Abstract
Whether temporary angiotensin II (AngII) blockade at the prediabetic stage attenuates renal injury in type 2 diabetic OLETF rats later in life was investigated. OLETF rats were treated with an AT1 receptor antagonist (olmesartan, 0.01% in food), angiotensin-converting enzyme inhibitor (temocapril, 0.01% in food), a combination of the two, or hydralazine (25 mg/kg per d) at the prediabetic stage (4 to 11 wk of age) and then monitored without further treatment until 50 wk of age. At 11 wk of age, blood glucose levels and urinary protein excretion (UproteinV) were similar between OLETF and control LETO rats. However, OLETF rats showed higher kidney AngII contents and type IV collagen mRNA expression than LETO rats at this age. These decreased with olmesartan, temocapril, and a combination of these but not with hydralazine. At 50 wk of age, diabetic OLETF rats showed higher BP, UproteinV, and intrarenal AngII levels than LETO rats. Temporary AngII blockade did not affect glucose metabolism or the development of hypertension in OLETF rats but significantly suppressed proteinuria and ameliorated glomerular injury. However, no parameters were affected by temporary hydralazine treatment. The present study demonstrated that intrarenal AngII and type IV collagen expression are already augmented long before diabetes becomes apparent in OLETF rats. Furthermore, temporary AngII blockade at the prediabetic stage attenuates the progression of renal injury in these animals. These data suggest that early AngII blockade could be an effective strategy for preventing the development of type 2 diabetic renal injury later in life.
Diabetic nephropathy is a major complication in diabetes and a leading cause of end-stage renal failure, which causes disabilities and a high mortality rate in patients with this disease (1). The mechanisms underlying the development of diabetic nephropathy are extremely complex; however, the potential role of the renin-angiotensin system (RAS) has been suggested (2-13). Recent studies indicate that in diabetes, intrarenal generation of angiotensin II (AngII) is elevated despite suppressed circulating RAS (5,6). Furthermore, AT1 receptor blockers (ARB) or angiotensin-converting enzyme inhibitors (ACEI) have been shown to attenuate the progression of diabetic nephropathy (3,4,7-13). Several clinical trials have shown that ARB are more effective than traditional antihypertensive therapies in reducing renal failure progression in patients with type 2 diabetes and that the renoprotective effects of ARB are independent of their antihypertensive actions (8-11). Of interest, it has also been shown that ACEI treatment of normotensive patients with diabetes and little or no proteinuria (early stages of diabetic nephropathy) results in long-term stabilization of plasma creatinine levels and urinary protein excretion rates (UproteinV) (12,13). These observations suggest that angiotensin blockade has clinical benefits for patients who have diabetes and have no or early signals of renal disease.
It has been shown that early administration of ACEI to young spontaneously hypertensive rats (SHR) has long-term antihypertensive effects even after treatment is discontinued (14,15). However, no similar effect could be found with administration of other antihypertensive drugs, including calcium antagonists, β-blockers, and direct vasodilators (15-17). Further studies showed that a single application of ACE antisense to neonatal rats attenuated the development of hypertension and endothelial dysfunction in SHR (18). Recently, Nakaya et al. (19,20) revealed that administration of ARB or ACEI during a limited time window before puberty results in a prolonged reduction in BP and renoprotection in stroke-prone SHR and Dahl saltsensitive hypertensive rats. Collectively, these data suggest that sensitivity to angiotensin blockade exists before the development of hypertension as well as associated renal injury. However, it is still not clear whether the renoprotective effects of angiotensin blockade are due to its antihypertensive effect or direct mechanisms. In addition, the effects of brief periods of ARB or ACEI treatment on renal injury in type 2 diabetes have not been examined.
This study investigates whether temporary AngII blockade at the prediabetic stage provides long-lasting protection against renal injury in type 2 diabetic rats. OLETF rats, which exhibit pathologic features of renal injury similar to those of human type 2 diabetes (21-23), were treated with an ARB, olmesartan (22,24), an ACEI, temocapril (22), or a nonspecific vasodilator, hydralazine (24), for only 7 wk at the prediabetic stage (4 to 11 wk of age) and then monitored without further treatment until 50 wk of age. The present study demonstrates for the first time that intrarenal AngII and reactive oxygen species (ROS) levels as well as type IV collagen gene expression are already augmented in young OLETF rats, long before diabetes becomes apparent. In addition, temporary AngII blockade at the prediabetic stage suppresses the development of renal injury in these animals, independent of its effects on BP and glucose metabolism. These data suggest that early AngII blockade could be an effective strategy for preventing the development of type 2 diabetic renal injury later in life.
Materials and Methods
Animals
All experimental procedures were performed according to the guidelines for the care and use of animals established by Kagawa Medical University. Male 4-wk-old OLETF rats and LETO rats (genetic control of OLETF rats; n = 12) were supplied by Otsuka Pharmaceutical Co. Ltd. (Tokushima, Japan). After basal measurements were obtained, OLETF rats were randomly treated with one of the following combinations: tap water + standard diet (n = 14); tap water + olmesartan (0.01% in food; Sankyo Co. Ltd., Tokyo, Japan); n = 14); tap water + temocapril (0.01% in food; Sankyo Co. Ltd.; n = 14); tap water + olmesartan (0.01% in food) + temocapril (0.01% in food; n = 14); or standard diet + tap water containing hydralazine (25 mg/kg per d; n = 14). The doses of olmesartan, temocapril, and hydralazine were determined on the basis of results from previous studies on rats (22,24). The medications were stopped at 11 wk of age. Half of the LETO and OLETF rats in each group then were killed, and the remaining rats were monitored without medication until they were killed at 50 wk of age.
