Abstract
This report addresses the concept that permissible HLA mismatching, i.e. mismatches that do not generate an allogeneic response, in hematopoietic stem cell transplantation (HCT) can be determined with structural similarity of polymorphic regions. We have applied the triplet version of a structural algorithm called HLAMatchmaker that considers short sequences involving polymorphic amino acid residues on the molecular surface as key elements of immunogenic epitopes. The triplet matching effect was analyzed in a National Marrow Donor Program dataset consisting of 744 unrelated hematopoietic cell transplantation cases with one HLA-A,-B or -C mismatch and 1690 fully HLA-A,-B,-C,-DR,-DQ allele matched cases. In multivariate models adjusting for other significant clinical risk factors, the degree of triplet mismatching did not significantly correlate with patient survival, engraftment or acute graft versus host disease. Other structurally based strategies should be pursued to identify permissible HLA mismatches in HCT.
Introduction
Although the outcome of unrelated donor hematopoietic cell transplants (HCT) can be optimized by matching for HLA-A,-B,-C, and -DRB1 alleles, many patients have no access to such matched donors. A recent analysis of the National Marrow Donor Program (NMDP) experience has shown that about one-quarter of patients received one allele mismatched HCT primarily involving the HLA-A,-B and -C loci [1]. Patients with such transplants have, however, less successful engraftment, more acute graft versus host (GVH) disease and lower survival rates [1–4]. This problem might be overcome with HCT from donors with “permissible” mismatches that do not increase patient morbidity and mortality. Permissive mismatches can be defined as HLA allele differences between the donor and recipient that do not elicit an allogeneic GVH or rejection response.
At least two studies have considered structural similarity among HLA allelic products as a means of predicting improved outcome. In a previous analysis of NMDP data, HLA mismatching within a serological cross-reactive group (CREG) at HLA-A or –B was not associated with a survival benefit in comparison to mismatches outside a CREG [5]. The findings suggest that serologically defined similarities between mismatched HLA class I antigens are not indicative of permissive mismatches. A second approach to define permissive mismatching used an algorithm, Histocheck, that applies the so-called distance index of Risler [6] to assess functional similarities between amino acid substitutions on disparate HLA molecules [7]. Similarity as measured by a low Histocheck score offered no benefit to patients with bone marrow transplants from class I mismatched donors [8]. At present, the criteria for HLA mismatch permissibility are not readily defined [9].
HLAMatchmaker is a matching algorithm that considers the structural basis of epitopes on HLA antigens. Each class I HLA antigen is viewed as a string of short sequences (triplets) involving polymorphic amino acid residues on the molecular surface. These residues are hypothesized to comprise key elements of epitopes that can induce HLA-specific antibodies [10, 11]. Donor-recipient compatibility is assessed by determining which triplets in corresponding sequence positions are different. The degree of structural compatibility of an HLA mismatch can vary between zero or few triplets to more than 15 triplets. The triplet matching concept has clinical relevance as suggested by an analysis of two large kidney transplant databases showing that HLA-A, -B mismatched kidneys that are compatible at the triplet level exhibit almost identical graft survival rates as the zero HLA-A, -B antigen mismatches defined by conventional criteria [12]. Moreover, the degree of humoral sensitization correlates with the number of mismatched triplets on immunizing HLA antigens [13–15]. HLAMatchmaker has also been used in refining and expanding platelet donor selection for refractory, thrombocytopenic patients [16, 17].
We have addressed the hypothesis that matching at the structural level using HLAMatchmaker permits the identification of permissible mismatches for HCT. This report describes our analysis of the effect of class I HLA mismatches with minimal mismatched triplets on HCT outcome.
Materials and Methods
NMDP database and patient characteristics
Patients reported to the NMDP who were transplanted between 1988 and 2003 and fully HLA-typed through the NMDP’s ongoing retrospective high resolution typing project were included in this analysis (Table 1). Eligible diagnoses included acute lymphoblastic leukemia (ALL, N=581), acute myeloid leukemia (AML, N=676), chronic myeloid leukemia (CML, N=954) and myelodysplastic syndrome (MDS, N=223). Early stage disease (N=1046) was defined as AML and ALL in first complete remission, CML in first chronic phase and MDS subtype refractory anemia. Intermediate stage disease (N=937) was AML or ALL in second or subsequent complete remission or in first relapse, and CML in accelerated phase or second chronic phase. Advanced phase disease (N=448) was AML in second or higher relapse or primary induction failure, CML in blast phase, MDS subtypes refractory anemia with excess blasts or in transformation, or MDS, not otherwise classified. All patients received standard myeloablative conditioning regimens.
Table 1.
