Abstract
Background
Reinforcement of behavioral responses involves a complex cerebral circuit engaging specific neuronal networks that are modulated by cortical oversight systems affiliated with emotion, memory, judgment, and decision making (collectively referred to in this study as the “extended reward and oversight system” or ”reward-network”). We examined whether reward-network brain volumes are reduced in alcoholics, and how volumes of subcomponents within this system are correlated with memory and drinking-history.
Methods
Morphometric analysis was performed on magnetic resonance brain scans in 21 abstinent long-term chronic alcoholic men and 21 healthy control men, group-matched on age, verbal IQ, and education. We derived volumes of total brain and volumes of cortical and subcortical reward-related structures including the dorsolateral-prefrontal, orbitofrontal, and cingulate cortices, and the insula, as well as the amygdala, hippocampus, nucleus accumbens septi (NAc), and ventral diencephalon.
Results
Morphometric analyses of reward-related regions revealed decreased total reward-network volume in alcoholic subjects. Volume reduction was most pronounced in right dorsolateral-prefrontal cortex, right anterior insula, and right NAc, as well as left amygdala. In alcoholics, NAc and anterior insula volumes increased with length-of-abstinence, and total reward-network and amygdala volumes correlated positively with memory scores.
Conclusions
The observation of decreased reward-network volume suggests that alcoholism is associated with alterations in this neural reward system. These structural reward system deficits, and their correlation with memory scores, elucidate underlying structural-functional relationships between alcoholism and emotional and cognitive processes.
Keywords: Alcoholism, MRI, reward system, amygdala, nucleus accumbens, dorsolateral-prefrontal cortex
Introduction
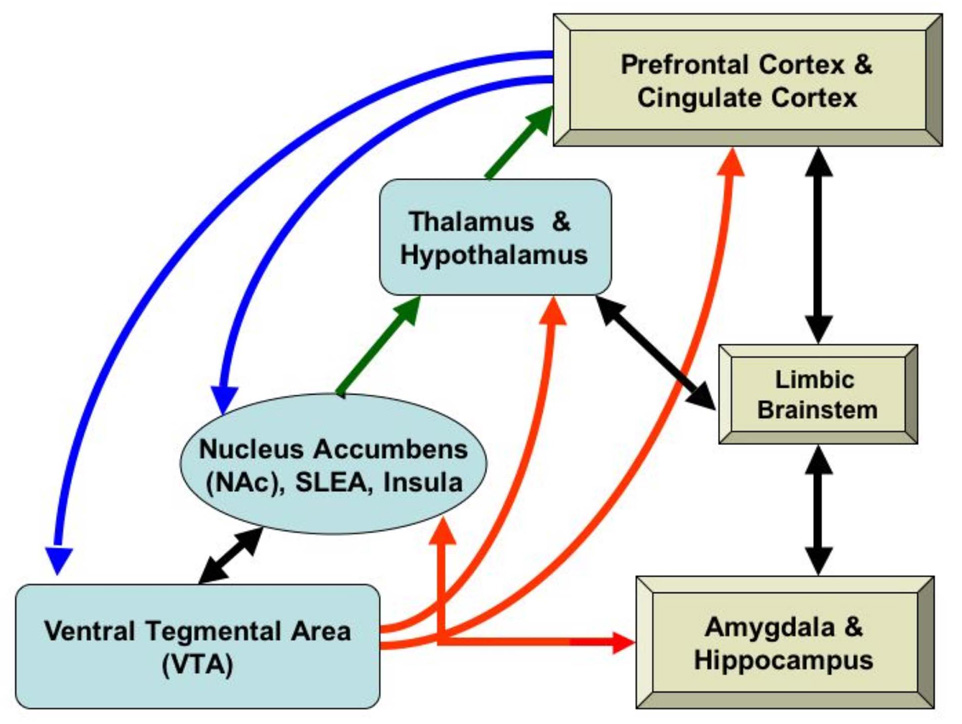
Emotional, memory, and motivational abnormalities in alcoholism are associated with changes in the mesocorticolimbic system (1, 2), a complex multi-functional network responsive to positive and negative reinforcement. Positive reinforcement (reward) increases the probability of a subsequent response, and drugs of abuse are at least as potent as natural reinforcers (e.g., food (3)). Circuitry involved in the development of reinforced behaviors is a central part of this network (see Figure 1). Principal components of the mesocorticolimbic reward circuit include amygdala, hippocampus, nucleus accumbens (ventral striatum), and ventral diencephalon(including basal forebrain, ventral tegmentum, and hypothalamus), and cortical areas with modulating and oversight functions, such as dorsolateral-prefrontal, orbitofrontal, temporal pole, subcallosal, and cingulate cortices, parahippocampal gyri, and the insula (2, 4–15). We hypothesized that morphometric abnormalities would present in alcoholic subjects in these subcortical gray-matter limbic and paralimbic regions, which mediate primary reward functions, together with associated cortical centers, which are important for executive functioning, emotional judgment and responses, decision making, and oversight (2, 16). Collectively, this cortical/subcortical circuit is referred to in this study as the “extended reward and oversight system” or the ”reward-network”. However, in addition to reward functions, this system is associated with motivation and evaluation; approach and avoidance; impulsivity and inhibition; reward and punishment.