Systolic BP (SBP) was measured in conscious rats by tail-cuff plethysmography (BP-98A; Softron Co., Tokyo, Japan), and 24-h urine samples were collected using metabolic cages. After decapitation, half of one kidney was homogenized in cold methanol and processed for measurements of kidney AngII content (24-27). The other half of this kidney was fixed in 10% buffered paraformaldehyde for histologic examination. The remaining kidney was snap-frozen in liquid nitrogen and stored at -80°C.
Real-Time Reverse Transcription-PCR
The mRNA expression of angiotensinogen in renal cortical tissues was analyzed quantitatively by real-time PCR using an Mx3000P System with a Brilliant Single-Step QRT-PCR Master Mix Kit (Stratagene, La Jolla, CA) (26). The following rat angiotensinogen primers were used: sense 5′-AGGCAAGAGGTGTAGCCAGT-3′ and antisense 5′-AGGACCTTATGTCCGTCCAG-3′. The following rat angiotensinogen probe was used: 5′-TCTTTCTACCTTGGATCGTTGGATCCC-3′. The mRNA expression of glyceraldehyde-3-phosphate dehydrogenase, renin, p22phox, gp91phox, types I and IV collagen, TGF-β, and connective tissue growth factor (CTGF) were analyzed by real-time PCR using a LightCycler FastStart DNA Master SYBR Green I kit (28). The oligonucleotide primer sequences and PCR conditions for p22phox, gp91phox, and types I and IV collagen are summarized in Table 1. TGF-β and CTGF mRNA expression were measured by using TaqMan Gene Expression Assay kits (Applied Biosystems, Foster City, CA). All data are expressed as the relative differences between OLETF and LETO rats after normalization to glyceraldehyde-3-phosphate dehydrogenase expression.
Table 1.
Primer sequences and PCR conditionsa
| Gene | Primer | Product Size (bp) | Annealing Temperature/Time (°C/s) | Extension Temperature/Time (°C/s) |
|---|---|---|---|---|
| GAPDH (NM_017008) |
F: 5′-TGAACGGGAAGCTCACTGG-3′ R: 5′-TCCACCACCCTGTTGCTGTA-3′ |
307 | 63/5 | 72/10 |
| Renin (NM_012642) |
F: 5′-TTGTGTGAGGAGGGCTGTAT-3′ R: 5′-TGCTGAGAGTGTAGGTCCTG-3′ |
199 | 58/5 | 72/8 |
| p22phox (NM_024160) |
F: 5′-TCCACTTACTGCTGTCCGT-3′ R: 5′-TCAATGGGAGTCCACTGCT-3′ |
127 | 62/5 | 72/6 |
| gp91phox (NM_023965) |
F: 5′-TGGTGATGTTAGTGGGAGC-3′ R: 5′-CTTTCTTGCATCTGGGTCT-3′ |
196 | 63/5 | 72/10 |
| Type I collagen (col1a1: Z78279) |
F: 5′-TCACCTACAGCACGCTTG-3′ R: 5′-GGTCTGTTTCCAGGGTTG-3′ |
245 | 56/5 | 72/10 |
| Type IV collagen (col4a3: L47281) |
F: 5′-GAGGGTGCTGGACAAGCTCTT-3′ R: 5′-TAAATGGACTGGCTCGGAATTC-3′ |
67 | 62/5 | 72/4 |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western Blot Analysis
AT1 and AT2 receptor protein levels in the renal cortical tissues were analyzed by Western blotting using antibodies against the AT1 and AT2 receptors (Santa Cruz Biotechnology, Santa Cruz, CA), as described previously (24,25,27). To check for equal loading, we reprobed membranes with an antibody against β-actin (Sigma Chemical, St. Louis, MO). Data are expressed as the relative differences between OLETF rats and LETO rats after normalization to β-actin expression.
Histologic Examination
Kidneys were fixed with 10% formalin (pH 7.4), embedded in paraffin, sectioned into 4-μm slices, and stained with hematoxylin-eosin or periodic acid-Schiff (PAS) reagents. The severity of glomerular sclerosis in the PAS-stained sections was determined using a semiquantitative score from 0 to 4: 0, no matrix expansion; 1, minor; 2, weak; 3, moderate; and 4, strong (25,28). The diameters of the glomeruli in each experimental group were measured using a visual caliper (SVS 30000; Showa Electric Laboratory, Fukuoka, Japan) (28). For all measurements, 245 to 315 randomly selected glomeruli were examined.
Other Analytical Procedures
AngII content in the kidneys was measured by RIA as described previously (24-27), and UproteinV was determined using a protein assay kit (microTP-test; Wako Co., Osaka, Japan). Blood glucose levels were measured with a glucose analyzer (Sanwa-Kagaku, Co. Ltd., Nagoya, Japan), and insulin levels were measured with a RIA kit (Amersham Biosciences, Piscataway, NJ). The degree of lipid peroxidation was determined using biochemical assays of the thiobarbituric acid reactive substances (TBARS) in the renal cortical tissues, as described previously (24,27,28). Renal cortical tissue collagen content was determined on the basis of hydroxyproline concentration (25,28).
Statistical Analyses
Values are presented as means ± SEM. Statistical comparisons of the differences were performed using one- or two-way ANOVA combined with Newman-Keuls post hoc test. P < 0.05 was considered statistically significant.