Patient characteristics of different mismatched groups in GVH direction
| 0Allele MM |
0Triplet MM |
1Triplet MM |
2–3Triplet MM |
4–5 Triplet MM |
6+Triplet MM |
|
|---|---|---|---|---|---|---|
| Number of patients | 1690 | 241 | 140 | 143 | 102 | 118 |
| Number of centers | 105 | 72 | 59 | 54 | 44 | 47 |
| Median age (years) (Range) | 35(<1–65) | 32(<1–60) | 35(1–58) | 30(<1–57} | 32(1–59) | 28(2–65) |
| Male gender | 960 (57%) | 124 (51%) | 73 (52%) | 80 (56%) | 56 (55%) | 64 (54%) |
| ≥ 90 Karnofsky Score | 1225 (75%) | 168 (71%) | 101(74%) | 100 (72%) | 79 (81%) | 86 (74%) |
| Disease at transplant | ||||||
| AML | 452 (27%) | 72 (30%) | 41 (29%) | 51 (36%) | 27 (27%) | 33 (28%) |
| ALL | 382 (23%) | 64 (26%) | 30 (22%) | 40 (28%) | 33 (32%) | 32 (27%) |
| CML | 695 (41%) | 89 (37%) | 62 (44%) | 38 (26%) | 32 (31%) | 38 (32%) |
| MDS | 161 ( 9%) | 16 ( 7%) | 7 ( 5%) | 14 (10%) | 10 (10%) | 15 (13%) |
| Disease status | ||||||
| Early | 761 (45%) | 96 (40%) | 64 (46%) | 41 (29%) | 36 (35%) | 48 (41%) |
| Intermediate | 631 (37%) | 104 (43%) | 54 (38%) | 72 (50%) | 34 (33%) | 42 (36%) |
| Advanced | 296 (18%) | 41 (17%) | 22 (16%) | 30 (21%) | 32 (32%) | 27 (23%) |
| Graft type | ||||||
| Marrow | 1561 (92) | 223 (93) | 126 (90) | 128 (90) | 97 (95) | 109 (92) |
| Peripheral blood | 129 ( 8) | 18 ( 7) | 14 (10) | 15 (10) | 5 ( 5) | 9 ( 8) |
| Donor/Recipient CMV match | ||||||
| Negative/Negative | 619 (37) | 73 (30) | 51 (37) | 53 (37) | 31 (30) | 44 (37) |
| Negative/Positive | 463 (27) | 71 (30) | 39 (28) | 36 (25) | 29 (28) | 32 (27) |
| Positive/Negative | 277 (16) | 44 (18) | 24 (17) | 25 (17) | 17 (17) | 15 (13) |
| Positive/Positive | 280 (17) | 49 (20) | 24 (17) | 28 (20) | 22 (22) | 23 (20) |
| Unknown | 51 ( 3) | 4 ( 2) | 2 ( 1) | 1 ( 1) | 3 ( 3) | 4 ( 3) |
| Donor/Recipient sex match | ||||||
| Male/Male | 665 (39) | 82 (34) | 44 (31) | 46 (32) | 30 (29) | 44 (37) |
| Male/Female | 407 (24) | 61 (25) | 35 (25) | 29 (20) | 29 (28) | 33 (28) |
| Female/Male | 295 (18) | 42 (18) | 29 (21) | 34 (24) | 26 (26) | 20 (17) |
| Female/Female | 323 (19) | 56 (23) | 32 (23) | 34 (24) | 17 (17) | 21 (18) |
| GVHD prophylaxis | ||||||
| FK506 ± other | 339 (20) | 58 (24) | 33 (23) | 29 (20) | 19 (19) | 17 (14) |
| CsA + MTX ± other | 984 (58) | 133 (55) | 77 (55) | 64 (45) | 55 (54) | 66 (56) |
| CsA± other (No MTX) | 63 ( 4) | 4 ( 2) | 4 ( 3) | 4 ( 3) | 1 ( 1) | 4 ( 3) |
| T-cell depletion | 285 (17) | 44 (18) | 26 (19) | 43 (30) | 27 (26) | 30 (26) |
| Other | 19 ( 1) | 2 ( 1) | 0 | 3 ( 2) | 0 | 1 ( 1) |
Patients undergoing conditioning regimens of lower intensity, second or subsequent transplantation, or surviving patients who did not provide signed, informed consent to allow analysis of their clinical data or HLA typing of stored NMDP Research Repository samples were excluded. All surviving recipients included in this analysis were retrospectively contacted and provided informed consent for participation in the NMDP research program. Informed consent was waived by the NMDP Institutional Review Board for all deceased recipients. To adjust for the potential bias introduced by exclusion of non-consenting surviving patients, a modeling process randomly excluded the same percentage of deceased patients using a biased coin randomization with exclusion probabilities based on characteristics associated with not providing consent for use of the data in survivors [1].
HLA matching at the triplet level
High resolution typing was performed retrospectively through the NMDP Donor/Recipient Pair Project for HLA-A, B, C, DRB1, and DQB1, as previously described [12]. To evaluate the role of triplet mismatching at the HLA class I loci (HLA-A, B and C) the study population was restricted to cases either fully matched for HLA-A, B, C, DRB1 and DQB1 alleles (10/10 matches) or with a single mismatch at HLA class I (9/10 allele matches). A total of 2434 donor-recipient pairs were included in the analysis, 1690 of which were 10/10 HLA matches. There were 744 donor-recipient pairs that were 9/10 HLA matched with one class I mismatch: 242 (33%) for HLA-A, 97 (13%) for HLA-B and 405 (54%) for HLA-C (Table 1).
The triplet version of HLAMatchmaker was used to assess the degree of structural compatibility between one-allele mismatches [10]. Table 1 shows the distribution of the numbers of mismatched triplets in the Host Versus Graft (HVG) and GVH direction among the allele mismatches for HLA-A, B and C. The 0-triplet mismatch group comprised relatively more HLA-B allele mismatches than the other triplet mismatch groups.
Clinical endpoints
The primary endpoint of this study was patient survival; engraftment and acute GVH disease were secondary endpoints. Two parameters were used to assess successful engraftment: blood neutrophil counts of ≥500/mm3 for 3 consecutive days and blood platelet counts reaching ≥20,000 × 109/L. The incidence of grades II–IV and III–IV acute GVH disease was determined during the first 100 days post-transplant. Events were summarized by the cumulative incidence estimate with death as a competing risk.
Statistical methods
The statistical analyses of transplant outcome parameters comparing matching in HVG and GVH directions were similar to those in a previous report on CREG matching [5]. To compare pre-transplant characteristics for discrete factors, the number of cases and their respective percentages were calculated and Chi-Square testing was done to compare the HLA matched, triplet matched and triplet mismatched groups. For continuous factors, the median and ranges were calculated and the Kruskal-Wallis test was used to analyze differences between HLA matched, triplet matched and triplet mismatched groups. Probabilities of disease-free survival were calculated using the Kaplan-Meier estimator with variance estimated by Greenwood's formula. Comparisons of survival curves were done with the log-rank test.
