Abstract
Objective
Our objective was to summarize and critically review data on the prevalence of posttraumatic stress disorder (PTSD) in general intensive care unit (ICU) survivors, risk factors for post-ICU PTSD, and the impact of post-ICU PTSD on health-related quality of life (HRQOL).
Methods
We conducted a systematic literature review using Medline, EMBASE, Cochrane Library, CINAHL, PsycINFO, and a hand-search of thirteen journals.
Results
Fifteen studies were eligible. The median point prevalence of questionnaire-ascertained “clinically significant” PTSD symptoms was 22% (n = 1,104), and the median point prevalence of clinician-diagnosed PTSD was 19% (n = 93). Consistent predictors of post-ICU PTSD included prior psychopathology, greater ICU benzodiazepine administration, and post-ICU memories of in-ICU frightening and/or psychotic experiences. Female sex and younger age were less consistent predictors, and severity of critical illness was consistently not a predictor. Post-ICU PTSD was associated with substantially lower HRQOL.
Conclusions
The prevalence of PTSD in ICU survivors is high and negatively impacts survivors’ HRQOL. Future studies should comprehensively address how patient-specific factors (e.g., pre-ICU psychopathology), ICU management factors (e.g., administration of sedatives), and ICU clinical factors (e.g., in-ICU delirium) relate to one another and to post-ICU PTSD. Clinicians caring for the growing population of ICU survivors should be aware of PTSD risk factors and monitor patients’ needs for early intervention.
Keywords: stress disorder, posttraumatic, critical care, intensive care unit, risk factors, outcome assessment (health care)
INTRODUCTION
Millions of patients require intensive care unit (ICU) treatment annually (approximately 4.4 million in the United States [1] and over 130,000 in the United Kingdom [personal communication – Cathy Welch, statistical research assistant for the Intensive Care National Audit and Research Centre] alone), and with recent advances in critical care medicine, more patients are surviving ICU stays [2]. With this increase in survival, research in the field has begun to focus on longer-term outcomes of ICU-treated patients, including mental health, health-related quality of life (HRQOL), and cognitive outcomes [3–5].
Critical illnesses and their requisite ICU therapies expose patients to extreme stressors, including respiratory insufficiency, pain with endotracheal intubation and suctioning, release of inflammatory cytokines, strain on the hypothamic-pituitary-adrenal (HPA) axis, administration of exogenous catecholamines, and delirium with associated psychotic experiences, all in the context of a limited ability to communicate and reduced autonomy. Critical illnesses are also, by definition, life-threatening, and many patients recall extremely frightening ICU experiences [6]. Thus, post-traumatic stress disorder (PTSD) is a potential concern, and a number of studies have appeared regarding post-ICU PTSD [7, 8]. In this report, we present results of a systematic review of: 1) the prevalence of PTSD following general ICU treatment, 2) potential risk factors for post-ICU PTSD, and 3) the relationship of post-ICU PTSD symptoms to HRQOL. As we have recently conducted a separate systematic review of psychopathology in acute lung injury/acute respiratory distress syndrome (ALI/ARDS) survivors [9], we chose not to include studies that focused exclusively on this patient group. Also, we chose not to include studies that focused exclusively on survivors from trauma, neurological, coronary, or surgical ICUs, given the possibility of potentially confounding risk factors for PTSD (e.g., trauma, myocardial infarction, or anesthesia/surgery). Our report differs from prior reviews on this topic [7, 8] in its exclusion of both ALI/ARDS-specific and specialty ICU-specific studies. A particularly important difference, however, is that we systematically review potential risk factors for post-ICU PTSD, whereas prior reviews focused on prevalence and methodological issues without systematically reviewing potential risk factors [7, 8].
METHODS
Search Strategy
We searched Medline (1966–2007), EMBASE (1974–2007), CINAHL (1982–2007), the Cochrane Library (2007, Issue 1), and PsycInfo (1967–2007) as of October 26, 2007. Our search strategy included the following terms mapped to the appropriate MeSH/EMTREE subject headings and “exploded”: (“mental disorders” OR “psychometrics”) AND (“respiratory distress syndrome, adult” OR “critical care” OR “critical illness” OR “intensive care units” OR “sepsis”). The following terms were also included as text words: (“depress*” OR “stress” OR “anxi*”) AND (“respiratory distress syndrome” OR “ARDS” OR “acute lung injury” OR “ALI”). In addition, we manually searched the tables of contents of 13 general medicine, psychiatry, and critical care journals (see Appendix 1) from January, 2000 to October, 2007. The search was limited to English-language articles. Articles dealing with neonatal or pediatric intensive care were excluded.
Appendix 1.
Manually Searched Journals from January, 2000 to October, 2007
| American Journal of Critical Care |
| American Journal of Psychiatry |
| American Journal of Respiratory and Critical Care Medicine |
| Archives of General Psychiatry |
| Biological Psychiatry |
| Critical Care |
| Critical Care Medicine |
| General Hospital Psychiatry |
| Intensive Care Medicine |
| JAMA |
| New England Journal of Medicine |
| Psychosomatic Medicine |
| Psychosomatics |
We did not include studies which focused exclusively on depression and/or non-specific anxiety outcomes, and we did not include studies limited to ALI/ARDS survivors. However, adding related terms helped identify several eligible, otherwise unidentified, studies.
Study Selection
Two authors (D.S.D. and S.V.D.) independently and sequentially reviewed citations, abstracts, and full text articles to select eligible studies. All citations or abstracts selected by either author were included in the next step of the selection process. At each step, the authors calculated interobserver agreement using percent agreement and the kappa statistic. Disagreements regarding eligibility of full text articles were resolved by consensus among all authors.
Articles were selected for review if they met the following criteria: 1) the study population was comprised of adult ICU survivors, and 2) PTSD assessments were conducted using validated measures at >1 month following ICU discharge. Studies focusing solely on survivors of ALI/ARDS or of specialty ICUs (i.e., trauma/surgical, coronary or neurological ICUs) were excluded, as were abstracts, case reports, and review articles.
Data Abstraction
For each eligible study, two authors (D.S.D. and J.M.G.) independently abstracted information regarding characteristics of the study cohorts, PTSD measures, potential risk factors, and associations between PTSD symptoms and HRQOL. The authors calculated interobserver agreement for data abstraction using percent agreement and the kappa statistic. Authors of eligible studies were contacted for additional information, when necessary.
RESULTS
Search Results and Study Characteristics
The authors reviewed 16,301 citations, 1,908 abstracts and 193 full-text articles (Figure 1). Fifteen articles were eligible for data abstraction [10–24] (Table 1). The percent agreement (kappa statistic) for each stage of the study selection and data abstraction process were: citation review, 91% (κ = 0.54); abstract review 98% (κ = 0.84); full text review 99% (κ = 0.89); and data abstraction, 98.7% (κ= 0.98).
Figure 1. Flow Diagram of Literature Search Results.