Results
BP, Body Weight, and Blood Glucose
The temporal profiles of SBP, body weight, and postprandial blood glucose are depicted in Figure 1, A through C. SBP remained unaltered in LETO rats, whereas OLETF rats progressively developed hypertension (Figure 1A). Temporary treatment with olmesartan, temocapril, a combination of these, or hydralazine at the prediabetic stage had no effect on the development of hypertension. From 15 to 50 wk of age, the body weight of untreated OLETF rats was higher than that of LETO rats (Figure 1B). None of the temporary treatments affected the body weight of OLETF rats (Figure 1B). OLETF rats showed higher postprandial blood glucose levels than LETO rats from 15 to 50 wk of age (Figure 1C). As shown in Table 2, the SBP and postprandial blood glucose levels were similar between LETO and OLETF rats at 11 wk of age. Treatment with olmesartan, temocapril, a combination of these, or hydralazine resulted in similar reductions in SBP but did not alter the postprandial blood glucose levels in OLETF rats. The data at 50 wk of age are shown in Table 3.
Figure 1.

Profiles of systolic BP (SBP; A), body weight (BW; B), postprandial blood glucose (PPBG; C), and urinary protein excretion (UproteinV; D). OLETF rats develop hypertension, obesity, diabetes, and proteinuria. In these animals, temporary treatment with olmesartan, temocapril, a combination of these, or hydralazine at the prediabetic stage (4 to 11 wk of age) does not affect the development of hypertension, obesity, or high glucose levels later in life. However, the progression of proteinuria is markedly suppressed by temporary treatment with olmesartan, temocapril, or a combination of these but not by treatment with hydralazine.
Table 2.
Systolic blood pressure, postprandial blood glucose level, left kidney weight, renal cortical thiobarbituric acid reactive substances, and mRNA expression of p22phox and gp91phox in LETO and OLETF rats at 11 wk of agea
| LETO | OLETF | OLETF + Olmesartan | OLETF + Temocapril | OLETF + Olmesartan + Temocapril | OLETF + Hydralazine | |
|---|---|---|---|---|---|---|
| SBP (mmHg) | 121 ± 7 | 131 ± 6 | 117 ± 2c | 118 ± 3c | 112 ± 4c | 117 ± 4c |
| PPBG (mmol/L) | 6.6 ± 0.1 | 7.1 ± 0.4 | 6.9 ± 0.3 | 6.7 ± 0.4 | 6.5 ± 0.3 | 6.6 ± 0.2 |
| LKW (g) | 1.12 ± 0.03 | 1.39 ± 0.02b | 1.35 ± 0.02b | 1.35 ± 0.03b | 1.36 ± 0.02b | 1.42 ± 0.04b |
| TBARS (nmol/mg protein) | 0.10 ± 0.02 | 0.22 ± 0.01b | 0.13 ± 0.02c | 0.15 ± 0.01b,c | 0.14 ± 0.02c | 0.20 ± 0.01b |
| p22phox mRNA (fold) | 1.00 ± 0.03 | 2.81 ± 0.19b | 1.38 ± 0.15c | 1.44 ± 0.15c | 1.31 ± 0.14c | 1.98 ± 0.08b |
| gp91phox mRNA (fold) | 1.00 ± 0.14 | 1.30 ± 0.11 | 0.90 ± 0.17 | 1.09 ± 0.16 | 0.82 ± 0.11 | 1.35 ± 0.15 |
Values are means ± SEM. SBP, systolic BP; PPBG, postprandial blood glucose level; LKW, left kidney weight; TBARS, thiobarbituric acid reactive substances.
P < 0.05 versus LETO.
P < 0.05, OLETF versus OLETF + olmesartan, temocapril, olmesartan plus temocapril or hydralazine.
Table 3.
Fasting blood glucose, plasma insulin, LKW, kidney angiotensin II levels, renal cortical TBARS, and mRNA expression of p22phox and gp91phox in LETO and OLETF rats at 50 wk of agea
| LETO | OLETF | OLETF + Olmesartan | OLETF + Temocapril | OLETF + Olmesartan + Temocapril | OLETF + Hydralazine | |
|---|---|---|---|---|---|---|
| FBG (mmol/L) | 5.3 ± 0.5 | 12.9 ± 1.1b | 11.5 ± 1.2b | 13.1 ± 1.4b | 12.1 ± 1.0b | 13.3 ± 1.4b |
| Insulin (ng/ml) | 5.16 ± 0.62 | 9.10 ± 1.35b | 8.23 ± 1.28b | 9.11 ± 1.03b | 8.14 ± 0.92b | 8.84 ± 0.76b |
| LKW (g) | 1.32 ± 0.03 | 2.41 ± 0.08b | 1.93 ± 0.11b,c | 1.98 ± 0.11b,c | 2.08 ± 0.10b,c | 2.41 ± 0.04b |
| AngII (fmol/g) | 139 ± 12 | 271 ± 24b | 247 ± 20b | 239 ± 29b | 235 ± 18b | 246 ± 18b |
| TBARS (nmol/mg protein) | 0.12 ± 0.02 | 0.23 ± 0.02b | 0.21 ± 0.01b | 0.23 ± 0.03b | 0.22 ± 0.03b | 0.24 ± 0.03b |
| p22phox mRNA (fold) | 1.00 ± 0.10 | 2.06 ± 0.28b | 1.74 ± 0.29b | 1.62 ± 0.31b | 2.08 ± 0.10b | 1.69 ± 0.32b |
| gp91phox mRNA (fold) | 1.00 ± 0.04 | 2.57 ± 0.17b | 2.03 ± 0.40b | 2.20 ± 0.23b | 2.43 ± 0.38b | 2.20 ± 0.18b |
Values are means ± SEM. FBG, fasting blood glucose; AngII, angiotensin II
P < 0.05 versus LETO.
P < 0.05: OLETF versus OLETF + olmesartan, temocapril, olmesartan plus temocapril or hydralazine.
UproteinV, Renal Cortical Collagen, TGF-β, and CTGF
The temporal profile of UproteinV is depicted in Figure 1D The UproteinV of OLETF rats progressively increased with age. The progression of proteinuria was markedly suppressed by temporary treatment with olmesartan, temocapril, or a combination of these but not by treatment with hydralazine.