Multivariate analyses were performed using the proportional hazards model to compare the HLA matched, triplet matched and triplet mismatched groups. Models were fit to determine which risk factors may be related to a given outcome. All variables were tested for the affirmation of the proportional hazards assumption. Factors violating the proportional hazards assumption were adjusted using time-dependent variables or stratification before the stepwise model building approach was used in developing models for the primary and secondary outcomes. Five patients were excluded from the multivariate models because of missing clinical and/or outcome data.
Results
Engraftment
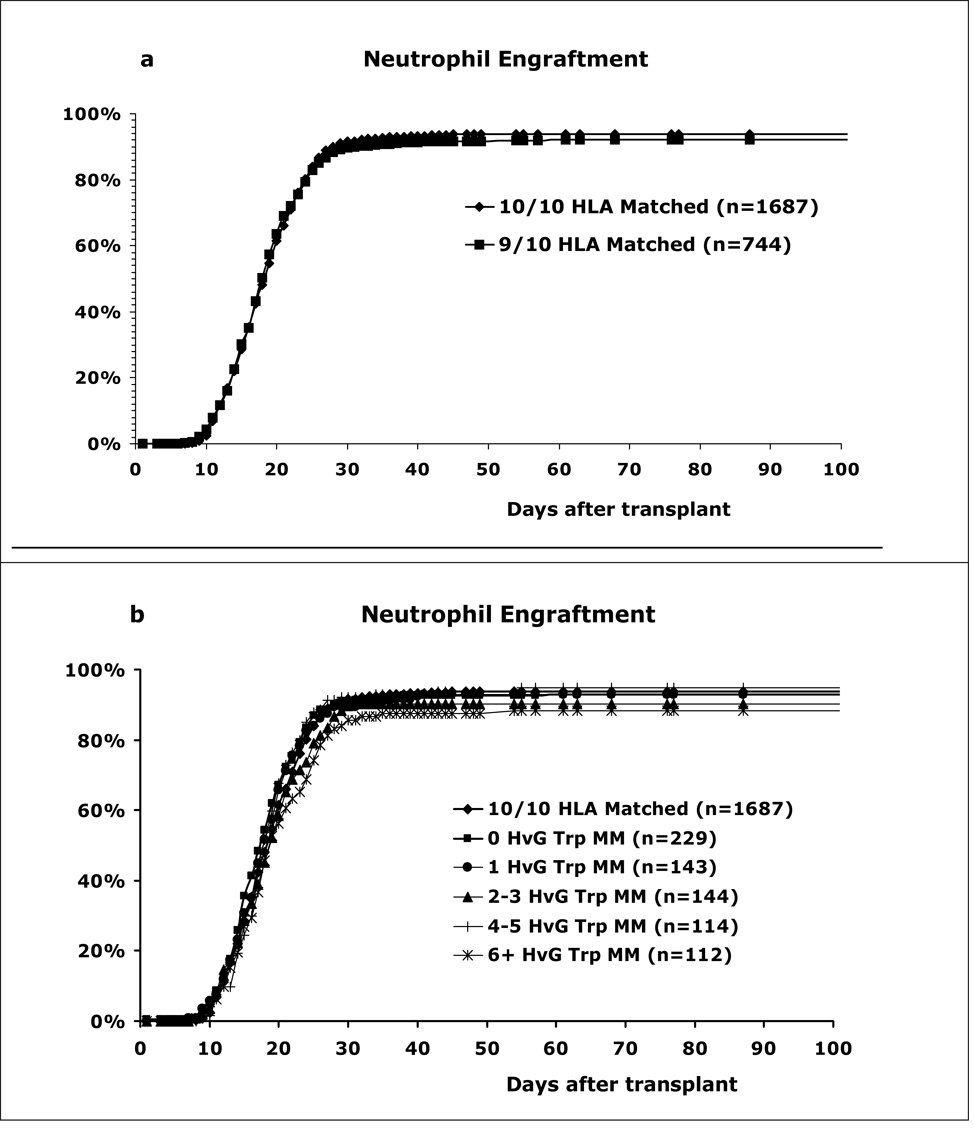
Figures 1a and 1b show the unadjusted cumulative percentages of patients with successful neutrophil engraftment for the 10/10 matched and Host versus Graft (HVG) direction triplet mismatched groups. There was no significant difference in neutrophil engraftment rates at 28 days between the 10/10 and 9/10 allele matched groups (p=0.33). On day 60 post-transplant, the cumulative incidence of neutrophil engraftment was 94% for the 10/10 group, 93% for the 0-triplet mismatches, 93% for the 1-triplet mismatches, 90% for the 2–3 triplet mismatches, 95% for the 4–5 triplet mismatches and 88% for the ≥6 triplet mismatches. The differences between the groups were not statistically significant.
Figure 1. Matching effect on neutrophil engraftment.
(a) The 9/10 matches have slightly lower rates than 10/10 matches (at 28 days p=0.33, NS). (b) Effect of triplet mismatching in the HVG direction. Although the 6+ triplet mismatch group had the lowest neutrophil engraftment rate, the differences between the different match groups were not statistically significant.
Figures 2a and 2b show the unadjusted cumulative percentages of patients with blood platelet counts reaching ≥20,000/mm3 for the 10/10 matched and HVG direction triplet mismatched groups.. The 9/10 matches had significantly lower success rates than the 10/10 matches (after 100 days: 65% versus 72%, p=0.001). At 100 days, the engraftment rates were 67% for the 0-triplet mismatches, 63% for the 1-triplet mismatches, 68% for the 2–3 triplet mismatches, 61% for the 4–5 triplet mismatches and 61% for the ≥6 triplet mismatches. The platelet engraftment differences observed between the triplet mismatched groups were not statistically significant.
Figure 2. Matching effect on platelet engraftment.
