studies suggesting a linkage between variations in the AGT gene and hypertension [75,85].
Figure 3.
Tubular renin-angiotensin system in proximal and distal nephron segments. In angiotensin (Ang) II hypertension, increased proximal tubular secretion of Ao spills over into the distal nephron and increases Ang II effects on distal tubular reabsorption. ACE—angiotensin-converting enzyme; CD—collecting duct; Cnt—connecting tubule; DT—distal tubule; PT—proximal tubule.
Acknowledgment
We are grateful to Debbie Olavarrieta for excellent secretarial support in preparing the manuscript and figures. Research support provided by grants from the NHLBI, Louisiana Board of Regents, and the National Kidney Foundation.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Navar LG, Hamm LL. The kidney in blood pressure regulation. In: Wilcox CS, editor. Atlas of Diseases of the Kidney. Hypertension and the Kidney. Current Medicine, Inc.; Philadelphia: 1999. pp. 1.1–1.22. [Google Scholar]
- 2••.Wolf G. The Renin-Angiotensin System and Progression of Renal Diseases. Karger; Hamburg: 2002. [Of major importanceThis book provides current concepts in several areas including basic functions of the RAS, Ang II in renal growth and development, pro-inflammatory actions of Ang II, Ang II and renal fibrosis, and the role of Ang II in specific diseases such as diabetes, allograft dysfunction, atherosclerosis, and ureteral obstruction.] [Google Scholar]
- 3•.Adamczak M, Zeier M, Dikow R, Ritz E. Kidney and hyper-tension. Kidney Int. 2002;61(Suppl 80):62–67. doi: 10.1046/j.1523-1755.61.s80.28.x. [Of importanceThis review discusses the role of the kidney in hypertension. In particular, the summary of studies showing renoprotective properties of ACE inhibitors and Ang II receptor type 1 antagonists is interesting.] [DOI] [PubMed] [Google Scholar]
- 4.Wang C-T, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in Ang II-infused hyper-tensive rats. Am J Physiol Renal Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 5.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf G, Schneider A, Wenzel U, et al. Regulation of glomerular TGF-b expression in the contralateral kidney of two-kidney, one-clip hypertensive rats. J Am Soc Nephrol. 1998;9:763–772. doi: 10.1681/ASN.V95763. [DOI] [PubMed] [Google Scholar]
- 7•.Wolf G, Wenzel U, Burns KD, et al. Angiotensin II activates nuclear transcription factor-kappaB through AT1 and AT2 receptors. Kidney Int. 2002;61:1986–1995. doi: 10.1046/j.1523-1755.2002.00365.x. [Of importanceThis paper shows that Ang II activates nuclear factor-κB, which is a key transcriptional factor in inflammatory diseases, through Ang II type 2 receptors as well as its type 1 receptors. This suggests that Ang II type 1 receptor antagonists may not antagonize Ang II-induced inflammatory actions mediated by AT2 receptors.] [DOI] [PubMed] [Google Scholar]
- 8.Hannken T, Schroeder R, Zahner G, et al. Reactive oxygen species stimulate p44/42 mitogen-activated protein kinase and induce p27Kipl: Role in angiotenin II mediated hypertrophy of proximal tubular cells. J Am Soc Nephrol. 2000;11:1387–1397. doi: 10.1681/ASN.V1181387. [DOI] [PubMed] [Google Scholar]
- 9.Hilgers KF, Hartner A, Porst M, et al. Monocyte chemoattractant protein-1 and macrophage infiltration in hypertensive kidney injury. Kidney Int. 2000;58:2408–2419. doi: 10.1046/j.1523-1755.2000.00424.x. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Ortega M, Lorenzo O, Egido J. Angiotensin III increases MCP-1 and activates NF-kB and AP-1 in cultured mesangial and mononuclear cells. Kidney Int. 2000;57:2285–2298. doi: 10.1046/j.1523-1755.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Ortega M, Lorenzo O, Ruperez M, et al. Angiotensin II activates nuclear transcription factor kB through AT1 and AT2 in vascular smooth muscle cells. Circ Res. 2000;86:1266–1272. doi: 10.1161/01.res.86.12.1266. [DOI] [PubMed] [Google Scholar]