At 11 wk of age, UproteinV and the renal cortical collagen contents were similar among all groups (Figures 1D and 2A). The mRNA expression of type I collagen in the renal cortical tissues was also similar between LETO and OLETF rats. In OLETF rats, however, treatment with olmesartan or olmesartan plus temocapril significantly decreased type I collagen gene expression (Figure 2B). Temocapril also tended to decrease type I collagen gene expression, but the change was not statistically significant. Furthermore, hydralazine did not alter type I collagen gene expression. However, renal cortical type IV collagen mRNA expression was approximately twofold higher in OLETF rats than in LETO rats at this age (Figure 2C). In addition, the augmentation of type IV collagen gene expression was prevented by treatment with olmesartan, temocapril, or a combination of these but not by treatment with hydralazine (Figure 2C). Renal cortical mRNA expression of TGF-β was not significantly changed in OLETF rats (Figure 2D), whereas CTGF mRNA expression was approximately fourfold higher in OLETF rats (Figure 2E). The augmentation of CTGF collagen gene expression was prevented by treatment with olmesartan, temocapril, or a combination of these but not by treatment with hydralazine (Figure 2E).
Figure 2.
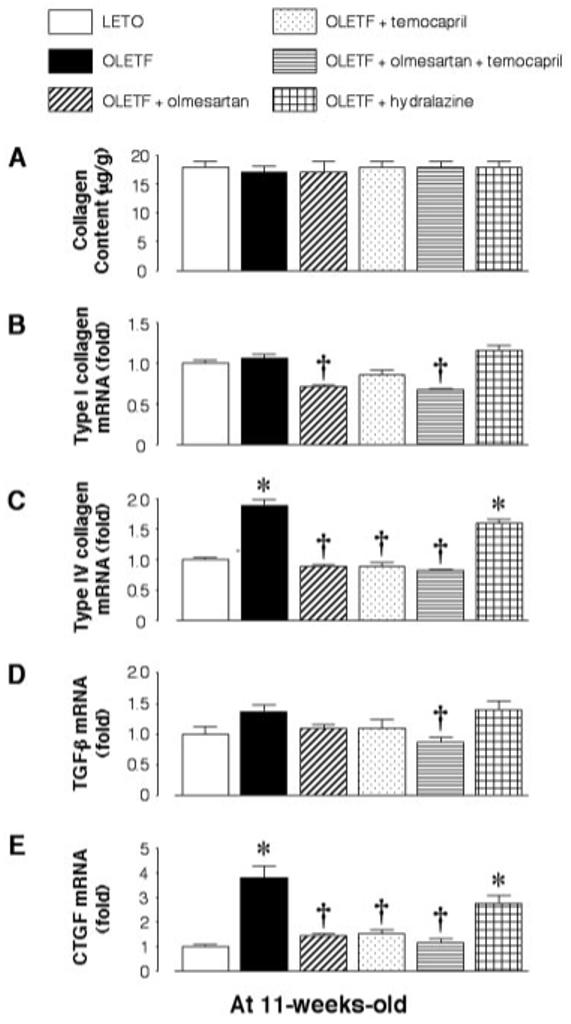
(A) Renal cortical collagen content, and mRNA expression of type I collagen (B), type IV collagen (C), TGF-β (D), and connective tissue growth factor (CTGF; E) in OLETF rats at 11 wk of age. The renal cortical collagen contents and mRNA expression of type I collagen and TGF-β are similar between OLETF and control LETO rats. However, renal cortical type IV collagen and CTGF mRNA expression is already augmented in OLETF rats at this age. The augmented type IV collagen and CTGF mRNA expression is normalized by treatment with olmesartan, temocapril, or a combination of these but not by treatment with hydralazine. *P < 0.05 versus LETO; †P < 0.05, OLETF versus OLETF + olmesartan, temocapril, or olmesartan plus temocapril.
At 50 wk of age, the renal cortical collagen content in untreated OLETF rats was significantly higher than that in LETO rats. In OLETF rats, the renal cortical collagen contents were significantly decreased by temporary treatment with olmesartan, temocapril, or a combination of these but not by treatment with hydralazine (Figure 3A). Similar results were observed for the renal cortical mRNA expression of types I and IV collagen, TGF-β, and CTGF (Figure 3, B through E).
Figure 3.
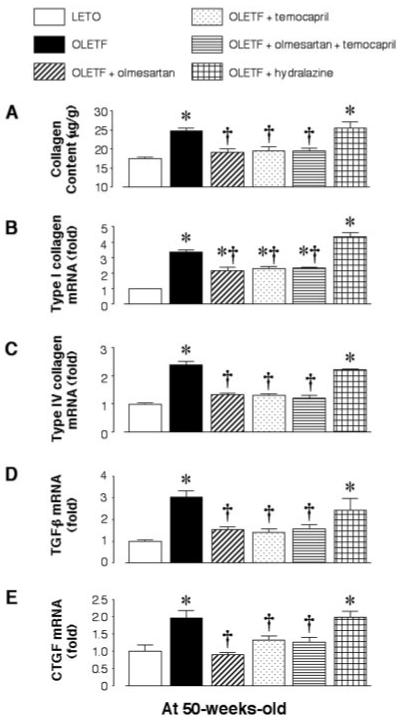
(A) Renal cortical tissue collagen content and mRNA expression of type I collagen (B), type IV collagen (C), TGF-β (D), and CTGF (E) in OLETF rats at 50 wk of age. OLETF rats show increased levels at 50 wk of age. In OLETF rats, temporary treatment with olmesartan, temocapril, or a combination of these at the prediabetic stage (4 to 11 wk of age) reduces these levels, whereas temporary treatment with hydralazine does not. *P < 0.05 versus LETO; †P < 0.05, OLETF versus OLETF + olmesartan, temocapril, or olmesartan plus temocapril.