(a) The 9/10 match group had a significantly lower engraftment rate than the 10/10 matches (p=0.001 after 100 days). (b) Effect of triplet matching; the 4–5 and 6 triplet mismatch groups had the lowest platelet engraftment rates but the differences between the triplet groups were not statistically significant
Acute GVH disease
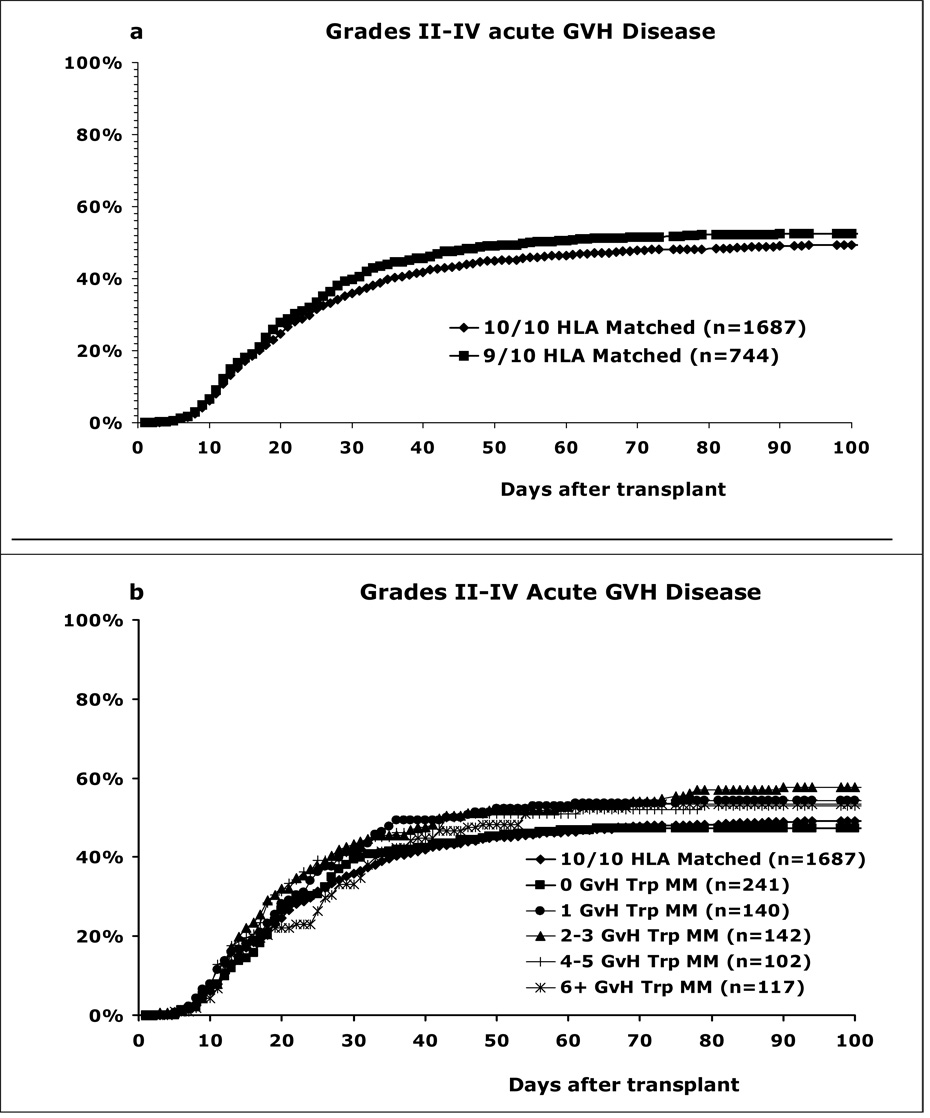
The cumulative incidence of grade II–IV acute GVH disease was higher for the 9/10 matches than the 10/10 matches, 52% versus 49% after 100 days, but this difference was not statistically significant (p=0.15) (Figure 3a). However, in a multivariate analysis adjusting for other risk factors, the 9/10 matches had a significantly higher risk for grade II–IV (p=0.008) and grade III–IV (p<0.001) GVH disease than the 10/10 matches (Table 3). Among the 9/10 matches, the 0-triplet mismatches in the GVH direction had a similar incidence of grade II–IV acute GVH disease as the 10/10 matches: 47% versus 49% after 100 days (Figure 3b). The other triplet mismatches had a higher incidence of acute GVH disease, namely 1 triplet (54%), 2–3 triplets (58%), 4–5 triplets (53%) and ≥6 triplets (53%), but were not statistically different.
Figure 3. HLA matching effect on acute grade II–IV GVH disease.
(a) The 9/10 match group had a statistically insignificant higher incidence than the 10/10 matches; 52% versus 49% after 100 days, p=0.15. (b) Effect of triplet mismatching in the GVH direction. There was no statistically significant correlation between the number of mismatched triplets and the incidence of acute GVH disease.
Table 3.
Multivariate analysis comparing acute GVH disease for 10/10 matches with the 9/10 match groups with triplet mismatches in the GVH direction.
| Match Group | N | Relative risk (95% Confidence Interval) | P - value |
|---|---|---|---|
| Grade II–IV GVH disease | |||
| 10/10 matches | 1687 | 1.00 | 0.008 |
| 0 Triplet mismatches | 241 | 1.04 (0.85 – 1.26) | 0.72 |
| 1 Triplet mismatches | 140 | 1.16 (0.92 – 1.46) | 0.22 |
| 2–3 Triplet mismatches | 142 | 1.46 (1.16 – 1.84) | 0.001 |
| 4–5 Triplet mismatches | 102 | 1.36 (1.03 – 1.79) | 0.031 |
| ≥ 6 Triplet mismatches | 117 | 1.17 (0.91 – 1.50) | 0.23 |
| All triplet groups | 0.16 | ||
| Grade III–IV GVH disease | |||
| 10/10 matches | 1687 | 1.00 | < 0.0001 |
| 0 Triplet mismatches | 241 | 1.23 (0.97–1.57) | 0.10 |
| 1 Triplet mismatches | 140 | 1.46 (1.09–1.95) | 0.01 |
| 2–3 Triplet mismatches | 142 | 1.93 (1.47–2.54) | 0.0001 |
| 4–5 Triplet mismatches | 102 | 1.60 (1.14–2.250 | 0.007 |
| ≥ 6 Triplet mismatches | 117 | 1.39 (1.00–1.92) | 0.048 |
| All triplet groups | 0.13 | ||
Adjusted factors: disease, GVH disease prophylaxis, graft type, Karnofsky score, year of transplant, recipient gender.