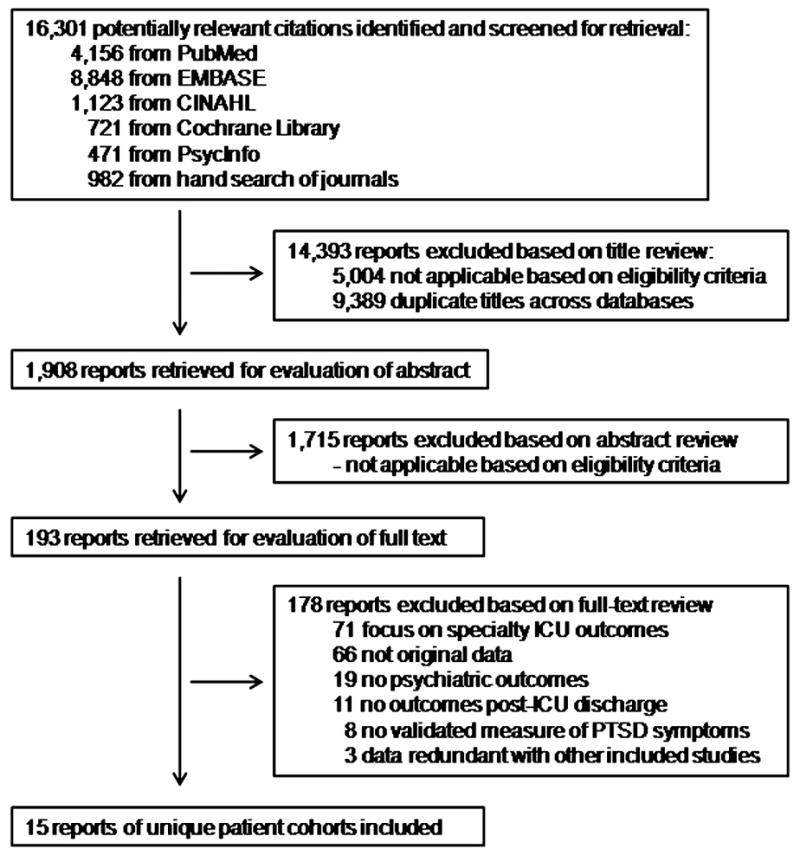
ICU = intensive care unit, PTSD = posttraumatic stress disorder
Table 1.
Study cohort characteristics, ordered by follow-up time
| Mean (Standard Deviation) or Median (Interquartile Range) [Absolute Range] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Study type |
N Enrolled | Inclusion (I) and exclusion (E) criteria |
Sex (%) | Age in years |
Days in hospital |
Days in ICU |
Days of mechanical ventilation |
APACHE II score |
Follow-up in months |
| Perrins et al. 1998 [10] | Prospective cohort | 72 | I: > 18 years of age, general ICU LOS at least 24 hours
E: history of mental illness, history of head trauma, lack of consent, active bereavement, non-English speaking |
- | 49 (16) | 32 (19) | 6 (5) | - | - | 1.5, 6, 12 |
| Jones et al. 2001 [11] | Prospective cohort | 45 | I: general medical and surgical ICU LOS at least 24 hours, mechanically ventilated
E: admitted following suicide attempt or head injury, ongoing or previous psychotic illness |
♂: 44%
♀: 56% |
57 [17–82] | - | 8 [1–60] | - | 17 [4–28] | 2 |
| Samuelson et al. 2007 [12] | Prospective cohort | 250 | I: > 18 years of age, mechanically ventilated, general ICU LOS at least 24 hours
E: head injury, psychotic illness, mental retardation, intoxication, admitted following suicide attempt, hearing/speech disability, non-Swedish speaking, transferred to another hospital, mechanically ventilated at d/c, mechanically ventilated > 24 hours pre-admission |
♂: 52%
♀: 48% |
63 (13) | - | 6 (6), 4 | 4 (5), 2 | 18 | 2 |
| Jones et al. 2007 [13] | Prospective cohort | 304 | I: > 18 years of age, mechanically ventilated, mixed general ICU LOS at least 48 hours
E: prior PTSD, admitted following suicide attempt, preexisting or concomitant psychotic illness, resides >30km from hospital, unresolved confusion, enrolled in another research study |
♂: 62%
♀: 38% |
61 [17–86] | - | 7 [2–76] | 3 [2–90] | 16 [3–36] | 3 |
| Cuthbertson et al. 2004 [14] | Prospective cohort | 111 | I: in general ICU
E: - |
♂: 56%
♀: 44% |
58 [18–87] | - | 6 [1–51] | 2 [0–44] | 18 [4–38] | 3 |
| Jones et al 2003 [15] | Randomized controlled trial | 69, 57 a, 126 b | I: mechanically ventilated, mixed general ICU LOS at least 48 hours
E: burn injury, neurosurgical patient, preexisting psychotic illness, speech/reading difficulty, life expectancy < 6 months |
♂:54%,58% a
♀: 46%,42% a |
57 (17) [17–77]
59 (16) a [17–84] a |
- | 14 (20) [2–114]
13 (18) a [2–110] a |
- | 17 (5)
16 (5) a |
2, 6 |
| Sukantarat et al. 2007 [16] | Prospective cohort | 51 | I: general ICU LOS at least 72 hours
E: major medical or surgical therapy following d/c, reluctance to undergo detailed testing, still in hospital at time of study, lived at a distance |
♂: 43%
♀: 57% |
57 (14)
60 (49,66) [27–82] |
- | 17 (17)
9 [3–78] |
12 (17) | 15 (6) | 3, 9 |
| Griffiths et al. 2006 [17] | Prospective cohort | 127 | I: general medical ICU LOS at least 3 days, > 18 years of age
E: unable to read/understand English |
♂: 66%
♀: 34% |
57 [17–85] | - | 14, 12 [2–101] | - | - | 3 |
| Girard et al. 2007 [18] | Prospective cohort | 275 | I: - in medical or coronary care ICU
E: neurologic disease impairing cognitive function, mental retardation, non-English speaking, sensory deficits impairing communication |
♂: 47%
♀: 53% |
52 (39,65) | - | 10 (5,13) | 5 (3,12) | 25 (20,31) | 6 |
| Nickel et al. 2004 [19] | Cross-sectional | 41 | I: Age 18–65 years, general medical ICU LOS at least 24 hours
E: - |
♂: 68%
♀: 32% |
47 | 12 (5) | - | - | 12 (11) | 6 [3–15] |
| Rattray et al. 2005 [20] | Prospective cohort | 109 | I: > 18 years of age, general ICU LOS at least 24 hours
E: living > 100 miles away |
- | 55 (18) | 28 (15,57) | 5 (2,13) | - | 18 (5) | 0.75, 6, 12 |
| Kress et al. 2003 [21] | Case-control | 13, 19 a, 32 b | I: mechanically ventilated in medical ICU, received IV drip sedation
E: - |
♂: 31%, 42% a
♀: 69%, 58% a |
50 (16), 47 (20) a | 18 (13), 19 (10) a | 7 (6), 13 (10) a | 6 (6)
10 (7)a |
16 (6), 18 (7) a | 14 (7), 12 (5) a |
| Scragg et al. 2001 [22] | Cross-sectional | 142 | I: general ICU survivor between 10/1/95–10/1/97
E: head trauma, accidental or non-accidental injury, admitted for routine surgery w/o complications. |
♂: 53%
♀: 47% |
57 [19–90] | - | [1–33] | - | - | 13 (6) [3–21] |
| Schelling et al. 2001 [23] | Randomized controlled trial | 9, 11 a, 20 b | I: septic shock treated in multidisciplinary ICU
E: preexisting neurologic or psychiatric disease, non-German speaking |
♂: 33%, 45%a
♀: 67%, 55% a |
48 [23–76], 55 [25–75] a | - | 23 [17–72], 32 [11–99] a | - | 24 [14–29], 22 [14–35] a | 31 [21–49] |
| Schelling et al. 1999 [24] | Case-control | 27, 27a, 54 b | I: septic shock treated in multidisciplinary ICU
E: preexisting neurologic and/or psychiatric disease, history of head trauma, surgery, history of CPR, non-German speaking, d/c from hospital < 6 months from start of study |
♂: 33%, 33% a
♀: 67%, 67% a |
54 (25,76), 53 (25,75) a | - | 35 (8,114), 35 (7,235) a | - | 23 (16,45), 23 (14,35) a | 48 [1–12], 120 [2–9] a |
Abbreviations (in alphabetical order): CPR = cardiopulmonary resuscitation; d/c = discharge; ICU = intensive care unit; LOS = length-of-stay; w/o = without; “-” = not reported.