- 12•.Price DA, Fisher ND, Lansang MC, et al. Renal perfusion in blacks: alterations caused by insuppressibility of intrarenal renin with salt. Hypertension. 2002;40:186–189. doi: 10.1161/01.hyp.0000024349.85680.87. [Of importanceRenal function was measured in 19 blacks and 22 whites on both high- and low-salt diets; responses to Ang II infusions and captopril were also determined on high salt diets. Whites had higher renal plasma flow than blacks, but the values were comparable during a low-salt diet. Responses to Ang II and captopril on a low-salt diet were comparable. It is suggested that intrarenal RAS in blacks is inadequately suppressed in response to a high-salt diet.] [DOI] [PubMed] [Google Scholar]
- 13.Fisher NDL, Price DA, Litchfield WR, et al. Renal response to captopril reflects state of local renin system in healthy humans. Kidney Int. 1999;56:635–641. doi: 10.1046/j.1523-1755.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- 14.Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411–1420. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- 15.Fridman K, Andersson OK, Wysocki M, et al. Acute effects of candesartan cilexetil (the new angiotensin II antagonist) on systemic and renal haemodynamics in hypertensive patients. Eur J Clin Pharmacol. 1998;54:497–501. doi: 10.1007/s002280050503. [DOI] [PubMed] [Google Scholar]
- 16.Gansevoort RT, Mimran A, de Zeeuw D, Jover B. AT1 receptor antagonists and the kidney. In: Epstein M, Brunner HR, editors. Angiotensin II Receptor Antagonists. Hanley & Belfus, Inc.; Philadelphia: 2001. pp. 295–316. [Google Scholar]
- 17.Cervenka L, Horacek V, Vaneckova I, et al. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension. 2002;40:735–741. doi: 10.1161/01.hyp.0000036452.28493.74. [DOI] [PubMed] [Google Scholar]
- 18.Cervenka L, Wang C-T, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT 1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension. 1999;33:102–107. doi: 10.1161/01.hyp.33.1.102. [DOI] [PubMed] [Google Scholar]
- 19•.Tokuyama H, Hayashi K, Matsuda H, et al. Differential regulation of elevated renal angiotensin II in chronic renal ischemia. Hypertension. 2002;40:34–40. doi: 10.1161/01.hyp.0000022060.13995.ed. [Of importanceIntrarenal Ang II was measured in both kidneys of 2K1C Goldblatt hypertensive rats, and it was shown that both kidneys had elevated Ang II contents. Importantly, ACE activity was only increased in nonclipped kidneys, while the clipped kidneys had elevated chymase levels.] [DOI] [PubMed] [Google Scholar]
- 20.Sadjadi J, Puttaparthi K, Welborn MB, III, et al. Upregulation of autocrine-paracrine renin-angiotensin systems in chronic renovascular hypertension. J Vasc Surg. 2002;36:386–392. doi: 10.1067/mva.2002.125016. [DOI] [PubMed] [Google Scholar]
- 21.Jacinto SM, Mullins JJ, Mitchell KD. Enhanced renal vascular responsiveness to angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol Renal Physiol. 1999;276:F315–F322. doi: 10.1152/ajprenal.1999.276.2.F315. [DOI] [PubMed] [Google Scholar]