A multivariate analysis showed that the risk of grade II–IV GVH disease for the 0, 1, 4–5 and ≥6 triplet mismatches and the risk of grade III–IV GVH disease for the 0 and ≥6 triplet mismatches was not statistically different when compared to the 10/10 matched cohort (Table 3). However, when the individual triplet mismatch groups were compared none of the groups were significantly different from each other (p=0.16 and p=0.13 for grades II–IV and III–IV, respectively). Altogether, this multivariate analysis suggests that the degree of triplet mismatching among the 9/10 matches does not correlate with the risk for acute GVH disease.
Disease-free Survival
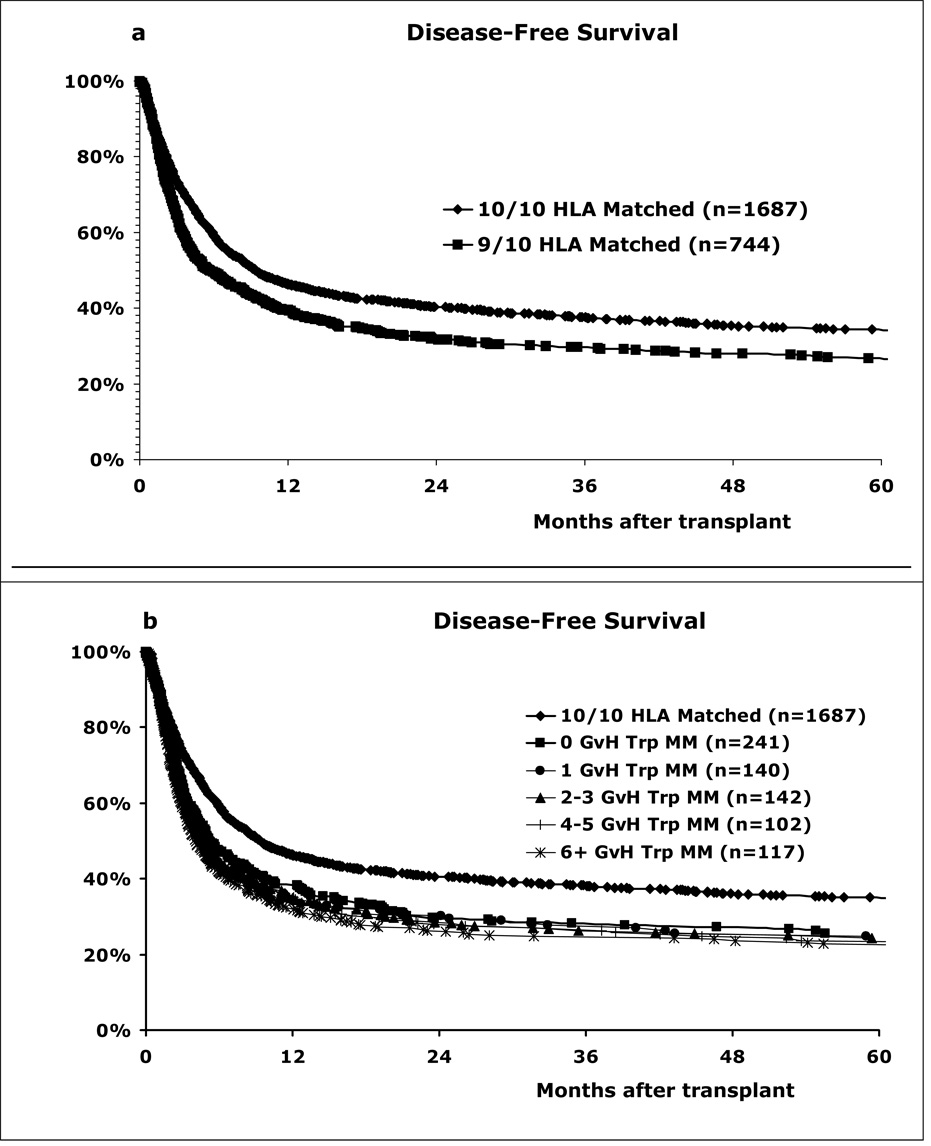
Disease-free survival (DFS) was significantly lower for the 9/10 matches than the 10/10 matches (Figure 4a). Two-year DFS was 32% and 40% for 9/10 and 10/10 groups, respectively (p<0.001). Triplet matching in the GVH direction within the 9/10 matches did not improve DFS (Figure 4b). The two-year unadjusted DFS rates were 31% for the 0-triplet mismatches, 36% for the 1-triplet mismatches, 34% for the 2–3 triplet mismatches, 29% for the 4–5 triplet mismatches and 29% for the ≥6 triplet mismatches. A similar analysis showed that triplet mismatching in the HVG direction did not benefit DFS (data not shown).
Figure 4. HLA matching effect on disease-free patient survival.
(a) The 9/10 matches had significantly lower survival rates than the 10/10 matches (p=0.001). (b) Triplet matching did not increase patient survival rates
Multivariate analysis showed that the overall risk of treatment failure (relapse or death, the inverse of DFS) (p=0.008) was significantly higher after 9/10 than after10/10 matched transplants (Table 4). The evaluation of individual triplet groups revealed that the difference in DFS between some triplet mismatch groups and the 10/10 matched group did not reach statistical significance (p>0.01), i.e. 1, 2–3 and ≥6 triplet mismatches. However, when the individual triplet mismatch groups were compared, none were significantly different from each other (p=0.62). Altogether, this multivariate analysis suggests that the degree of triplet mismatching among the 9/10 matches does not influence the likelihood of DFS.