control group values (for randomized-controlled trials and case-control studies);
entire sample
Table 1 shows baseline descriptive data for the fifteen studies, ordered by timing of follow-up assessment. Follow-up periods ranged from approximately 6 weeks to 7 years, though most studies had PTSD assessments within the first year post-ICU. The studies enrolled 1,745 unique patients; 14 patients were included in two studies [23, 24]. Of the fifteen studies reviewed, nine were prospective cohort studies [10–14, 16–18, 20]; two were randomized, controlled trials [15, 23]; two were case-control studies [21, 24]; and two were cross-sectional studies [19, 22]. Eight of the studies were conducted in the UK [10, 11, 14–17, 20, 22], three in Germany [19, 23, 24], two in the US [18, 21], one in Sweden [12], and one in multiple European countries [13]. Though ICU admission diagnoses were categorized in different ways across studies, it is possible to make some general observations. Pulmonary syndromes (e.g. pneumonia) were common primary admission diagnoses, affecting >20% of patients in at least nine studies [11–13, 17–21. 23]. Gastrointestinal syndromes (e.g., hemorrhage or peritonitis) were also common primary admission diagnoses, affecting >20% of patients in at least eight studies [12, 13, 16, 17, 20, 23, 24]. Surgery was a primary admission diagnosis in >20% of patients in at least four studies [12–14, 16]; sepsis, three studies [21, 23, 24], and trauma, one study [23]. Three studies did not provide details regarding ICU admission diagnoses [10, 15, 22].
Personality Characteristics and Prior Psychiatric History
Three studies used the Spielberger State-Trait Anxiety Inventory–Trait scale (STAI-T) to assess levels of trait anxiety [11, 15, 21], though only the first assessed its relationship to PTSD symptoms. One study assessed pre-existing personality disorders using a semi-structured interview [19]; in that study, personality disorders were included as pre-existing psychiatric disorders, together with Axis I conditions.
Seven of the studies excluded patients with a pre-ICU psychiatric illness, apparently based upon chart review [10–13, 15, 23, 24] (Table 1). In three of these, patients were only excluded if they had a pre-existing psychotic illness or were admitted after a suicide attempt [11, 12, 15]; a fourth also excluded patients with a pre-ICU history of PTSD (assessed post-ICU) [13].
Five of the studies reported information regarding patients’ psychiatric histories (Table 2). In one study, psychiatrists made diagnoses of pre-ICU psychopathology using semi-structured interviews [19]; the lifetime prevalence of any pre-existing Axis I or Axis II psychiatric disorder was 42%, and the most common pre-existing disorder was major depressive disorder. In three studies apparently employing chart reviews and/or unspecified questions [11, 13, 21], prevalences of pre-ICU psychiatric disorders ranged from 18% to 24%. In one study, psychiatric history was ascertained with a question regarding prior mental health treatment-seeking with a physician; 14% endorsed this [14].
Table 2.
Measurements of posttraumatic stress disorder symptoms, ordered by follow-up time
| Study | Past psychiatric illness |
Instrument | Follow-up in months |
N at follow-up | Mean (sd) or Median (IQR) [Range] Score | Cut-off score | Point prevalence |
|---|---|---|---|---|---|---|---|
| Perrins et al. 1998 [10] | - | IES | 1.5 | 44 | 14, 12 | 19 | 31% |
| 6 | 41 | 12, 12 | 32% | ||||
| 12 | 38 | 12, 8 | 27% | ||||
| Jones et al. 2001 [11] | 18% | IES | 2 | 30 | 16, 11 | 19 | 23% |
| Samuelson et al. 2007 [12] | - | IES-R | 2 | 226 | 8(12), 3, [0–83] | 30 | 8% |
| Jones et al. 2007 [13] | 24% | PDS | 3 | 238 | - | DSM-IV criteria | 9% |
| Cuthbertson et al. 2004 [14] | 14% | DTS | 3 | 78 | 8 [0–87] | 27 | 22% |
| Jones et al. 2003 [15] | - | IES | 2 | 63, 51 a, 114 b | 20, 14 b | - | - |
| 6 | 58, 44 a, 102 b | 23, 20 b | 19 | 53%, 48% a, 51% b | |||
| Sukantarat et al. 2007 [16] | - | IES-I | 3 | 51 | 13 (9) | 21 | (24%) c |
| 9 | 45 | 12 (10) | (20%) c | ||||
| IES-A | 3 | 51 | 12 (9) | 18 | (36%) c | ||
| 9 | 45 | 12 (9) | (38%) c | ||||
| IES-Total | 3 | 51 | - | 26 | 35% | ||
| 9 | 45 | - | 62% | ||||
| Griffiths et al. 2006 [17] | - | TSQ | 3 | 108 | - | 6 | 52% |
| Girard et al. 2007 [18] | - | PTSS-10 | 6 | 43 | - | 35 | 14% |
| Nickel et al. 2004 [19] | 42% | SCID-PTSD | 6 | 41 | n/a | n/a | 10% |
| PTSS-10 | 6 | 41 | - | 35 | 17% | ||
| Rattray et al. 2005 [20] | - | IES-I | 0.75 | 109 | 7 (3) | 20 | (18%) c |
| 6 | 61 | 6 (3) | |||||
| 12 | 80 | 7 (3) | |||||
| IES-A | 0.75 | 109 | 6 (3) | 20 | (20%) c | ||
| 6 | 61 | 4 (5) | |||||
| 12 | 80 | 6 (4) | |||||
| Kress et al. 2003 [21] | 38%, 11% a
22% b |
IES | 14, 12 a | 13, 19 a, 32 b | 11 (15), 27 (19) a | - | - |
| Interview d | n/a | n/a | 0%, 32%a, 19% b | ||||
| Scragg et al. 2001 [22] | - | IES | 13 (6) [3–21] | 77 | - | 30 | 16% |
| Schelling et al. 2001 [23] | - | SCID-PTSD | n/a | n/a | 11%, 64% a, 40% b | ||
| PTSS-10 | 31 | 9, 11a, 20 b | 27, 36 a | 35 | 11%, 64% a, 40% b,e | ||
| Schelling et al. 1999 [24] | - | PTSS-10 | 48, 120 b | 27, 27 a, 54 b | - | 35 | 18%, 59% a, 39% b |
Abbreviations (in alphabetical order): DTS = Davidson Trauma Scale; IES = Impact of Events Scale; IES-A = Impact of Events Scale-Avoidance Subscale; IES-I = Impact of Events Scale-Intrusion Subscale; IES-R = Impact of Events Scale-Revised; n/a = not-applicable; PDS = Posttraumatic Diagnostic Scale; PTSD = post-traumatic stress disorder; PTSS-10 = Post Traumatic Symptom Scale-10; PTSS-14 = Post Traumatic Symptom Scale-14; SCID = Structured Clinical Interview for DSM-IV; TSQ = Trauma Stress Questionnaire; “-” = not reported.
control group value (for randomized-controlled trials and case-control studies);
entire sample;
clinically significant symptoms of intrusion or avoidance;
PTSD diagnosed using an unspecified structured interview with a clinical psychologist;
not included in questionnaire-based median prevalence estimate.
Prevalence of Posttraumatic Stress Symptoms/Disorder
At least one PTSD measure was completed by 1,251 patients. Ten of the studies utilized in-person assessments [11, 13, 15–21, 23]; three, mailed questionnaires [10, 22, 24]; and two, telephone interviews [12, 14]. Three studies employed clinicians to diagnose PTSD using DSM-IV criteria (Table 2); in two studies, psychiatrists administered the Structured Interview for DSM-IV (SCID) [19, 23], and, in one, a clinical psychologist administered an unspecified structured interview [21]. In two of these studies, the investigators also administered questionnaires to assess PTSD symptoms [19, 23]. The remaining twelve studies only used questionnaires to assess PTSD symptoms. Questionnaires included the Impact of Events Scale (IES) [10, 11, 15, 16, 20–22]; the Impact of Events Scale-Revised version (IES-R) [12]; the Posttraumatic Symptom Scale-10 (PTSS-10) [18, 19, 23, 24]; the Posttraumatic Diagnostic Scale (PDS) [13]; the Davidson Trauma Scale (DTS) [14]; and the Trauma Stress Questionnaire (TSQ) [17].