Histologic Findings
The glomerular histologic findings with hematoxylin-eosin and PAS staining are shown in Figure 4, A and B, respectively. In 11-wk-old OLETF rats, there were no obvious glomeruli alterations (data not shown). However, mesangial expansion accompanied by an accumulation of extracellular matrix and glomerular capillary wall thickening occurred at 50 wk of age. Some glomeruli showed developed diabetic glomerulopathy accompanied by diffuse mesangial matrix expansion. However, there were no obvious findings except minor alterations as a result of aging in the glomeruli of LETO rats even at 50 wk of age. The semiquantitative analyses data showed that the glomerular sclerosis index in untreated OLETF rats (50 wk of age) was significantly greater compared with LETO rats (Figure 4C). Temporary treatment with olmesartan, temocapril, or a combination of these significantly decreased the glomerular sclerosis index in OLETF rats, whereas this value was similar between the untreated and hydralazine-treated OLETF rats at 50 wk of age (Figure 4C). Figure 4D shows the glomerular size distribution and reveals that the glomeruli tended to be larger in OLETF rats than in LETO rats. In OLETF rats, temporary treatment with olmesartan, temocapril, a combination of these, or hydralazine did not remarkably alter the glomerular size distribution.
Figure 4.
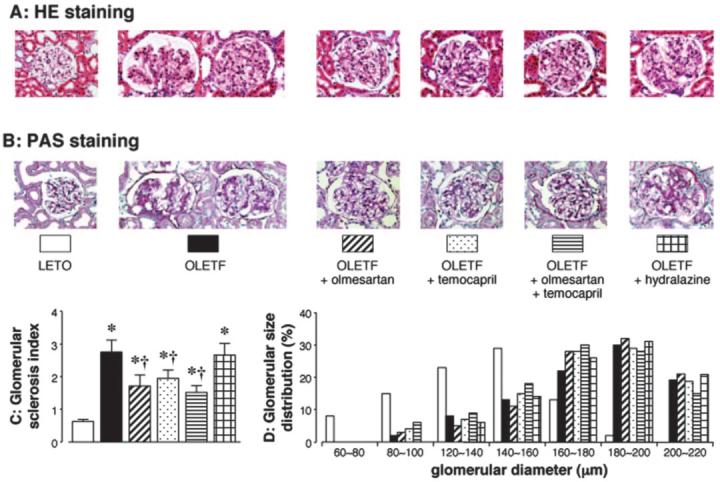
Photomicrographs of glomeruli with hematoxylin-eosin (HE; A) and periodic acid-Schiff (PAS; B) reagents. In diabetic OLETF rats, mesangial expansion accompanied by an accumulation of extracellular matrix and glomerular capillary wall thickening occurs at 50 wk of age. Some glomeruli show developed glomerulopathy accompanied by diffuse mesangial matrix expansion. These glomerular changes are ameliorated by temporary treatment (4 to 11 wk of age) with olmesartan, temocapril, or a combination of these but not by treatment with hydralazine. However, there are no obvious findings except minor alterations as a result of aging in the glomeruli of the control LETO rats even at 50 wk. The glomerular sclerosis index (C) and glomerular size distribution (D) determined as described in the Materials and Methods section. *P < 0.05 versus LETO; †P < 0.05, OLETF versus OLETF + olmesartan, temocapril, or olmesartan plus temocapril. Original magnification, ×200 in A and B.
Angiotensinogen, Renin, AngII, and AT1 and AT2 Receptors
At 11 wk of age, OLETF rats showed higher renal angiotensinogen and renin mRNA levels than LETO rats (Figure 5, A and B). Treatment with olmesartan, temocapril, or olmesartan plus temocapril significantly decreased angiotensinogen mRNA levels but increased renin mRNA levels in OLETF rats. Hydralazine slightly decreased angiotensinogen expression but increased renin expression in OLETF rats (Figure 5, A and B). OLETF rats already showed higher kidney AngII contents than LETO rats at this age (Figure 5C). The kidney AngII content decreased with daily treatments of olmesartan, temocapril, or olmesartan plus temocapril, whereas hydralazine had no effect. The protein expression of AT1 (approximately 46 kD) and AT2 (approximately 44 kD) receptors was similar among all groups (data not shown).
Figure 5.
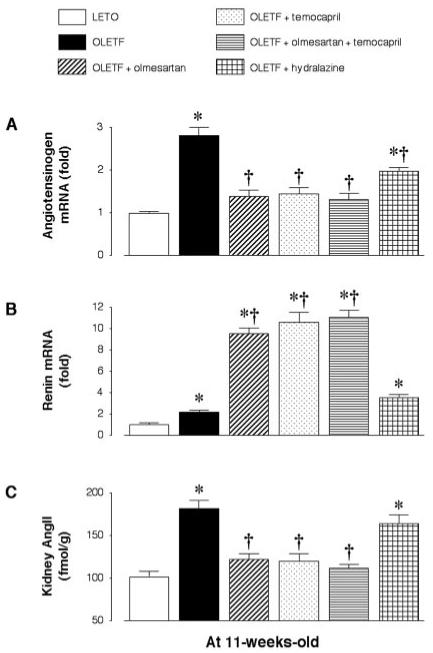
Renal cortical mRNA expression of angiotensinogen (A) and renin (B), as well as kidney angiotensin II (AngII; C) levels in OLETF rats at 11 wk of age. Compared with control LETO rats, OLETF rats already show higher renal angiotensinogen and renin mRNA expression as well as kidney AngII levels at the prediabetic stage. Augmented intrarenal AngII levels in OLETF rats are decreased by treatment with olmesartan, temocapril, or olmesartan plus temocapril but not by treatment with hydralazine. *P < 0.05 versus LETO; †P < 0.05, OLETF versus OLETF + olmesartan, temocapril, or olmesartan + temocapril.