Table 4.
Multivariate analysis comparing disease-free survival for 10/10 and 9/10 matches with triplet mismatches in the GVH direction.
| Match Group | N | Relative risk (95% Confidence Interval) | P - value |
|---|---|---|---|
| 10/10 matches | 1687 | 1.00 | 0.008 |
| 0 Triplet mismatches | 241 | 1.26 (1.07 – 1.48) | 0.005 |
| 1 Triplet mismatches | 140 | 1.13 (0.91 – 1.39) | 0.263 |
| 2–3 Triplet mismatches | 142 | 1.17 (0.95 – 1.43) | 0.147 |
| 4–5 Triplet mismatches | 102 | 1.39 (1.10 – 1.75) | 0.005 |
| ≥6 Triplet mismatches | 117 | 1.14 (0.91 – 1.42) | 0.264 |
| All Triplet groups | 0.62 |
Adjusted factors: disease, disease stage, recipient age, GVH disease prophylaxis, Karnofsky score, recipient gender.
Discussion
This study confirms previous reports that a single class I HLA mismatch has an adverse effect on HCT outcome as manifested by less engraftment, more acute GVH disease and lower patient survival [1, 3, 4]. The goal of this analysis was to identify structurally similar permissive class I mismatches that result in the same outcome as the fully matched transplants or, at least, with better outcomes than class I mismatches with higher structural dissimilarity. However, the results of this analysis suggest that matching for HLAMatchmaker assigned triplets does not benefit HCT outcome.
HLA Matchmaker considers compatibility at the humoral immune level, i.e. a structural identification of antibody accessible epitopes exposed on the HLA molecular surface. The original version uses triplets, i.e. linear sequences of three amino acids, at least one of which is an antibody-accessible polymorphic residue [10]. The triplet version does not address compatibility at the cellular immune level involving T-cell activation influenced by structural polymorphisms in the peptide-binding groove and on the HLA molecular surface. Alloreactive T-cells play a dominant role in GVH disease and their T-cell receptors recognize peptides presented by HLA molecules. One might expect that zero-triplet mismatches would be structurally more similar to the corresponding self-alleles of the patient and may therefore bind the same peptide repertoire unless the peptide-binding groove has significant amino acid differences.
The literature has many reports describing alloreactive T-cells that are specific for serological HLA antigens that can be structurally defined by triplets. For instance, alloreactive T-cell clones have been described that are specific for HLA-A2, B7 or Bw4 [18–22]; these antigens carry unique triplets: 66RKV, 177DK and 82ALR, respectively (the numbers indicate the HLA protein sequence position and amino acids are shown with the standard one-letter code). Although triplet matching improves outcome in renal and corneal transplantation [12, 23] and reduces humoral sensitization [13–15], we must conclude that triplet matching is not useful in the selection of mismatched donors for HCT.
It should be pointed out that HLAMatchmaker considers a considerable proportion of polymorphic residues involved with peptide binding. The peptide-binding sites of class I HLA molecules comprise 32–36 amino acid residues [24–27], 25 of them are polymorphic. HLAMatchmaker includes 18 of these polymorphic residues to define triplets accessible to antibodies; polymorphisms in non-exposed sequence positions 24, 73, 95, 97, 99, 113 and 114 are not considered. Certain positions in the peptide-binding groove have been reported to be relevant for HCT. For instance, amino acid differences in sequence positions 116 and 156 have been shown to increase the risk of GVH disease and patient mortality after HCT [28–30]. This structural matching algorithm includes triplets in positions 116 and 156.
At this time, the application of a structurally based HLA mismatch permissibility algorithm for HCT remains elusive. Since HCT outcome is primarily affected by cellular immune mechanisms mediated by various types of T-lymphocytes and Natural Killer (NK) cells, the algorithm must take in account all amino acid polymorphisms of HLA. This includes the residues in the peptide-binding groove that are important in determining the repertoire of HLA bound peptides [27, 31, 32], the amino acids on the α1 and α2 helices that contact the TCR of alloreactive lymphocytes [33–36] and the HLA polymorphisms that affect NK function [37–40]. With this complexity, the development of a permissible mismatch strategy represents a considerable challenge.
Table 2.