In determining the median point prevalence of questionnaire-ascertained “clinically significant” PTSD symptoms post-ICU across studies, we addressed three challenges as follows. First, some studies collected PTSD data at more than one time point; in those instances, we used the median value from each study. Second, some studies had treatment or case groups vs. control groups; in those instances, we used the prevalence for the entire sample, reasoning that the control groups in these studies are not necessarily more representative than the treatment or case groups. Third, two studies included 14 of the same patients [23, 24]; in this instance, we excluded the smaller study (n = 20) [23]. Notably, not all of the studies using the IES used the same threshold or “cut-off score” to define “clinically significant” PTSD symptoms. The median point prevalence of questionnaire-ascertained “clinically significant” PTSD symptoms post-ICU was 22% (range 8% to 51%) (12 studies, n = 1,104). The median point prevalence of clinician-ascertained PTSD post-ICU was 19% (range 10% to 39%) (3 studies, n = 93). Of note, two studies employed both the PTSS-10 and the SCID [19,23]: in one of these studies, three of seven patients with “clinically significant” PTSD symptoms (PTSS-10 scores > 35) were not diagnosed with SCID PTSD, though these patients appeared to exhibit subthreshold PTSD-related phenomena [19]; in the other, all eight patients with PTSS-10 scores > 35 had SCID PTSD [23]. In the four studies that assessed PTSD symptoms in the same patients longitudinally, no substantial differences were evident in mean/median scores over 6 to 12 months [10, 15, 16, 20]; however, only one of these studies explicitly tested for the effect of time (not significant) [20].
Potential Risk Factors for Posttraumatic Stress Symptoms/Disorder
Two demographic factors were significant predictors of post-ICU PTSD in some studies: female sex in two of seven studies [10, 12, 14, 18, 20, 23, 24] and younger age in four of eight studies [12, 14, 16, 18, 20, 22–24] (Table 3). No study found an association with male sex or older age. Two of the larger studies (n >100) apparently did not assess associations with demographic factors [13, 15].
Table 3.
Potential risk factors for post-traumatic stress symptoms/disorder
| Study (n) | Measure of Association | Potential risk factor | Outcome: post-traumatic stress symptoms/disorder |
|---|---|---|---|
| Perrins et al. 1998 [10] (n = 44) | Mann-Whitney U
Kruskal-Wallis one-way ANOVA |
a) Sex
b) Trauma c) Respiratory failure d) No recall of the ICU a e) Lack of social support f) Admitted to ICU via emergency department g) Admitted conscious from a ward to ICU |
a) n.s.
b) high IES and IES-A scores at 6 weeks, p < 0.05 c) high IES score at 6 weeks, p < 0.05 d) n.s. e) n.s. f) high IES score at 6 weeks, p < 0.05 g) high IES-A scores at 6 weeks, p < 0.02 |
| Jones et al. 2001 [11] (n = 30) | T-test
Kruskal-Wallis χ2 Mann-Whitney U Spearman’s correlation |
a) History of anxiety or depression
b) ICU diagnostic group c) No factual memories of ICU+“delusional memories” vs. factual memories of ICU+”delusional memories” or no “delusional memories” 2 weeks post-ICU d) Trait anxiety (from State-Trait Anxiety Inventory) a e) ICU LOS, APACHE II |
a) p = 0.88
b) p = 0.34 c) median 41 vs. 11 IES score, p = 0.001 d) r = 0.49 with IES-I, p = 0.007 r = 0.13 with IES-A, p = 0.48 e) n.s. |
| Samuelson et al. (n = 226) | Mann-Whitney U or χ2 or Fisher’s exact test
Multivariable logistic regression – Model 1 Multivariable logistic regression – Model 2 |
a) Younger age
b) Female sex c) APACHE II d) ICU LOS e) Days of mechanical ventilation f) Propofol in ICU g) Midazolam in ICU h) Ketobemidone in ICU i) Neuromuscular blocker in ICU Proportion of total ICU stay with MAAS score… j) …0–2 k) …3 l) …4–6 m) Complete amnesia of ICU 5 days post-ICU n) “Delusional memories” of ICU 5 days post-ICU o) “Delusional memories” of ICU without factual recall 5 days post-ICU p) # of recalled extremely stressful events 5 days-post ICU q) Recall of feeling fearful in ICU 5 days post-ICU r) Nightmares 5 days post-ICU s) HAD anxiety score 5 days post-ICU t) HAD depression score 5 days post-ICU u) Female sex v) MAAS score 4–6 w) Recall of greater # of extremely stressful events 5 days post-ICU x) Female sex y) MAAS score 4–6 z) Recall of feeling fearful in ICU 5 days post-ICU |
Comparisons: PTSD vs. no PTSD
a) mean 57 vs. 64 years, p = 0.04 b) 74% vs. 46%, p = 0.04 c) median 13 vs. 18, p = 0.09 d) median 2.7 vs. 3.6, p = 0.42 e) median 1.8 vs. 1.6, p = 0.97 f) 95% vs. 93%, p = 1.00 g) 68% vs. 30%, p = 0.02 h) 79% vs. 82%, p = 0.76 i) 5% vs. 13%, p = 0.71 j) median 22% vs. 27%, p = 0.66 k) median 62% vs. 67%, p = 0.48 l) median 11% vs. 0%, p = 0.06 m) 16% vs. 19%, p = 1.00 n) 42% vs. 33%, p = 0.57 o) 0% vs. 3%, p = 1.00 p) median 3 vs. 1, p = 0.03 q) 37% vs. 8%, p = 0.001 r) 16% vs. 13%, p = 0.72 s) median 5 vs. 2, p = 0.006 t) median 7 vs. 4, p = 0.001 u) OR = 4.7, p = 0.005 v) OR = 1.74 per 0.1 higher MAAS, p = 0.005 w) OR = 1.1, p = 0.008 x) OR = 4.9, p = 0.004 y) OR = 1.77 per 0.1 higher MAAS, p = 0.005 z) OR = 7.0, p = 0.002 |
| Jones et al. 2007 [13] (n = 238) | Spearman’s correlation | a) Previous psychiatric history
b) Amount of sedation in ICU c) Amount of opiates in ICU d) Physical restraint in ICU e) Mean hours restrained in ICU f) “Delusional memories” of ICU 2 weeks post-ICU |
a) r = 0.23, p = 0.0003
b) r = 0.15, p = 0.02 c) r = 0.28, p < 0.0001 d) r = 0.33, p < 0.0001 e) r = 0.19, p = 0.003 f) r = 0.23, p = 0.0003 |
| Cuthbertson et al. 2004 [14] | χ2 or Spearman’s correlation
Mann-Whitney U Spearman’s correlation |
a) Admission diagnosis group; patient concern regarding severity/likelihood of death from critical illness/injury a; sex; ICU LOS; APACHE II score
b) Age c) Saw physician for mental health pre-ICU a d) Days of mechanical ventilation |
a) n.s.
