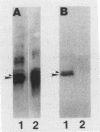
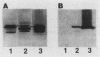
Abstract
Anticarbohydrate monoclonal antibodies were tested for their ability to bind to various strains of Neisseria. A monoclonal antibody that binds to the ganglio-series glycosphingolipid, ganglio-N-triaosylceramide, also bound to strains of Neisseria gonorrhoeae but not to other species of Neisseria. An antibody specific for the globo-series glycosphingolipid, globotriaosylceramide, also bound to strains of N. gonorrhoeae, Neisseria meningitidis, Neisseria lactamica, and Branhamella catarrhalis but not to any other strains of nonpathogenic Neisseria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buehler J., Macher B. A. Glycosphingolipid immunostaining: detection of antibody binding with an avidin-biotin enzyme system. Anal Biochem. 1986 Nov 1;158(2):283–287. doi: 10.1016/0003-2697(86)90551-8. [DOI] [PubMed] [Google Scholar]
- Galili U., Basbaum C. B., Shohet S. B., Buehler J., Macher B. A. Identification of erythrocyte Gal alpha 1-3Gal glycosphingolipids with a mouse monoclonal antibody, Gal-13. J Biol Chem. 1987 Apr 5;262(10):4683–4688. [PubMed] [Google Scholar]
- Griffiss J. M., Schneider H., Mandrell R. E., Yamasaki R., Jarvis G. A., Kim J. J., Gibson B. W., Hamadeh R., Apicella M. A. Lipooligosaccharides: the principal glycolipids of the neisserial outer membrane. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S287–S295. doi: 10.1093/cid/10.supplement_2.s287. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Jacquemart F., Millot G., Goujet-Zalc C., Mahouy G., Zalc B. Production and characterization of a mouse monoclonal antibody to the glycolipid asialo-GM1. Hybridoma. 1988 Aug;7(4):323–331. doi: 10.1089/hyb.1988.7.323. [DOI] [PubMed] [Google Scholar]
- John C. M., Griffiss J. M., Apicella M. A., Mandrell R. E., Gibson B. W. The structural basis for pyocin resistance in Neisseria gonorrhoeae lipooligosaccharides. J Biol Chem. 1991 Oct 15;266(29):19303–19311. [PubMed] [Google Scholar]
- Kannagi R., Cochran N. A., Ishigami F., Hakomori S., Andrews P. W., Knowles B. B., Solter D. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2(12):2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R., Fukuda M. N., Hakomori S. A new glycolipid antigen isolated from human erythrocyte membranes reacting with antibodies directed to globo-N-tetraosylceramide (globoside). J Biol Chem. 1982 Apr 25;257(8):4438–4442. [PubMed] [Google Scholar]
- Kasai K., Galton J., Terasaki P. I., Wakisaka A., Kawahara M., Root T., Hakomori S. I. Tissue distribution of the Pk antigen as determined by a monoclonal antibody. J Immunogenet. 1985 Aug-Oct;12(4-5):213–220. doi: 10.1111/j.1744-313x.1985.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Griffiss J. M. Neisseria lactamica and Neisseria meningitidis share lipooligosaccharide epitopes but lack common capsular and class 1, 2, and 3 protein epitopes. Infect Immun. 1989 Feb;57(2):602–608. doi: 10.1128/iai.57.2.602-608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Hu Z., Westerink M. A., Poolman J. T., Griffiss J. M. Electromorphic characterization and description of conserved epitopes of the lipooligosaccharides of group A Neisseria meningitidis. Infect Immun. 1988 Oct;56(10):2631–2638. doi: 10.1128/iai.56.10.2631-2638.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Griffiss J. M., Macher B. A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988 Jul 1;168(1):107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Kim J. J., John C. M., Gibson B. W., Sugai J. V., Apicella M. A., Griffiss J. M., Yamasaki R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991 May;173(9):2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Lesse A. J., Sugai J. V., Shero M., Griffiss J. M., Cole J. A., Parsons N. J., Smith H., Morse S. A., Apicella M. A. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J Exp Med. 1990 May 1;171(5):1649–1664. doi: 10.1084/jem.171.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., McLaughlin R., Aba Kwaik Y., Lesse A., Yamasaki R., Gibson B., Spinola S. M., Apicella M. A. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect Immun. 1992 Apr;60(4):1322–1328. doi: 10.1128/iai.60.4.1322-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Williams G. D., Pier G. B. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982 Jan;128(1):13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- Sidberry H., Kaufman B., Wright D. C., Sadoff J. Immunoenzymatic analysis by monoclonal antibodies of bacterial lipopolysaccharides after transfer to nitrocellulose. J Immunol Methods. 1985 Feb 11;76(2):299–305. doi: 10.1016/0022-1759(85)90307-2. [DOI] [PubMed] [Google Scholar]
- Solomon F. R., Higgins T. J. A monoclonal antibody with reactivity to asialo GM1 and murine natural killer cells. Mol Immunol. 1987 Jan;24(1):57–65. doi: 10.1016/0161-5890(87)90111-8. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Virji M., Weiser J. N., Lindberg A. A., Moxon E. R. Antigenic similarities in lipopolysaccharides of Haemophilus and Neisseria and expression of a digalactoside structure also present on human cells. Microb Pathog. 1990 Dec;9(6):441–450. doi: 10.1016/0882-4010(90)90062-u. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Hakomori S. Gangliosides of human erythrocytes. A novel ganglioside with a unique N-acetylneuraminosyl-(2 leads to 3)-N-acetylgalactosamine structure. Biochemistry. 1979 Nov 27;18(24):5502–5504. doi: 10.1021/bi00591a037. [DOI] [PubMed] [Google Scholar]
- Yamasaki R., Bacon B. E., Nasholds W., Schneider H., Griffiss J. M. Structural determination of oligosaccharides derived from lipooligosaccharide of Neisseria gonorrhoeae F62 by chemical, enzymatic, and two-dimensional NMR methods. Biochemistry. 1991 Oct 29;30(43):10566–10575. doi: 10.1021/bi00107a028. [DOI] [PubMed] [Google Scholar]
- Yamasaki R., Nasholds W., Schneider H., Apicella M. A. Epitope expression and partial structural characterization of F62 lipooligosaccharide (LOS) of Neisseria gonorrhoeae: IgM monoclonal antibodies (3F11 and 1-1-M) recognize non-reducing termini of the LOS components. Mol Immunol. 1991 Nov;28(11):1233–1242. doi: 10.1016/0161-5890(91)90010-h. [DOI] [PubMed] [Google Scholar]
- Young W. W., Jr, MacDonald E. M., Nowinski R. C., Hakomori S. I. Production of monoclonal antibodies specific for two distinct steric portions of the glycolipid ganglio-N-triosylceramide (asialo GM2). J Exp Med. 1979 Oct 1;150(4):1008–1019. doi: 10.1084/jem.150.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurewicz E. C., Matsuura F., Moghissi K. S. Structural characterization of neutral oligosaccharides of human midcycle cervical mucin. J Biol Chem. 1982 Mar 10;257(5):2314–2322. [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]