- 22.Mackie FE, Meyer TW, Campbell DJ. Effects of antihypertensive therapy on intrarenal angiotensin and bradykinin levels in experimental renal insufficiency. Kidney Int. 2002;61:555–563. doi: 10.1046/j.1523-1755.2002.00141.x. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert RE, Wu LL, Kelly DJ, et al. Pathological expression of renin and angiotensin II in the renal tubule after subtotal nephrectomy. Implications for the pathogenesis of tubulointerstitial fibrosis. Am J Pathol. 1999;155:429–440. doi: 10.1016/S0002-9440(10)65139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Y, Sigmund CD. Androgen-dependent regulation of human angiotensinogen expression in KAP-hAGT transgenic mice. Am J Physiol Renal Physiol. 2001;280:F54–F60. doi: 10.1152/ajprenal.2001.280.1.F54. [DOI] [PubMed] [Google Scholar]
- 25.Lake-Bruse KD, Sigmund CD. Transgenic and knockout mice to study the renin-angiotensin system and other interactng vasoactive pathways. Curr Hypertens Rep. 2000;2:211–216. doi: 10.1007/s11906-000-0084-1. [DOI] [PubMed] [Google Scholar]
- 26.Smithies O, Kim H-S, Takahashi N, Edgell MH. Importance of quantitative genetic variations in the etiology of hyper-tension. Kidney Int. 2000;58:2265–2280. doi: 10.1046/j.1523-1755.2000.00411.x. [DOI] [PubMed] [Google Scholar]
- 27.Navar LG, Harrison-Bernard LM, Imig JD, Mitchell KD. Renal actions of angiotensin II at AT1 receptor blockers. In: Epstein M, Brunner HR, editors. Angiotensin II Receptor Antagonists. Hanley & Belfus, Inc.; Philadelphia: 2000. pp. 189–214. [Google Scholar]
- 28.Harrison-Bernard LM, Navar LG, Ho MM, et al. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol Renal Physiol. 1997;273:F170–F177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z-Q, Millatt LJ, Heiderstadt NT, et al. Differential regulation of renal angiotensin subtype AT1A and AT2 receptor protein in rats with angiotensin-dependent hypertension. Hypertension. 1999;33:96–101. doi: 10.1161/01.hyp.33.1.96. [DOI] [PubMed] [Google Scholar]
- 30.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 31.Sharma M, Sharma R, Greene AS, et al. Documentation of angiotensin II receptors in glomerular epithelial cells. Am J Physiol Renal Physiol. 1998;274:F623–F627. doi: 10.1152/ajprenal.1998.274.3.f623. [DOI] [PubMed] [Google Scholar]
- 32.Silldorff EP, Hilbun LR, Pallone TL. Angiotensin II constriction of rat vasa recta is partially thromboxane dependent. Hypertension. 2002;40:541–546. doi: 10.1161/01.hyp.0000033467.04939.dd. [DOI] [PubMed] [Google Scholar]
- 33.Pallone TL, Huang JM. Control of descending vasa recta pericyte membrane potential by angiotensin II. Am J Physiol Renal Physiol. 2002;282:F1064–F1074. doi: 10.1152/ajprenal.00306.2001. [DOI] [PubMed] [Google Scholar]
- 34.Bouby N, Hus-Citharel A, Marchetti J, et al. Expression of type 1 angiotensin II receptor subytpes and angiotensin II-induced calcium mobilization along the rat nephron. J Am Soc Nephrol. 1997;8:1658–1667. doi: 10.1681/ASN.V8111658. [DOI] [PubMed] [Google Scholar]
- 35.Ruan XP, Wagner C, Chatziantoniou C, et al. Regulation of angiotensin II receptor AT1 subtypes in renal afferent arterioles during chronic changes in sodium diet. J Clin Invest. 1997;99:1072–1081. doi: 10.1172/JCI119235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng HF, Becker BN, Burns KD, Harris RC. Angiotensin II upregulates type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest. 1995;95:2012–2019. doi: 10.1172/JCI117886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amiri F, Garcia R. Renal angiotensin II receptor regulation in two-kidney, one clip hypertensive rats. Effect of ACE inhibition. Hypertension. 1997;30(part 1):337–344. doi: 10.1161/01.hyp.30.3.337. [DOI] [PubMed] [Google Scholar]
- 38.Amiri F, Haddad G, Garcia R. Renal angiotensin II receptor regulation and renin-angiotensin system inhibition in one-kidney, one clip hypertensive rats. J Hypertens. 1999;17:279–286. doi: 10.1097/00004872-199917020-00013. [DOI] [PubMed] [Google Scholar]