At 50 wk of age, renal cortical angiotensinogen mRNA expression was similar between OLETF and LETO rats (data not shown). However, mRNA renin expression was significantly higher in OLETF rats than in LETO rats (by 2.1 ± 0.2-fold). Temporary treatment with olmesartan, temocapril, a combination of these, or hydralazine had no effect on mRNA angiotensinogen and renin expression (data not shown). Untreated OLETF rats had higher kidney AngII contents than LETO rats (Table 2). Temporary treatment with olmesartan, temocapril, a combination of these, or hydralazine had no effect on these values (Table 2). The protein levels of AT1 and AT2 receptors were similar among all groups (data not shown).
TBARS Content and mRNA Expression of NAD(P)H Oxidase Components
At 11 wk of age, OLETF rats already showed higher renal cortical TBARS contents and mRNA p22phox expression compared with LETO rats (Table 2). In the OLETF rats, treatment with olmesartan, temocapril, or a combination of these decreased the renal cortical TBARS content and p22phox expression. Hydralazine also significantly decreased renal cortical p22phox mRNA expression but not TBARS content. However, mRNA gp91phox expression did not differ among the groups (Table 2).
At 50 wk of age, untreated OLETF rats showed higher renal cortical TBARS contents as well as mRNA p22phox and gp91phox expression compared with LETO rats (Table 3). Temporary treatment with olmesartan, temocapril, a combination of these, or hydralazine did not alter these levels.
Discussion
The RAS has been implicated in the pathophysiology of diabetic nephropathy, largely on the basis of the ability of ARB or ACEI to reduce proteinuria and the progression of renal injury in patients (7-13) and animals (21,22,29) with diabetes. The present study demonstrates that renal injury is associated with increases in intrarenal AngII levels in type 2 diabetic OLETF rats (50 wk of age). In addition, expression of AngII receptors was maintained in the kidneys of diabetic OLETF rats. The key finding of the present study is that intrarenal AngII levels are already augmented in young OLETF rats (11 wk of age) before the manifestation of diabetes. Furthermore, the augmentation of the intrarenal AngII content was prevented by concurrent administration of an ARB or ACEI. In addition, brief treatment with an ARB or ACEI during a limited time window at the prediabetic stage (4 to 11 wk of age) attenuated the development of renal injury later in life, independent of its effects on BP and glucose metabolism. These data suggest a potential contribution of augmented intrarenal AngII levels at the prediabetic period to the development of type 2 diabetic renal injury.
Uehara et al. (21) showed that the onset of proteinuria and renal histologic changes was delayed by daily treatments with ACEI (from 6 to 22 wk of age) in OLETF rats. In the present study, however, the renoprotective effects of temporary angiotensin blockade cannot be explained simply as a consequence of the delay in the onset of renal injury. If temporary angiotensin blockade for 7 wk (4 to 11 wk of age) merely delays the onset of renal injury, then a rightward shift of 7 wk in the time course of proteinuria development should be observed in the treated animals. However, such rightward shifts in the UproteinV levels were not observed in the ARB-or ACEI-treated animals in the present study. It also seems likely that the antihypertensive effects of ARB and ACEI during the treatment period are not the main cause of this phenomenon. The present results revealed that temporary treatment with a nonspecific vasodilator (hydralazine) resulted in a similar reduction in BP to treatments with ARB and ACE but did not attenuate renal injury in OLETF rats. In addition, the antihypertensive effects of these agents were diminished soon after the cessation of treatment.
Consistent with previous observations in hypertensive rats (25,27,30), continuous AngII blockade decreased AngII kidney content in young OLETF rats (11 wk of age). These data suggest that some of the renoprotective effects of temporary angiotensin blockade are accompanied by reductions in intrarenal AngII levels during this treatment period. However, increases in intrarenal AngII levels in diabetic OLETF rats (50 wk of age) are not prevented by temporary ARB and ACEI treatments. Furthermore, neither AT1 nor AT2 receptor expression in the renal cortical tissues was altered by these treatments. These data suggest that the renoprotective effects of temporary angiotensin blockade are not due to the sustained suppression of intrarenal RAS activity.
AngII induces cellular changes through NAD(P)H oxidasemediated ROS production (29,30); ROS have also emerged as important mediators in the pathogenesis of diabetic nephropathy (29,31-33). Similar to the alterations in intrarenal AngII levels, p22phox expression and TBARS content were augmented in both prediabetic (11 wk of age) and diabetic (50 wk of age) OLETF rats. We also observed that augmented renal p22phox expression and TBARS content were reduced by daily ARB or ACEI treatments at 11 wk of age. However, the augmentation of ROS in diabetic OLETF rats (50 wk of age) was not prevented by temporary treatment with ARB or ACEI. Collectively, these results suggest the potential contribution of ROS to the development of renal injury in OLETF rats; however, the renoprotective effects of temporary angiotensin blockade at the prediabetic stage cannot be explained by sustained suppression of intrarenal ROS.