Figure 1.
Brain regions involved in the extended reward and oversight system. Prefrontal and cingulate regions connect to the nucleus accumbens (NAc) in the ventral striatum, the midbrain ventral tegmental area (VTA), and reciprocally with other limbic system structures (limbic brainstem, amygdala, and hippocampus). Limbic structures also interconnect with the NAc and to the basal forebrain (substantia innominata, or sublenticular extended amygdala: SLEA). The VTA projects to the NAc (reciprocally), to the thalamus and hypothalamus, and to prefrontal cortex. The NAc projects to the thalamus, which projects to prefrontal cortex.
In alcoholics, brain regions previously studied with magnetic resonance imaging (MRI)include frontal lobes, cingulate cortex, striatum, amygdala, hippocampus, hypothalamus, and cerebellum (17–20). Most studies demonstrated alcoholism-related structural changes, including atrophy and white-matter damage (17, 18, 21–23). However, no prior study comprehensively assessed the reward-network as an interconnected system in its entirety and in its subcomponents. Brain regions that have received relatively little attention in alcoholism include nucleus accumbens septi (NAc) and ventral diencephalon. Studying groups of anatomical regions, which are components of structural and functional circuits, is an important avenue in identifying a biomarker for a disease (15, 24).
Using MRI in the present study, we analyzed brains of abstinent long-term chronic alcoholic subjects (AL) and group-matched healthy nonalcoholic controls (NC) to test the hypothesis that alcoholism is associated with volumetric changes in the reward-network. We also explored relationships of volumetric alterations of the reward-network with memory, IQ, and drinking-history.
Methods
Subjects
Participants were right-handed men from the Boston area. Handedness was determined by a handedness questionnaire (25) and the Edinburgh Inventory (26). The study included 21 AL individuals, abstinent from alcohol at least four weeks, and 21 healthy NC subjects (Table 1).Participants were native English speakers, with comparable socioeconomic backgrounds. Groups were comparable with respect to demographic variables.
Table 1.
Demographic, clinical, and neuropsychological testing data.
|
Nonalcoholic Comparison Subjects n=21 * mean ± SD |
Subjects with Alcoholism n=21 * mean ± SD |
p-value | |
|---|---|---|---|
| Age at Scan | 54.0 ± 11.8 | 50.7 ± 11.7 | 0.36 |
| Years of Education | 14.5 ± 2.0 | 13.5 ± 2.1 | 0.13 |
| IQ and Memory | |||
| Full Scale IQ (a) | 109.2 ± 10.4 | 103.8 ± 12.0 | 0.13 |
| Verbal IQ | 109.3 ± 11.6 | 106.8 ± 12.0 | 0.50 |
| Performance IQ | 107.7 ± 10.7 | 99.7 ± 12.6 | 0.04 |
| General Memory (b) | 103.3 ± 14.2 | 99.4 ± 11.5 | 0.34 |
| Working Memory | 105.6 ± 13.8 | 109.3 ± 18.1 | 0.46 |
| WAIS-III Performance Subtests | |||
| Digit Symbol | 10.4 ± 2.8 | 8.2 ± 2.3 | 0.01 |
| Block Design | 11.1 ± 2.6 | 10.3 ± 2.9 | 0.35 |
| Picture Arrangements | 11.3 ± 2.7 | 10.4 ± 2.3 | 0.27 |
| Object Assembly | 9.9 ± 3.1 | 10.3 ± 2.9 | 0.69 |
| Executive Functioning | |||
| WCST Perseverative Errors (%) (c) | 44.5 ± 33.2 | 42.4 ± 28.2 | 0.83 |
| FAS Word Generation (%) (d) | 44.5 ± 19.9 | 39.0 ± 24.5 | 0.44 |
| Time (sec) to Complete Trails A (e) | 33.9 ± 12.8 | 33.8 ± 14.9 | 0.98 |
| Time (sec) to Complete Trails B | 87.3 ± 32.0 | 77.5 ± 36.0 | 0.36 |
| Depression Inventories | |||
| POMS (f) | 37.9 ± 4.2 | 42.4 ± 8.5 | 0.04 |
| MAACL (g) | 46.4 ± 5.7 | 57.2 ± 30.8 | 0.13 |
| Hamilton (h) | 1.2 ± 1.2 | 4.5 ± 5.4 | 0.01 |
| Anxiety Measure | |||
| MAACL | 43.4 ± 5.0 | 48.2 ± 12.3 | 0.11 |
| Drinking History | |||
| Quantity Frequency Index (QFI) | 0.4 ± 0.5 | 11.3 ± 9.4 | <0.0001 |
| Years of Consuming 21+ Drinks per Week | - | 18.3 ± 8.6 | |
| Length of Sobriety (years) | - | 5.9 ± 10.4 |
WAIS-III (30)
WMS-III (31)
FAS (Controlled Oral Word Association Test: (42))
Trails A & B (38)
POMS (Profile of Mood States: (33))
MAACL (Multiple Affect Adjective Check List: (34))
Hamilton Depression Scale (32).