Distribution of Triplet Mismatch Groups among HLA Class I Mismatched Donor-Recipient Pairs
| Mismatch Group |
HLA-A Mismatches |
MLA-B Mismatches |
HLA-C Mismatches |
Total |
|---|---|---|---|---|
| 242 (33%) | 97 (13%) | 405 (54%) | 744 | |
| HVG Direction | ||||
| 0 Triplets | 67 (29%) | 76 (33%) | 86 (38%) | 229 |
| 1 Triplet | 45 (32%) | 9 (6%) | 89 (62%) | 143 |
| 2–3 Triplets | 32 (22%) | 5 (3%) | 108 (75%) | 145 |
| 4–5 Triplets | 37 (32%) | 4 (4%) | 73 (64%) | 114 |
| 6+ Triplets | 61 (54%) | 3 (3%) | 49 (43%) | 113 |
| GVH Direction | ||||
| 0 Triplets | 73 (30%) | 73 (30%) | 95 (40%) | 241 |
| 1 Triplet | 44 (31%) | 14 (10%) | 82 (59%) | 140 |
| 2–3 Triplets | 37 (26%) | 6 (4%) | 100 (70%) | 143 |
| 4–5 Triplets | 36 (35%) | 1 (1%) | 65 (64%) | 102 |
| 6+ Triplets | 52 (44%) | 3 (3%) | 63 (53%) | 118 |
Acknowledgement
The authors would like to thank Dr. Carolyn K. Hurley for providing advice and a critical review of the manuscript. This study was supported by Grant RO1-AI-55933 from the National Institutes of Health to Dr. Duquesnoy and funding from the National Marrow Donor Program and the Department of the Navy, Office of Naval Research Grants #N00014-99-2-0006 and #N00014-05-1-0859 to the National Marrow Donor Program. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Office of Naval Research or the National Marrow Donor Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee S, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 2.Sasazuki T, Juji T, Morishima Y, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. N Engl J Med. 1998;339:1177. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- 3.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 4.Petersdorf E, Hansen J, Martin P, et al. Major histocompatibility complex class I alleles and antigens in hematopoietic cell transplantation. N Engl J Med. 2001;345:1794. doi: 10.1056/NEJMoa011826. [DOI] [PubMed] [Google Scholar]
- 5.Wade JA, Hurley CK, Takemoto SK, et al. HLA Mismatching Within or Outside of Cross Reactive Groups (CREG) Is Associated with Similar Outcomes After Unrelated Donor Bone Marrow Transplant. Blood. 2007;109:4064. doi: 10.1182/blood-2006-06-032193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Risler JL, Delorme MO, Delacroix H, Henaut A. Amino acid substitutions in structurally related proteins. A pattern recognition approach. Determination of a new and efficient scoring matrix. J Mol Biol. 1988;204:1019. doi: 10.1016/0022-2836(88)90058-7. [DOI] [PubMed] [Google Scholar]
- 7.Elsner H-A, Blasczyk R. Sequence similarity matching: proposal of a structure-based rating system for bone marrow transplantation. Eur J Immunogenet. 2002;29:229. doi: 10.1046/j.1365-2370.2002.00301.x. 229–236. [DOI] [PubMed] [Google Scholar]
- 8.Shaw BE, Barber LD, Madrigal JA, Cleaver S, Marsh SGE. Scoring for HLA matching? A clinical test of HistoCheck. Bone Marrow Transplant. 2004;34:367–368. doi: 10.1038/sj.bmt.1704586. [DOI] [PubMed] [Google Scholar]
- 9.Petersdorf EW, Gooley T, Malkki M, Horowitz M The International Histocompatibility Working Group in Hematopoietic Cell T. Clinical significance of donor-recipient HLA matching on survival after myeloablative hematopoietic cell transplantation from unrelated donors. Tissue Antigens. 2007;69 Suppl 1:25. doi: 10.1111/j.1399-0039.2006.759_2.x. [DOI] [PubMed] [Google Scholar]
- 10.Duquesnoy RJ. HLAMatchmaker: A Molecularly Based Algorithm For Histocompatibility Determination. I. Description of the Algorithm. Hum Immunol. 2002;63:339. doi: 10.1016/s0198-8859(02)00382-8. [DOI] [PubMed] [Google Scholar]
- 11.Duquesnoy RJ, Marrari M. HLAMatchmaker: A Molecularly Based Algorithm For Histocompatibility Determination. II. Verification of the Algorithm and Determination of the Relative Immunogenicity of Amino Acid Triplet-Defined Epitopes. Hum Immunol. 2002;63:353. doi: 10.1016/s0198-8859(02)00381-6. [DOI] [PubMed] [Google Scholar]
- 12.Duquesnoy RJ, Takemoto S, De Lange P, Doxiadis IIN, Schreuder GMT, Claas FHJ. HLAMatchmaker: A Molecularly Based Algorithm For Histocompatibility Determination III. Effect of matching at the HLA-A,B amino acid triplet level on kidney transplant survival. Transplantation. 2003;75:884. doi: 10.1097/01.TP.0000055101.20821.AC. [DOI] [PubMed] [Google Scholar]
- 13.Dankers MKA, Witvliet MD, Roelen DL, et al. The Number of Amino Acid Triplet Differences between Patient aand Donor is Predictive for the Antibody Reactivity Against Mismatched HLA Antigens. Transplantation. 2004;I28:1236–1239. doi: 10.1097/01.tp.0000120385.03278.28. [DOI] [PubMed] [Google Scholar]
- 14.Mihaylova A, Baltadjieva D, Boneva P, et al. Clinical Relevance of Anti-HLA Antibodies Detected by Flow-Cytometry Bead-Based Assays—Single-Center Experience. Hum Immunol. 2006;67:787. doi: 10.1016/j.humimm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Goodman R, Taylor C, O’Rourke C, Lynch A, Bradley A, Key K. Utility of HLAMatchmaker and single-antigen HLA-antibody detection beads for identification of acceptable mismatches in highly sensitised patients awaiting kidney transplantation. Transplantation. 2006;81:1331. doi: 10.1097/01.tp.0000205202.56915.f5. [DOI] [PubMed] [Google Scholar]
- 16.Nambiar A, Duquesnoy RJ, Adams S, et al. HLAMatchmaker-Driven Analysis Of Response To HLA Matched Platelet Transfusions In Alloimmunized Patients. Blood. 2006;107:1680. doi: 10.1182/blood-2004-10-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duquesnoy RJ. “Match and Treat“, An effective strategy for transplanting highly sensitized pediatric transplant candidates? (Editorial) Pediatric Transplantation. 2007;11:3. doi: 10.1111/j.1399-3046.2006.00628.x. [DOI] [PubMed] [Google Scholar]