- 39.Harrison-Bernard LM, El-Dahr SS, O'Leary DF, Navar LG. Regulation of angiotensin II type 1 receptor mRNA and protein in angiotensin II-induced hypertension. Hypertension. 1999;33(part II):340–346. doi: 10.1161/01.hyp.33.1.340. [DOI] [PubMed] [Google Scholar]
- 40.Zhuo J, Ohishi M, Mendelsohn FAO. Roles of AT1 and AT2 receptors in the hypertensive Ren-2 gene trasngenic rat kidney. Hypertension. 1999;33(part II):347–353. doi: 10.1161/01.hyp.33.1.347. [DOI] [PubMed] [Google Scholar]
- 41.Harrison-Bernard LM, Zhuo J, Kobori H, et al. Intrarenal AT1 receptor and ACE binding in angiotensin II-induced hyper-tensive rats. Am J Physiol Renal Physiol. 2001;281:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imig JD, Navar GL, Zou LX, et al. Renal endosomes contain angiotensin peptides, converting enzyme, and AT1A receptors. Am J Physiol Renal Physiol. 1999;277:F303–F311. doi: 10.1152/ajprenal.1999.277.2.F303. [DOI] [PubMed] [Google Scholar]
- 43.Ingert C, Grima M, Coquard C, et al. Effects of dietary salt changes on renal renin-angiotensin system in rats. Am J Physiol Renal Physiol. 2002;283:F995–F1002. doi: 10.1152/ajprenal.00321.2001. [DOI] [PubMed] [Google Scholar]
- 44.Navar LG, Harrison-Bernard LM, Imig JD. Compartmentalization of intrarenal angiotensin II. In: Ulfendahl HR, Aurell M, editors. Renin-Angiotensin. Portland Press; London: 1998. pp. 193–208. [Google Scholar]
- 45•.van Kats JP, Schalekamp MA, Verdouw PD, et al. Intrarenal angiotensin II: interstitial and cellular levels and site of production. Kidney Int. 2001;60:2311–2317. doi: 10.1046/j.1523-1755.2001.00049.x. [Of importanceThis study determined the relative contribution of circulating and intrarenally produced Ang I to the intrarenal Ang II. The authors infused labeled Ang I and Ang II systemically and found that most of the intrarenal Ang II was locally generated Ang II rather than derived from circulating Ang I or Ang II.] [DOI] [PubMed] [Google Scholar]
- 46.Komine N, Khang S, Wead LM, et al. Effect of combining an ACE inhibitor and an angiotensin II receptor blocker on plasma and kidney tissue angiotensin II levels. Am J Kidney Dis. 2002;39:159–164. doi: 10.1053/ajkd.2002.29909. [DOI] [PubMed] [Google Scholar]
- 47.Zou L, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens. 1998;11:570–578. doi: 10.1016/s0895-7061(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 48•.Ingert C, Grima M, Coquard C, et al. Contribution of angiotensin II internalization to intrarenal angiotensin II levels in rats. Am J Physiol Renal Physiol. 2002;283:F1003–F1010. doi: 10.1152/ajprenal.00322.2001. [Of importanceComponents of the RAS were measured in kidneys from normal and low-salt fed rats with and without losartin treatment. Intrarenal Ang II levels increased more than Ang I levels. Losartan treatment decreased renal Ang II levels much more in low-salt rats than in normal salt rats, indicating that after salt restriction the increase in renal Ang II results mainly from uptake of Ang II via AT1 receptors.] [DOI] [PubMed] [Google Scholar]
- 49•.Zhuo JL, Imig JD, Hammond TG, et al. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [Of importanceAngiotensin II was measured in renal endosomes in normal rats, Ang II-infused rats, and Ang II-infused rats treated with candesartan. Ang II infusions caused marked increases in intact immunoreactive Ang II in endosomes, which were prevented by candesartan. The results demonstrate internalization of intact Ang II in endosomes of Ang II infused via an AT1 receptor–mediated process.] [DOI] [PubMed] [Google Scholar]
- 50.Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol Renal Physiol. 1997;273:F246–F253. doi: 10.1152/ajprenal.1997.273.2.F246. [DOI] [PubMed] [Google Scholar]