Although the present study provides no direct information regarding the mechanisms that are responsible for the beneficial actions of temporary angiotensin blockade on type 2 diabetic renal injury, several possibilities can be suggested. Okada et al. (23) showed that AngII stimulated DNA synthesis to a greater extent in mesangial cells that were isolated from young prediabetic OLETF rats (14 wk of age) than in those from older diabetic OLETF rats (50 wk of age). These findings suggest that a possible critical AngII-sensitive phase exists in the renal cells of OLETF rats at the prediabetic stage. The authors also indicated stage-specific gene regulation in the mesangial cells of OLETF rats (23). Thus, it can be speculated that AngII-induced gene regulation in the renal cells of prediabetic OLETF rats is affected by temporary treatment with ARB or ACEI. The present study revealed that, although the collagen contents in the renal cortical tissues remained unchanged in prediabetic OLETF rats (11 wk of age), type IV collagen gene expression was already enhanced at this age. In addition, gene expression of CTGF, which mediates downstream events of TGF-β (34) and stimulates fibroblast proliferation and extracellular matrix protein synthesis (35,36), was markedly increased in the kidneys of OLETF rats (11 wk of age). Furthermore, temporary AngII blockade at the prediabetic stage resulted in sustained reductions in these gene expression and collagen accumulation later in life. These data suggest that AngII-induced CTGF and collagen gene expression at the prediabetic stage may participate, at least in part, in the onset of renal injury in OLETF rats.
In summary, the present study demonstrates that intrarenal AngII and ROS levels as well as type IV collagen and CTGF gene expression are already augmented long before the first signs of diabetes become apparent in OLETF rats. Furthermore, temporary blockade of the RAS at the prediabetic stage suppresses the development of diabetic renal injury, independent of its effects on BP and glucose metabolism. These data support the hypothesis that early blockade of the RAS could be an effective strategy for preventing the development of type 2 diabetic renal injury later in life. However, more careful assessments of the morphology and intrarenal RAS (by using electron microscopy, HPLC separation, and receptor binding assays, etc.) will be required for confirmation. In addition, further studies on patients with type 2 diabetes will be necessary to address this hypothesis directly. In this regard, the ROADMAP (Randomized Olmesartan and Diabetes Microalbuminuria Prevention) study was initiated in September 2004 to determine the difference in time to microalbuminuria between patients with type 2 diabetes and normoalbuminuria allocated to olmesartan or conventional antihypertensive therapy (presented at the 19th Scientific Meeting of the International Society of Hypertension, February 2004). Importantly, this clinical trial will test whether the prevention of microalbuminuria with early olmesartan treatment leads to renal and other cardiovascular protection and improves disease morbidity and mortality later in life.
Acknowledgments
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15790136; A.N.); and by grants from the Salt Science Research Foundation (04C2), the Nankai-Ikueikai Foundation, Mitsui Life Social Welfare Foundation, Japan Research Foundation for Clinical Pharmacology, and the Kao Foundation for Arts and Sciences (to A.N.).
Part of this work was presented at the 36th Annual Meeting and Scientific Exposition of the American Society of Nephrology, San Diego, CA, October 2003; and the 58th Annual Fall Conference and Scientific Sessions of the Council for High Blood Pressure Research in association with the Council on the Kidney in Cardiovascular Disease, Chicago, IL, October 2004.
We thank Drs. Hidetoshi Kakari and Toshitaka Nakagawa (Kagawa Medical University) for excellent technical assistance. We are also grateful to Sankyo Co. Ltd. for supplying olmesartan and temocapril and to Otsuka Pharmaceutical Co. Ltd. for supplying OLETF and LETO rats.
References
- 1.Makino H, Nakamura Y, Wada J. Remission and regression of diabetic nephropathy. Hypertens Res. 2003;26:515–519. doi: 10.1291/hypres.26.515. [DOI] [PubMed] [Google Scholar]
- 2.Rincon-Choles H, Kasinath BS, Gorin Y, Abboud HE. Angiotensin II and growth factors in the pathogenesis of diabetic nephropathy. Kidney Int Suppl. 2002;82:S8–S11. doi: 10.1046/j.1523-1755.62.s82.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Berl T. Angiotensin-converting enzyme inhibitors versus AT1 receptor antagonist in cardiovascular and renal protection: The case for AT1 receptor antagonist. J Am Soc Nephrol. 2004;15(Suppl 1):S71–S76. doi: 10.1097/01.asn.0000093235.09769.9c. [DOI] [PubMed] [Google Scholar]
- 4.Deferrari G, Ravera M, Deferrari L, Vettoretti S, Ratto E, Parodi D. Renal and cardiovascular protection in type 2 diabetes mellitus: Angiotensin II receptor blockers. J Am Soc Nephrol. 2002;13(Suppl 3):S224–S229. doi: 10.1097/01.asn.0000032544.37147.ae. [DOI] [PubMed] [Google Scholar]
- 5.Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14:274–281. doi: 10.1016/s1043-2760(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 6.Burns KD. Angiotensin II and its receptors in the diabetic kidney. Am J Kidney Dis. 2000;36:449–467. doi: 10.1053/ajkd.2000.16192. [DOI] [PubMed] [Google Scholar]
- 7.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 8.Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: A blood pressure-independent effect. Circulation. 2002;106:672–678. doi: 10.1161/01.cir.0000024416.33113.0a. [DOI] [PubMed] [Google Scholar]