b) 49 vs. 62 years (PTSD vs. no PTSD), p = 0.04 c) p = 0.005 d) p = 0.01 |
| Jones et al. 2003 [15] (n = 102) | One-way ANOVA
Fisher’s exact test |
a) No self-help rehabilitation manual at 8 weeks (n = 114)
…at 6 months (n = 102) b) “Delusional memories” of ICU 2 weeks after ICU d/c (n = 52) |
a) p = 0.026
n.s. b) 60% vs. 28% (PTSD vs. no PTSD), p = 0.028 |
| Sukantarat et al. 2007 [16] (n = 51) | Spearman’s correlation | Age, ICU LOS, APACHE II score, TISS points | n.s. |
| Girard et al. 2007 [18] (n = 43) | Spearman’s correlation
Wilcoxon rank sum test Multivariable linear regression |
a) Age
b) Cumulative lorazepam dose in ICU c) Cumulative midazolam dose in ICU d) Cumulative fentanyl dose in ICU e) Cumulative morphine dose in ICU f) Cumulative propofol dose in ICU g) Days of mechanical ventilation h) ICU LOS i) Traumatic ICU memories a j) Neuropsychological test scores at follow-up k) APACHE II score l) Days of delirium in ICU m) Female vs. male sex n) Age o) Female sex p) Cumulative lorazepam dose in ICU q) APACHE II score r) Days of delirium in ICU |
a) r = −0.30, p = 0.04
b) r = 0.30, p = 0.05 c) r = −0.22, p = 0.16 d) r = 0.09, p = 0.56 e) r = 0.07, p = 0.66 f) r = −0.16, p = 0.30 g) r = 0.03, p = 0.83 h) r = 0.10, p = 0.51 i) r = 0.37, p = 0.02 j) r = −0.08, p = 0.63 k) r = 0.04, p = 0.80 l) r = 0.03, p = 0.84 m) median PTSS-10 score 22 vs. 17, p = 0.06 n) nonlinear effect, p = 0.04 o) β = 7.4, p = 0.02 p) β = 0.39, p = 0.001 q) β = 0.02, p = 0.90 r) β = 0.91, p = 0.31 |
| Nickel et al. 2004 [19] (n = 41) | Fisher’s exact test
Mann-Whitney U |
a) Previous psychiatric diagnosis a
b) APACHE II score |
a) SCID PTSD, p = 0.025
PTSS-10≥35, p = 0.11 b) 20 vs. 22 (SCID PTSD), p = 0.67 |
| Rattray et al. 2005 [20] (n = 60–80) | Repeated measures
ANOVA Pearson’s correlation Multivariable regression |
a) Time since hospital d/c (d/c, 6 months, 12 months)
b) Sex c) Age d) APACHE II score e) ICU LOS f) Hospital LOS g) Awareness of surroundings in ICU (hospital d/c) h) Frightening ICU memories (hospital d/c) i) Satisfaction with ICU care (hospital d/c) j) Recall of ICU experience (hospital d/c) k) IES-A at hospital d/c l) IES-I at hospital d/c |
IES scores (square roots)
a) 2.4 vs. 2.1 vs. 2.4 (IES-A), p = 0.34 2.7 vs. 2.4 vs. 2.6 (IES-I), p = 0.42 b) n.s. c) r = −0.25 with IES-A at hospital d/c, p = 0.02 r = −0.38 with IES-A at 6 mos, p = 0.003 r = −0.33 with IES-A at 12 mos, p = 0.003 r = −0.41 with IES-I at hospital d/c, p = 0.0002 r = −0.27 with IES-I at 6 mos, p = 0.04 r = −0.32 with IES-I at 12 mos, p = 0.004 d) n.s. e) r = 0.26 with IES-I at 12 months, p = 0.02 f) r = −0.25 with IES-I at hospital d/c, p = 0.02 g) n.s. h) r = 0.43 with IES-A at hospital d/c, p < 0.0001 r = 0.43 with IES-A at 6 mos, p = 0.0006 r = 0.47 with IES-A at 12 mos, p < 0.0001 r = 0.53 with IES-I at hospital d/c, p < 0.0001 r = 0.39 with IES-I at 6 mos, p = 0.002 r = 0.41 with IES-I at 12 mos, p = 0.0002 i) n.s. j) r = −0.29 with IES-I at hospital d/c, p = 0.009 k) βst = 0.42 with IES-A at 6 months, p = 0.001 k) βst = 0.19 with IES-A at 6 months, p = 0.0001 βst = 0.19 with IES-A at 12 months, p = 0.10 l) βst = 0.46 with IES-I at 6 months, p = 0.001 βst = 0.50 with IES-I at 12 months, p ≤ 0.0005 |
| Kress et al. 2003 [21] (n = 32) | T-test
MANOVA Fisher’s exact test |
No daily sedative interruption in the ICU vs. daily sedative interruption in the ICU | 27 vs. 11 IES points, p = 0.02
16 vs. 8 IES-A points & 14 vs. 6 IES-I points, p = 0.055 32% versus 0% with PTSD, p = 0.06 |
| Scragg et al. 2001 [22] (n = 77) | Pearson’s correlation & multiple regression with transformed variables | Age, ICU LOS, Time since ICU d/c | n.s. |
| Schelling et al. 2001 [23] (n = 20) | Mann-Whitney U, χ2, or Fisher’s Exact test
Spearman’s correlation |
a) Sex
b) Age c) Cause of sepsis d) Average GCS score e) Serum cortisol in ICU f) No hydrocortisone vs. hydrocortisone in ICU g) Duration of septic shock h) Total norepinephrine in ICU i) ICU LOS |
a) p = 0.61
b) median 55 vs. 52 years (PTSD vs. no PTSD), p = 0.85 c) p > 0.15 d) 12.9 vs. 12.5 (PTSD vs. no PTSD), p = 0.79 e) 15.7 vs. 55.4 μg/dL (PTSD vs. no PTSD), p = 0.02 f) 36 vs. 27, p = 0.30 64% vs. 11% with PTSD, p = 0.02 g) r = −0.11, p = 0.63 h) r = −0.10, p = 0.68 I) r = 0.22, p = 0.36 |
| Schelling et al. 1999 [24] (n = 54) | Mann-Whitney U or χ2 | a) Sex
b) Age c) Cause of sepsis d) ICU LOS in days e) At least one traumatic ICU memory vs. none a f) No hydrocortisone vs. hydrocortisone in ICU g) In those with at least one traumatic memory, a no hydrocortisone vs. hydrocortisone in ICU h) In those with no traumatic memories, a no hydrocortisone vs. hydrocortisone in ICU |
a) 44% female, 28% male (PTSD vs. no PTSD), p = 0.24
b) median 53 vs. 55 years (PTSD vs. no PTSD), p = 0.13 c) p = 0.56 d) median 45 vs. 29 (PTSD vs. no PTSD), p = 0.046 e) median 31 vs. 21, p = 0.03 f) 59% vs. 18% with PTSD, p = 0.002 g) median 36 vs. 26, p = 0.041 62% vs. 21% with PTSD, p = 0.009 h) median 22 vs. 20, p = 1.00; 33% vs. 14% with PTSD, p = 0.35 |
Abbreviations (in alphabetical order): ANOVA = analysis of variance; APACHE II = Acute Physiology and Chronic Health Evaluation II; β = linear regression coefficient in multivariable model; βst = standardized linear regression coefficient in multivariable model; CI = confidence interval; d/c = discharge; “delusional memories” = memories of in-ICU psychotic/nightmare experiences; GCS = Glasgow Coma Scale; GP = general practitioner; HAD = Hospital Anxiety and Depression Scale; ICU = intensive care unit; IES-A = Impact of Events Scale-Avoidance Subscale; IES-I = Impact of Events Scale-Intrusion Subscale; LOS = length of stay; MAAS = Motor Activity Assessment Scale; MANOVA = multivariate analysis of variance; n.s. = not significant; OR = odds ratio; TISS = Therapeutic Intervention Scoring System; “-” = not reported.