- 18.Wallace LE, Houghton MA, Rickinson AB, et al. Allospecific T cell recognition of HLA-A2 antigens: evidence for group-specific and subgroup-specific epitopes. Immunogenetics. 1985;21(3):201. doi: 10.1007/BF00375373. [DOI] [PubMed] [Google Scholar]
- 19.Elliott TJ, Eisen HN. Cytotoxic T lymphocytes recognize a reconstituted class I histocompatibility antigen (HLA-A2) as an allogeneic target molecule. Proc Natl Acad Sci USA. 1990;87:5213. doi: 10.1073/pnas.87.13.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Seventer GA, Huis B, Melief CJ, et al. Fine specificity of human HLA-B7-specific cytotoxic T-lymphocyte clones. I. Identification of HLA-B7 subtypes and histotopes of the HLA-B7 cross-reacting group. Hum Immunol. 1986;16(4):375. doi: 10.1016/0198-8859(86)90064-9. [DOI] [PubMed] [Google Scholar]
- 21.Smith KD, Epperson DF, Lutz CT. Alloreactive cytotoxic T-lymphocyte-defined HLA-B7 subtypes differ in peptide antigen presentation. Immunogenetics. 1996;43(1–2):27. doi: 10.1007/BF00186601. [DOI] [PubMed] [Google Scholar]
- 22.Hiraiwa M, Yamamoto J, Matsumoto K, et al. T cell can recognize the allospecificities formed by the substitution of amino acids associated with HLA-Bw4/Bw6 public epitopes. Hum Immunol. 1991;32(1):41. doi: 10.1016/0198-8859(91)90115-p. [DOI] [PubMed] [Google Scholar]
- 23.Boehringer D, Reinhard T, Duquesnoy R, et al. Beneficial Effect of Matching at the HLA-A and B Amino-Acid Triplet Level on Rejection Free Survival in Penetrating Keratoplasty. Transplantation. 2004;(77):417. doi: 10.1097/01.TP.0000110415.10401.94. [DOI] [PubMed] [Google Scholar]
- 24.Saper M, Bjorkman P, Wiley DC. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991;219:277. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- 25.Smith KJ, Reid SW, Harlos K, et al. Bound water structure and polymorphic amino acids act together to allow binding of different peptides to MHC class I HLA-B53. Immunity. 1996;4:215. doi: 10.1016/s1074-7613(00)80430-6. [DOI] [PubMed] [Google Scholar]
- 26.Fan QOR, Garboczi DN, Winter CC, Wagtmann N, Long EO, Wiley DC. Direct binding of a soluble natural killer cell inhibitory receptor to a soluble human leukocyte antigen-Cw4 class I major histocompatibility complex molecule. Proc Natl Acad Sci USA. 1996;93(14):7178. doi: 10.1073/pnas.93.14.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doytchinova IA, Guan P, Flower DR. Identifying human MHC supertypes using bioinformatic methods. J Immunol. 2004;172:4314. doi: 10.4049/jimmunol.172.7.4314. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara GB, Bacigalupo A, Lamparelli T, et al. Bone marrow transplantation from unrelated donors: the impact of mismatches with substitutions at position 116 of the huam leukocyte antigen class I heavy chain. Blood. 2001;98:3150. doi: 10.1182/blood.v98.10.3150. [DOI] [PubMed] [Google Scholar]
- 29.Clayberger C, Rosen M, Parham P, Krensky AM. Recognition of an HLA public determinant (Bw4) by human allogeneic cytotoxic T lymphocytes. J Immunol. 1990;144(11):4172. [PubMed] [Google Scholar]
- 30.Fleischhauer K, Kernan NA, O'Reilly RJ, et al. Bone marrow-allograft rejection by T lymphocytes recognizing a single amino acid difference in HLA-B44. N Engl J Med. 1990;323(26):1818. doi: 10.1056/NEJM199012273232607. [DOI] [PubMed] [Google Scholar]
- 31.Falk KO, Rotzschke O, Stefanovic S, Jung G, Rammensee HG. Allele specific motifs revealed by sequencing of self-peptides eluted form MHC molecules. Nature. 1991;351:390. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 32.Sette A, Sidney J. Nine major HLA class I suprtypes account for the vast preponderance of HLA-A and HLA-B polymorphism. Immunogenetics. 1999;50:201. doi: 10.1007/s002510050594. [DOI] [PubMed] [Google Scholar]
- 33.Noun G, Reboul M, Abastado JP, et al. Strong alloantigenicity of the alpha-helices residues of the MHC class I molecule. J Immunol. 1998;161(1):148. [PubMed] [Google Scholar]
- 34.Pullen JK, Tallquist MD, Melvold RW, et al. Recognition of a single amino acid change on the surface of a major transplantation antigen is in the context of self peptide. J Immunol. 1994;152(7):3445. [PubMed] [Google Scholar]
- 35.Lombardi G, Matsui M, Moots R, et al. Limited regions of the alpha 2-domain alpha-helix control anti-A2 allorecognition: an analysis using a panel of A2 mutants. Immunogenetics. 1991;34(3):149. doi: 10.1007/BF00205817. [DOI] [PubMed] [Google Scholar]
- 36.Smith KD, Lutz CT. Alloreactive T Cell Recognition of MHC Class I Molecules. The T Cell Receptor Interacts with Limited Regions of the MHC Class I Long Alpha Helices. J Immunol. 1997;158:2805. [PubMed] [Google Scholar]
- 37.Gagne K, Brizard G, Gueglio B, et al. Relevance of KIR gene polymorphisms in bone marrow transplantation outcome. Hum Immunol. 2002;63(4):271. doi: 10.1016/s0198-8859(02)00373-7. [DOI] [PubMed] [Google Scholar]
- 38.Shilling HG, Young N, Guethlein LA, et al. Genetic control of human NK cell repertoire. J Immunol. 2002;169(1):239. doi: 10.4049/jimmunol.169.1.239. [DOI] [PubMed] [Google Scholar]
- 39.Charron D. Immunogenomics of hematopoietic stem cell transplantation. Transfus Clin Biol. 2003;10(3):156. doi: 10.1016/s1246-7820(03)00045-4. [DOI] [PubMed] [Google Scholar]
- 40.Hsu KC, Pinto-Agnello C, Gooley T, et al. Hematopoietic stem cell transplantation: killer immunoglobulin-like receptor component. Tissue Antigens. 2007;69 Suppl 1:42. doi: 10.1111/j.1399-0039.2006.759_5.x. [DOI] [PubMed] [Google Scholar]