- 9.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 10.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group Renoprotective effect of the angiotensin receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 11.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P, Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 12.Ravid M, Savin H, Jutrin I, Bental T, Katz B, Lishner M. Long-term stabilizing effect of angiotensin-converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Ann Intern Med. 1993;118:577–581. doi: 10.7326/0003-4819-118-8-199304150-00001. [DOI] [PubMed] [Google Scholar]
- 13.The EUCLID Study Group Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. Lancet. 1997;349:1787–1792. [PubMed] [Google Scholar]
- 14.Wu JN, Berecek KH. Prevention of genetic hypertension by early treatment of spontaneously hypertensive rats with the angiotensin converting enzyme inhibitor captopril. Hypertension. 1993;22:139–146. doi: 10.1161/01.hyp.22.2.139. [DOI] [PubMed] [Google Scholar]
- 15.Giudicelli JF, Freslon JL, Glasson S, Richer C. Captopril and hypertension development in the SHR. Clin Exp Hypertens. 1980;2:1083–1096. doi: 10.3109/10641968009037162. [DOI] [PubMed] [Google Scholar]
- 16.Unger T, Rettig R. Development of genetic hypertension. Is there a “critical phase”? Hypertension. 1990;16:615–616. doi: 10.1161/01.hyp.16.6.615. [DOI] [PubMed] [Google Scholar]
- 17.Nyborg NC, Mulvany MJ. Lack of effect of anti-hypertensive treatment with felodipine on cardiovascular structure of young spontaneously hypertensive rats. Cardiovasc Res. 1985;19:528–536. doi: 10.1093/cvr/19.9.528. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Katovich MJ, Gelband CH, Reaves PY, Phillips MI, Raizada MK. Sustained inhibition of angiotensin I-converting enzyme (ACE) expression and long-term antihypertensive action by virally mediated delivery of ACE antisense cDNA. Circ Res. 1999;85:614–622. doi: 10.1161/01.res.85.7.614. [DOI] [PubMed] [Google Scholar]
- 19.Nakaya H, Sasamura H, Hayashi M, Saruta T. Temporary treatment of prepubescent rats with angiotensin inhibitors suppresses the development of hypertensive nephrosclerosis. J Am Soc Nephrol. 2001;12:659–666. doi: 10.1681/ASN.V124659. [DOI] [PubMed] [Google Scholar]
- 20.Nakaya H, Sasamura H, Mifune M, Shimizu-Hirota R, Kuroda M, Hayashi M, Saruta T. Prepubertal treatment with angiotensin receptor blocker causes partial attenuation of hypertension and renal damage in adult Dahl salt-sensitive rats. Nephron. 2002;91:710–718. doi: 10.1159/000065035. [DOI] [PubMed] [Google Scholar]
- 21.Uehara Y, Hirawa N, Numabe A, Kawabata Y, Nagoshi H, Negoro H, Fujiwara S, Gomi T, Ikeda T, Goto A, Omata M. Angiotensin-converting enzyme inhibition delays onset of glucosuria with regression of renal injuries in genetic rat model of non-insulin-dependent diabetes mellitus. J Cardiovasc Pharmacol Ther. 1998;3:327–336. doi: 10.1177/107424849800300408. [DOI] [PubMed] [Google Scholar]
- 22.Koga K, Yamagishi S, Takeuchi M, Inagaki Y, Amano S, Okamoto T, Saga T, Makita Z, Yoshizuka M. CS-886, a new angiotensin II type 1 receptor antagonist, ameliorates glomerular anionic site loss and prevents progression of diabetic nephropathy in Otsuka Long-Evans Tokushima fatty rats. Mol Med. 2002;8:591–599. [PMC free article] [PubMed] [Google Scholar]
- 23.Okada M, Takemura T, Yanagida H, Yoshioka K. Response of mesangial cells to low-density lipoprotein and angiotensin II in diabetic (OLETF) rats. Kidney Int. 2002;61:113–124. doi: 10.1046/j.1523-1755.2002.00107.x. [DOI] [PubMed] [Google Scholar]
- 24.Yao L, Kobori H, Rahman M, Seth DM, Shokoji T, Fan Y, Zhang GX, Kimura S, Abe Y, Nishiyama A. Olmesartan improves endothelin-induced hypertension and oxidative stress in rats. Hypertens Res. 2004;27:493–500. doi: 10.1291/hypres.27.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in Dahl salt-sensitive rats. Kidney Int. 2004;65:972–981. doi: 10.1111/j.1523-1755.2004.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiyama A, Kobori H, Fukui T, Zhang GX, Yao L, Rahman M, Hitomi H, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Role of angiotensin II and reactive oxygen species in cyclosporine A-dependent hypertension. Hypertension. 2003;42:754–760. doi: 10.1161/01.HYP.0000085195.38870.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–848. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 29.Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: Effects of ACEI and ARB. Kidney Int. 2002;61:186–194. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 30.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000;91:21–27. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 31.Wilcox CS. Reactive oxygen species: Roles in blood pressure and kidney function. Curr Hypertens Rep. 2002;4:160–166. doi: 10.1007/s11906-002-0041-2. [DOI] [PubMed] [Google Scholar]
- 32.Ha H, Lee HB. Reactive oxygen species and matrix remodeling in diabetic kidney. J Am Soc Nephrol. 2003;14(Suppl 3):S246–S249. doi: 10.1097/01.asn.0000077411.98742.54. [DOI] [PubMed] [Google Scholar]
- 33.Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003;14(Suppl 3):S241–S245. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Blom IE, Sa S, Goldschmeding R, Abraham DJ, Leask A. CTGF expression in mesangial cells: Involvement of SMADs, MAP kinase, and PKC. Kidney Int. 2002;62:1149–1159. doi: 10.1111/j.1523-1755.2002.kid567.x. [DOI] [PubMed] [Google Scholar]
- 35.Riser BL, Denichilo M, Cortes P, Baker C, Grondin JM, Yee J, Narins RG. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol. 2000;11:25–38. doi: 10.1681/ASN.V11125. [DOI] [PubMed] [Google Scholar]
- 36.Yokoi H, Mukoyama M, Nagae T, Mori K, Suganami T, Sawai K, Yoshioka T, Koshikawa M, Nishida T, Takigawa M, Sugawara A, Nakao K. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol. 2004;15:1430–1440. doi: 10.1097/01.asn.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]