Factor assessed retrospectively
In-ICU disease and treatment factors were examined as prospective risk factors in thirteen studies. Agitation while in the ICU was a significant predictor in the one study in which this was examined [12]. Similarly, physical restraint in the ICU was a significant predictor in the one study in which this was examined [13]. Four studies examined benzodiazepine sedation (operationalized in various ways); the following were significant predictors: total dose of lorazepam in the ICU in one study [18], receipt/dose of midazolam in the ICU in one of two studies [12, 18], total dose of benzodiazepine in the ICU in one study [13], and absence of daily sedative interruption in the ICU in one study [21] (in this last study, infusions of benzodiazepines and other sedating drugs were interrupted simultaneously). Receipt/dose of opiates in the ICU was a significant predictor in one of three studies [12, 13, 18]. In two related studies of septic shock, not receiving hydrocortisone was a significant predictor of PTSD [23, 24]; in one of these studies, a low serum cortisol in the ICU was also a significant predictor [23]. Duration of mechanical ventilation was a significant predictor in one of three studies [12, 14, 18], and ICU length of stay was a significant predictor in one of nine studies [11, 12, 14, 16, 18, 20, 22–24]. Notably, one prospectively assessed potential risk factor was consistently not associated with later PTSD symptoms: severity of critical illness, as measured using the Acute Physiology and Chronic Health Evaluation II score (APACHE II– seven of seven studies) [11, 12, 14, 16, 18–20].
Several studies examined early post-ICU memories of in-ICU experiences as predictors of subsequent PTSD symptoms (these memories were assessed 5 or 14 days post-ICU or at hospital discharge). Early “delusional memories” (memories of in-ICU psychotic/nightmare experiences) predicted later PTSD symptoms in 3 of 4 studies [11–13, 15], and early memories of in-ICU frightening experiences predicted later PTSD symptoms in two of two studies [12, 20].
Pre-ICU anxiety or depressive disorder, assessed two weeks post-ICU, was a significant predictor of post-ICU PTSD in one of two studies [11, 13]. The study that did not find a significant association was small (n = 30) [11].
Four studies evaluated potential risk factors for PTSD symptoms cross-sectionally (i.e., risk factors were measured at the same time as PTSD symptoms). These included the following: recall of traumatic or frightening ICU experiences in two of two studies [18, 24]; higher trait anxiety in one of one study [11]; prior mental health treatment-seeking with a physician in one of one study [14]; and a pre-ICU history of psychiatric disorder in one of one study [19].
Associations of Posttraumatic Stress Symptoms/Disorder and Health-Related Quality of Life
Only four studies evaluated HRQOL [16, 18, 20, 24], and only two assessed relationships between PTSD symptoms and HRQOL [16, 18] (Table 4). Symptoms of PTSD were associated with lower HRQOL in both studies. In the Sukantarat et al. study, which employed the SF-36 [25], symptoms of PTSD were more strongly associated with mental health HRQOL domains (vitality, social functioning, emotional role, and general mental health) than with physical health HRQOL domains (physical functioning, role-physical, bodily pain, and general health).
Table 4.
Associations of posttraumatic stress symptoms/disorder and health-related quality of life
| Study (n) | Measure of Association | Follow-up in Months | Posttraumatic Stress Symptoms/Disorder Instrument | Health-Related Quality of Life Instrument(s) | Test Value(s) |
|---|---|---|---|---|---|
| Sukantarat et al.2007 [16] (n = 51) | Spearman’s correlation | 3 | IES-A | EQ-5D | r = −0.34, p < 0.005 |
| SF-36 PCS | r = −0.28, n.s. | ||||
| SF-36 MCS | r = −0.34, p < 0.05 | ||||
| IES-I | EQ-5D | r = −0.24, p > 0.05 | |||
| SF-36 PCS | r = −0.06, n.s. | ||||
| SF-36 MCS | r = −0.38, p < 0.05 | ||||
| 9 | IES-A | EQ-5D | r = −0.50, p < 0.001 | ||
| SF-36 PCS | r = −0.23, n.s. | ||||
| SF-36 MCS | r = −0.48, p < 0.001 | ||||
| IES-I | EQ-5D | r = −0.50, p < 0.001 | |||
| SF-36 PCS | r = −0.16, n.s. | ||||
| SF-36 MCS | r = −0.49, p < 0.001 | ||||
|
| |||||
| Girard et al. 2007 [18] (n = 43) | Spearman’s correlation | 6 | PTSS-10 | SF-12 | r = −0.57, p < 0.0001 |
Abbreviations (in alphabetical order): EQ-5D = Euro-quality of life; IES-A = Impact of Events Scale-Avoidance Subscale; IES-I = Impact of Events Scale-Intrusion Subscale; n.s. = not significant; PTSS-10 = Post Traumatic Symptom Scale-10; SF-12 = Short-Form-12; SF-36 MCS = Short Form-36 Mental Component Summary; SF-36 PCS = Short Form-36 Physical Component Summary.
DISCUSSION
This systematic review of PTSD in general ICU survivors highlights three important issues. First, the prevalence of substantial post-ICU PTSD symptoms is high, and symptoms appear to persist over time. Across studies, the median point prevalence of questionnaire-ascertained substantial PTSD symptoms was 22%, and the median point prevalence of clinician-diagnosed PTSD was 19%. These figures are quite a bit higher than the 3.5% one-year prevalence of PTSD in a recent study of US adults that utilized non-clinicians administering a structured interview [26]. In addition, these figures are as high as, or higher than, the median point prevalences of substantial PTSD symptoms in survivors of myocardial infarction (16%) or cardiac surgery (17%) in a recent review of studies that used three of the same PTSD measures [27]. Also, these figures are only slightly lower than the median point prevalence of substantial PTSD symptoms in ALI/ARDS survivors (28%) in a recent systematic review of studies that used two of the same PTSD questionnaires [9]. Importantly, since pre-ICU psychopathology appears a potent risk factor for post-ICU PTSD, and several of the studies reviewed here excluded patients with prior psychopathology, our prevalence estimates for post-ICU PTSD are very likely underestimates for the population of all ICU survivors.
Second, consistent predictors of post-ICU PTSD include pre-ICU psychopathology, greater ICU benzodiazepine administration, and post-ICU memories of in-ICU frightening and/or psychotic experiences. Female sex and younger age were less consistent predictors, and severity of critical illness was consistently not a predictor. Pre-trauma psychopathology, female sex, and younger age appear to be general risk factors for PTSD [28]. We note that, though we previously speculated that durations of mechanical ventilation and ICU length of stay may increase risk for post-ICU PTSD [9], we found little evidence for this here.
Third, post-ICU PTSD may have a substantial impact on quality of life. Though only two of the included studies addressed this issue, the results are consistent with findings in ALI/ARDS survivors [9] and the general PTSD literature [29, 30].
The existing literature has several important limitations. First, most of the studies relied exclusively on questionnaires (i.e., screening instruments) to estimate the burden of PTSD symptoms in ICU survivors. Only three of the fifteen studies utilized expert clinicians and diagnostic interviews, and, of the questionnaires employed, only the PTSS-10 has been validated against clinician diagnoses in the post-ICU setting [31]. Future studies of PTSD in general ICU survivors should utilize expert clinicians to conduct diagnostic interviews in larger cohorts, to more definitively estimate prevalence and incidence, and to evaluate the psychometric properties of other existing questionnaires, such as the IES, in this setting; as noted in the Results section, different investigators employed different thresholds in their studies.
Second, additional research is required to understand risk factors for post-ICU PTSD. We first consider patient-specific risk factors. Only one study related personality traits to PTSD symptoms [11], and, in that study, patients rated trait anxiety and PTSD symptoms simultaneously, so it remains unclear whether patients’ symptom states affected their self-reports of traits [32]. Nevertheless, the results are consistent with the general PTSD literature, which suggests that high neuroticism (a more general tendency to experience negative emotions) is a risk factor for PTSD [33–35]. State-related recall could also have biased patients’ reports of pre-ICU psychopathology, as it appears the latter was assessed after the ICU stay in each case; i.e., patients with more acute stress disorder or PTSD symptoms may have recalled pre-ICU psychiatric illness more readily.