- 51.Siragy HM, Howell NL, Ragsdale NV, Carey RM. Renal inter-stitial fluid angiotensin: Modulation by anesthesia, epinephrine, sodium depletion and renin inhibition. Hypertension. 1995;25:1021–1024. doi: 10.1161/01.hyp.25.5.1021. [DOI] [PubMed] [Google Scholar]
- 52.Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–1242. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- 53.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39:129–134. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- 54.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid angiotensin I and angiotensin II concentrations during local angiotensin-converting enzyme inhibition. J Am Soc Nephrol. 2002;13:2207–2212. doi: 10.1097/01.asn.0000026610.48842.cb. [DOI] [PubMed] [Google Scholar]
- 55.Nishiyama A, Seth DE, Navar LG. Renal interstitial concentrations of angiotensin I and angiotensin II in angiotensin II-infused hypertensive rats. J Am Soc Nephrol. 2001;12:574A. [Google Scholar]
- 56.Navar LG, Harrison-Bernard LM, Wang C-T, et al. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10:S189–S195. [PubMed] [Google Scholar]
- 57.Tank JE, Henrich WL, Moe OW. Regulation of glomerular and proximal tubule renin mRNA by chronic changes in dietary NaCl. Am J Physiol Renal Physiol. 1997;273:F892–F898. doi: 10.1152/ajprenal.1997.273.6.F892. [DOI] [PubMed] [Google Scholar]
- 58.Wang CT, Navar LG, Mitchell KD. Proximal tubular fluid angiotensin II levels in angiotensin II-induced hypertensive rats. J Hypertens. 2003 doi: 10.1097/00004872-200302000-00027. In press. [DOI] [PubMed] [Google Scholar]
- 59.Boer WH, Braam B, Fransen R, et al. Effects of reduced renal perfusion pressure and acute volume expansion on proximal tubule and whole kidney angiotensin II content in the rat. Kidney Int. 1997;51:44–49. doi: 10.1038/ki.1997.6. [DOI] [PubMed] [Google Scholar]
- 60.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol Renal Physiol. 1996;271:F143–F149. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 61•.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [Of importanceThis paper provides evidence that Ang II directly stimulates apical sodium channel activity in cortical collecting duct cells via Ang II type 1 receptors. These results indicate one means by which enhanced distal intratubular Ang II may contribute to augmentation of sodium reabsorption in distal nephron segments.] [DOI] [PubMed] [Google Scholar]
- 62.van Kats JP, de Lannoy LM, Danser AHJ, et al. Angiotensin II type 1 (AT1) receptor-mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. Hypertension. 1997;30(part 1):42–49. doi: 10.1161/01.hyp.30.1.42. [DOI] [PubMed] [Google Scholar]
- 63.Hein L, Meinel L, Pratt RE, et al. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: Evidence for selective sorting of receptor and ligand. Mol Endocrinol. 1997;11:1266–1277. doi: 10.1210/mend.11.9.9975. [DOI] [PubMed] [Google Scholar]
- 64••.Chen R, Mukhin YV, Garnovskaya MN, et al. A functional angiotensin II receptor-GFP fusion protein: evidence for agonist-dependent nuclear translocation. Am J Physiol Renal Physiol. 2000;279:F440–F448. doi: 10.1152/ajprenal.2000.279.3.F440. [Of major importanceChinese hamster ovary cells were transfected with an AT1A receptor linked to GFP. Upon stimulation with Ang II, the ligand receptor complex was internalized and shown to migrate to the nucleus and colocalize with a nuclear stain. The increased AT1A receptor localization in the nucleus when treated with Ang II suggests that receptor density in nucleus may be regulated by Ang II.] [DOI] [PubMed] [Google Scholar]
- 65.Linas SL. Role of receptor mediated endocytosis in proximal tubule epithelial function. Kidney Int. 1997;52(Suppl 61):S-18–S-21. [PubMed] [Google Scholar]
- 66.Becker BN, Cheng H-F, Harris RC. Apical ANG II-stimulated PLA2 activity and Na+ flux: a potential role for Ca2+-independent PLA2. Am J Physiol Renal Physiol. 1997;273:F554–F562. doi: 10.1152/ajprenal.1997.273.4.F554. [DOI] [PubMed] [Google Scholar]
- 67.Haller H, Lindschau C, Erdmann B, et al. Effects of intra-cellular angiotensin II in vascular smooth muscle cells. Circ Res. 1996;79:765–772. doi: 10.1161/01.res.79.4.765. [DOI] [PubMed] [Google Scholar]
- 68.Licea H, Walters MR, Navar LG. Renal nuclear angiotensin II receptors in normal and hypertensive rats. Acta Physioloica Hungarica. 2002;89:427–438. doi: 10.1556/APhysiol.89.2002.4.3. [DOI] [PubMed] [Google Scholar]
- 69.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 72.Ingelfinger JR, Jung F, Diamant D, et al. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol Renal Physiol. 1999;276:F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 73•.Rohrwasser A, Morgan T, Dillon HF, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [Of importanceThis study demonstrated AGT secretion at the apical side by polarized proximal tubule epithelia. It also showed renin expression in connecting cells. AGT was detected in urine and was directly correlated with AGT expression in proximal tubule and inversely with dietary sodium. The authors point out the potential importance of tubular RAS elements in the coordinated regulation of sodium reabsorption at various sites within the nephron.] [DOI] [PubMed] [Google Scholar]
- 74.Lantelme P, Rohrwasser A, Gociman B, et al. Effects of dietary sodium and genetic background on angiotensinogen and Renin in mouse. Hypertension. 2002;39:1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 75.Lalouel J-M, Rohrwasser A, Terreros D, et al. Angiotensinogen in essential hypertension: From genetics to nephrology. J Am Soc Nephrol. 2001;12:606–615. doi: 10.1681/ASN.V123606. [DOI] [PubMed] [Google Scholar]
- 76.Loghman-Adham M, Rohrwasser A, Helin C, et al. A conditionally immortalized cell line from murine proximal tubule. Kidney Int. 1997;52:229–239. doi: 10.1038/ki.1997.325. [DOI] [PubMed] [Google Scholar]
- 77.Navar LG, Imig JD, Zou L, Wang C-T. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17:412–422. [PubMed] [Google Scholar]
- 78.Jeunemaitre X, Ménard J, Clauser E, Corvol P. Angiotensinogen: molecular biology and genetics. In: Laragh JH, Brenner BM, editors. Hypertension: Patho-physiology, Diagnosis, and Management. edn 2. Raven Press, Ltd; New York: 2000. pp. 1653–1665. [Google Scholar]
- 79.Ding Y, Davisson RL, Hardy DO, et al. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem. 1997;272:28142–28148. doi: 10.1074/jbc.272.44.28142. [DOI] [PubMed] [Google Scholar]
- 80.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:142–144. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Henrich WL, McAllister EA, Eskue A, et al. Renin regulation in cultured proximal tubular cells. Hypertension. 1996;27:1337–1340. doi: 10.1161/01.hyp.27.6.1337. [DOI] [PubMed] [Google Scholar]
- 82.Casarini DE, Boim MA, Stella RCR, et al. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 83.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davisson RL, Ding Y, Stec DE, et al. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics. 1999;1:3–9. doi: 10.1152/physiolgenomics.1999.1.1.3. [DOI] [PubMed] [Google Scholar]
- 85.Fukamizu A. Genomic expression systems on hierarchy and network leading to hypertension: long on history, short on facts. Hypertens Res. 2000;23:545–552. doi: 10.1291/hypres.23.545. [DOI] [PubMed] [Google Scholar]