ICU-related risk factors also require clarification. First, it remains unclear to what extent and through what mechanism benzodiazepine sedation might relate causally to post-ICU PTSD. That is, benzodiazepine administration might reflect clinicians’ management of patients’ in-ICU anxiety/agitation, the latter of which may be a marker of risk for, or prodrome of, PTSD. Notably, one study found that pre-ICU anxiety or depression was associated with more in-ICU benzodiazepine administration [13]. Of the studies reviewed, the closest approximation to a clinical trial was a small study with a low follow-up proportion (n = 32, 40% of survivors) on the effects of sedative interruption during mechanical ventilation on subsequent psychopathology [21]. If benzodiazepines are a cause of post-ICU PTSD, an intermediate mechanism may be delirium with associated frightening psychotic experiences. That is, since psychotic symptoms and nightmares are common in delirious patients, and post-ICU memories of in-ICU psychotic experiences are strong predictors of PTSD, delirium may be a true risk factor for PTSD in the ICU setting. Only one of the eligible studies explicitly examined delirium as a risk factor for post-ICU PTSD [18]; although no significant association was evident, this could be due to a lack of statistical power (n = 43) or insensitivity of delirium operationalization or measurement (i.e., number of days of delirium measured with a daily clinical exam). Interestingly, a history of anxiety and/or depressive disorders prior to ICU admission predicted post-ICU memories of in-ICU psychotic experiences in two of two studies in which this was assessed [11, 13]; also, a growing number of studies provide evidence that pre-hospital depression is a risk factor for in-hospital delirium [36–38]. Thus, patients with a history of anxiety or depression may be particularly prone to in-ICU delirium and associated psychotic experiences, as well as subsequent PTSD, and the causal mechanisms remain unclear. Clarification of these issues is an important goal for future research that could facilitate prevention. For example, if delirium itself is causally related to post-ICU PTSD, minimization of delirium – e.g., via use of alternative sedatives [39] – would have important longer-term mental health benefits. We note that some form/level of sedation is required for mechanically ventilated patients, not just for humane practice but also to reduce oxygen demands and promote synchronous breathing with ventilators [40].
Additionally, how, and in what circumstances, systemic corticosteroid administration may prevent PTSD is also unclear. The small studies (n = 20–54) reviewed here suggest that stress-dose hydrocortisone is preventative in the context of septic shock [23, 24]. The authors speculated that administration of corticosteroids may have resulted in less endogenous catecholamine release (through an endogenous feedback mechanism) and less need for exogenous catecholamine administration in order to maintain adequate blood pressure; indeed, there was some evidence for the latter in the one reviewed study in which this was assessed [23]. Another mechanism the authors considered is that hydrocortisone (and minimization of catecholamines) could have advantageously affected memory formation and retrieval. We note that corticosteroids may also be preventative via minimization of systemic inflammation; i.e., some evidence suggests that inflammatory cytokines are associated with general medical illness-related psychopathology [41, 42]; to our knowledge, this potential mechanism has not been studied in the ICU setting. Importantly, though Schelling et al. have reported that stress-dose hydrocortisone was similarly beneficial in the context of cardiac surgery [43, 44], we know of no studies of PTSD-preventative effects of stress-dose corticosteroids in general ICU patients. Also, a recent study of surgical abdominal peritonitis patients did not find that ICU corticosteroid administration prevented later PTSD in survivors [45]. Thus, it is unclear whether administration of stress-dose corticosteroids would have PTSD-preventative effects in most critically ill patients.
Studies that integrate patient-specific and ICU-related risk factors are needed. Taking a step in this direction, Jones et al. developed a structural equation model of risk factors for post-ICU PTSD [13]; their model suggests that pre-ICU anxiety or depressive disorders, sedation and physical restraint, and recall of in-ICU nightmares/psychotic experiences have individual and combined effects on the development of post-ICU PTSD. More research is needed to understand the role that these and other potential etiologic factors (e.g., sepsis, hypoxia, delirium, and corticosteroid/catecholamine administration) may play. We feel that critical illness/ICU treatment is promising as a model to investigate the development of psychopathology in the context of extreme stress, as intensive monitoring of clinical state, physiology, hormones, and catecholamines are possible at the time of the stress. Though knowledge regarding the neurobiology of PTSD is increasing, inconsistent findings are common, and consideration of context (within and outside individuals) appears vital [46].
There are several potentially important limitations to this systematic review. First, confidence regarding the precision and validity of our findings should be tempered given the methodological issues described above. Second, though the results suggest some stability in levels of PTSD symptoms over the first 6–12 months post-ICU, we did not feel we could draw strong conclusions regarding the effect of time on prevalence of substantial PTSD symptoms, given heterogeneity in instruments (for which thresholds may not be equivalent), in thresholds within instrument (i.e., the IES), and in sample compositions. Notably, the studies of septic shock survivors had much longer follow-up periods than the other studies, and the prevalences of substantial PTSD symptoms were as high as those in studies with shorter follow-up periods. Though it is possible that septic shock is associated with a particularly high prevalence and/or persistence of substantial PTSD symptoms, we are unable to draw strong conclusions without additional data. Third, although we did not include studies that focused exclusively on survivors from trauma, neurological, coronary, or surgical ICUs, individual patients in the reviewed studies did have other potential risk factors for PTSD, including trauma, surgery, and heart disease; thus, we cannot conclude that the high prevalences reported are specific to critical illness/ICU treatment itself. Finally, despite a comprehensive search of 16,301 citations, potentially eligible studies may have been missed due to inconsistent indexing in electronic databases.
We conclude that, in general ICU survivors, substantial PTSD symptoms are common and may negatively impact HRQOL. Risk factors for post-ICU PTSD appear to include pre-ICU psychopathology, greater in-ICU benzodiazepine sedation, and in-ICU frightening/psychotic experiences, though further work is necessary to clarify causal mechanisms. In the meantime, clinicians should recognize that PTSD is common following intensive care, necessitating collaboration between intensivists, primary care physicians, and psychiatrists to ensure prompt, comprehensive evaluations and treatment.
Acknowledgments
Dr. Needham is supported by a Clinician-Scientist Award from the Canadian Institutes of Health Research and by the National Institutes of Health (Acute Lung Injury SCCOR Grant # P050 HL73994). Dr. Bienvenu is supported by a Career Development Award from the National Institutes of Health (K23 MH64543).
The authors thank Christina Jones, R.N., Ph.D., John P. Kress, M.D., Joan (Perrins) Maclean, R.N., Ph.D., Janice Rattray, R.N., Ph.D., Peter Scragg, Ph.D., and Kannika Sukantarat, Ph.D., for providing additional data from their studies included in this review.
Footnotes
The authors have no competing interests or potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Quality Measures Clearinghouse. Intensive care: percentage of adult patients having had an intensive care unit (ICU) stay whose hospital outcome is death. [Accessed March 21, 2008]; http://www.qualitymeasures.ahrq.gov/summary/summary.aspx?doc_id=8026.
- 2.Angus DC, Carlet J. Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29:368–77. doi: 10.1007/s00134-002-1624-8. [DOI] [PubMed] [Google Scholar]
- 3.Broomhead LR, Brett SJ. Clinical review: intensive care follow-up - what has it told us? Crit Care. 2002;6:411–17. doi: 10.1186/cc1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowdy DW, Eid MP, Sedrakyan A, et al. Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med. 2005;31:611–20. doi: 10.1007/s00134-005-2592-6. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130:869–78. doi: 10.1378/chest.130.3.869. [DOI] [PubMed] [Google Scholar]
- 6.Jones C, Griffiths RD, Humphris G. Disturbed memory and amnesia related to intensive care. Memory. 2000;8:79–94. doi: 10.1080/096582100387632. [DOI] [PubMed] [Google Scholar]
- 7.Jackson JC, Hart RP, Gordon SM, et al. Post-traumatic stress disorder and post-traumatic stress symptoms following critical illness in medical intensive care unit patients: assessing the magnitude of the problem. Crit Care. 2007;11:R27. doi: 10.1186/cc5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33:1506–18. doi: 10.1007/s00134-007-0730-z. [DOI] [PubMed] [Google Scholar]
- 9.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70:512–19. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrins J, King N, Collings J. Assessment of long-term psychological well-being following intensive care. Intensive Crit Care Nurs. 1998;14:108–16. doi: 10.1016/s0964-3397(98)80351-0. [DOI] [PubMed] [Google Scholar]
- 11.Jones C, Griffiths RD, Humphris G, Skirrow PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001;29:573–80. doi: 10.1097/00003246-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Samuelson KAM, Lundberg D, Fridlund B. Stressful memories and psychological distress in adult mechanically ventilated intensive care patients: a 2-month follow-up study. Acta Anaesthesiol Scand. 2007;51:671–8. doi: 10.1111/j.1399-6576.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones C, Backman C, Capuzzo M, et al. Precipitants of post-traumatic stress disorder following intensive care: a hypothesis generating study of diversity in care. Intensive Care Med. 2007;33:978–85. doi: 10.1007/s00134-007-0600-8. [DOI] [PubMed] [Google Scholar]
- 14.Cuthbertson BH, Hull A, Strachan M, Scott J. Post-traumatic stress disorder after critical illness requiring general intensive care. Intensive Care Med. 2004;30:450–55. doi: 10.1007/s00134-003-2004-8. [DOI] [PubMed] [Google Scholar]
- 15.Jones C, Skirrow P, Griffiths RD, et al. Rehabilitation after critical illness: a randomized, controlled trial. Crit Care Med. 2003;31:2456–61. doi: 10.1097/01.CCM.0000089938.56725.33. [DOI] [PubMed] [Google Scholar]
- 16.Sukantarat K, Greer S, Brett S, Williamson R. Physical and psychological sequelae of critical illness. Br J Health Psychol. 2007;12:65–74. doi: 10.1348/135910706X94096. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths J, Gager M, Alder N, et al. A self-report-based study of the incidence and associations of sexual dysfunction in survivors of intensive care treatment. Intensive Care Med. 2006;32:445–51. doi: 10.1007/s00134-005-0048-7. [DOI] [PubMed] [Google Scholar]
- 18.Girard TD, Shintani AK, Jackson JC, et al. Risk factors for post-traumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit Care. 2007;11:R28. doi: 10.1186/cc5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nickel M, Leiberich P, Nickel C, et al. The occurrence of posttraumatic stress disorder in patients following intensive care treatment: a cross-sectional study in a random sample. J Intensive Care Med. 2004;19:285–90. doi: 10.1177/0885066604267684. [DOI] [PubMed] [Google Scholar]
- 20.Rattray JE, Johnston M, Wildsmith JA. Predictors of emotional outcomes of intensive care. Anaesthesia. 2005;60:1085–92. doi: 10.1111/j.1365-2044.2005.04336.x. [DOI] [PubMed] [Google Scholar]
- 21.Kress JP, Gehlbach B, Lacy M, et al. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168:1457–61. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]
- 22.Scragg P, Jones A, Fauvel N. Psychological problems following ICU treatment. Anaesthesia. 2001;56:9–14. doi: 10.1046/j.1365-2044.2001.01714.x. [DOI] [PubMed] [Google Scholar]
- 23.Schelling G, Briegel J, Roozendaal B, et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50:978–85. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- 24.Schelling G, Stoll C, Kapfhammer HP, et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder and health-related quality of life in survivors. Crit Care Med. 1999;27:2678–83. doi: 10.1097/00003246-199912000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 26.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spindler H, Pedersen SS. Posttraumatic stress disorder in the wake of heart disease: prevalence, risk factors, and future research directions. Psychosom Med. 2005;67:715–23. doi: 10.1097/01.psy.0000174995.96183.9b. [DOI] [PubMed] [Google Scholar]
- 28.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clinical Psychol. 2000;68:748–66. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 29.Malik ML, Connor KM, Sutherland SM, et al. Quality of life and posttraumatic stress disorder: a pilot study assessing changes in SF-36 scores before and after treatment in a placebo-controlled trial of fluoxetine. J Trauma Stress. 1999;12:387–93. doi: 10.1023/A:1024745030140. [DOI] [PubMed] [Google Scholar]
- 30.Zatzick DF, Marmar CR, Weiss DS, et al. Posttraumatic stress disorder and functioning and quality of life outcomes in a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1997;154:1690–95. doi: 10.1176/ajp.154.12.1690. [DOI] [PubMed] [Google Scholar]
- 31.Stoll C, Kapfhammer HP, Rothenhausler HB, et al. Sensitivity and specificity of a screening test to document traumatic experiences and to diagnose post-traumatic stress disorder in ARDS patients after intensive care treatment. Intensive Care Med. 1999;25:697–704. doi: 10.1007/s001340050932. [DOI] [PubMed] [Google Scholar]
- 32.Bienvenu OJ, Stein MB. Personality and anxiety disorders: a review. J Personal Disord. 2003;17:139–51. doi: 10.1521/pedi.17.2.139.23991. [DOI] [PubMed] [Google Scholar]
- 33.Bramsen I, Dirkzwager AJ, van der Ploeg HM. Predeployment personality traits and exposure to trauma as predictors of posttraumatic stress symptoms: a prospective study of former peacekeepers. Am J Psychiatry. 2000;157:1115–19. doi: 10.1176/appi.ajp.157.7.1115. [DOI] [PubMed] [Google Scholar]
- 34.Breslau N. Epidemiologic studies of trauma, posttraumatic stress disorder, and other psychiatric disorders. Can J Psychiatry. 2002;47:923–9. doi: 10.1177/070674370204701003. [DOI] [PubMed] [Google Scholar]
- 35.Fauerbach JA, Lawrence JW, Schmidt CW, Munster AM, Costa PT. Personality predictors of injury-related posttraumatic stress disorder. J Nerv Ment Dis. 2000;188:510–7. doi: 10.1097/00005053-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 36.McAvay GJ, Van Ness PH, Bogardus ST, et al. Depressive symptoms and the risk of incident delirium in older hospitalized adults. J Am Geriatr Soc. 2007;55:684–91. doi: 10.1111/j.1532-5415.2007.01150.x. [DOI] [PubMed] [Google Scholar]
- 37.Minden SL, Carbone LA, Barsky A, et al. Predictors and outcomes of delirium. Gen Hosp Psychiatry. 2005;27:209–14. doi: 10.1016/j.genhosppsych.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Pompei P, Foreman M, Rudberg M, et al. Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc. 1994;42:809–15. doi: 10.1111/j.1532-5415.1994.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 39.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–53. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 40.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–41. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 41.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2005;56:819–24. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Musselman DL, Miller AH, Porter MR, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158:1252–7. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 43.Schelling G, Kilger E, Roozendaal B, et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biol Psychiatry. 2004;55:627–33. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Weis F, Kilger E, Roozendaal B, et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. Journal Thorac Cardiovasc Surg. 2006;131:277–82. doi: 10.1016/j.jtcvs.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 45.Boer KR, van Ruler O, van Emmeril AAP, et al. Factors associated with posttraumatic stress symptoms in a prospective cohort of patients after abdominal sepsis: a nomogram. Intensive Care Med. 2008;34:664–74. doi: 10.1007/s00134-007-0941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shalev AY, Segman RH. Commentary: biological findings in PTSD – too much or too little? Prog Brain Res. 2008;167:187–99. doi: 10.1016/S0079-6123(07)67013-7. [DOI] [PubMed] [Google Scholar]