One control subject did not receive neuropsychological or clinical tests, and one alcoholic subject did not complete the MAACL.
Abbreviations: SD - standard deviation; QFI – Quantity-Frequency Index (a drinking severity scale: (29))
Participation was solicited from newspaper and web-based advertisements and from Boston University Medical Center, Boston Veterans Affairs (VA) Healthcare System, and VA after-care programs. Twenty participants (12 NC, 8 AL) were veterans. This study was approved by the Institutional Review Boards of the participating institutions. Informed consent was obtained from each subject prior to neuropsychological testing and scanning. Participants were reimbursed for time and travel expenses. Neurobehavioral and psychiatric evaluations typically required from seven to nine hours over two or more days. Participants had frequent breaks, and sessions were discontinued and rescheduled if a subject indicated fatigue.
Neurobehavioral and Psychiatric Evaluations
Participants underwent medical history interview and vision testing, plus a series of questionnaires (e.g., handedness, alcohol and drug use) to ensure they met inclusion criteria. Participants performed a computer-assisted, shortened version of the Diagnostic Interview Schedule (DIS, (27)) that provides lifetime psychiatric diagnoses according to DSM-IV (28) criteria. Participants were excluded if any source (DIS scores, hospital records, referrals, or personal interviews) indicated they had one of the following: history of neurological dysfunction(e.g., major head injury with loss of consciousness greater than 15 minutes, stroke, epilepsy, or seizures unrelated to alcohol withdrawal); electroconvulsive therapy; major psychiatric disorder (e.g., schizophrenia or primary depression); symptoms of clinical depression within the six months prior to testing; current use of psychoactive medication; history of abuse of drugs besides alcohol; clinical evidence of active hepatic disease; history of serious learning disability or dyslexia; and uncorrected abnormal vision or hearing problem.
Participants received a structured interview regarding their drinking patterns, including length-of-abstinence and years-of-heavy-drinking. A Quantity Frequency Index (QFI), which factors the amount, type, and frequency of alcoholic usage over the last six months (for the nonalcoholics), or over the six months preceding cessation of drinking (for the alcoholics), was calculated for each participant (29). Heavy-drinking was quantified as greater than 21 drinks per week (one drink: 355 ml beer, 148 ml wine, or 44 ml hard liquor). The AL group had QFI of 11.2±9.4, had heavy-drinking for 18.3±8.5 years, and had been sober for 5.9±10.4 years. The NC group had QFI of 0.4±0.5. AL participants met DSM-IV (28) criteria for alcohol abuse and dependence for a period of at least five years in their lives, and had abstained from alcohol for at least four weeks prior to testing.
Tests of intelligence, memory, and affect were administered, including the Wechsler Adult Intelligence Scale, Third-edition (WAIS-III, for Verbal IQ, Performance IQ, and Full-Scale IQ: (30)), Wechsler Memory Scale, Third-edition (WMS-III, for General Memory and Working Memory: (31)), Hamilton Depression Scale (32), Profile of Mood States (POMS: (33)), and Multiple Affect Adjective Check List (MAACL: (34)). Subtests of the WAIS-III that have been reported sensitive to alcohol-related visuospatial dysfunction are Digit Symbol, Picture Arrangement, Block Design, and Object Assembly (35–37). In addition, subjects underwent the following tests sensitive to frontal brain systems: Trail Making Test versions A and B (38); a computerized version (39) of the Wisconsin Card Sorting Test (WCST: (40, 41)); and the Controlled Oral Word Association Test (COWAT or FAS test: (42)).
MRI Acquisition
MRI scans were obtained at Massachusetts General Hospital (MGH) on a Siemens 3-Tesla Trio scanner. Image acquisitions included sagittal scout, T2-TSE (to rule out gross pathology), and two T1-weighted MP-RAGE series for volumetric analysis: TR=2530ms, TE=3.31ms, TI=1100ms, flip-angle=7°, Field-of-View=256, slice-thickness=1.33mm, number-of-slices=128 contiguous, sagittal images of the entire brain, matrix=256×256, number-of-excitations=2. The two MP-RAGE series were averaged, then the averaged series was re-sliced in a standard coronal three-dimensional brain coordinate system (43). Images were re-formatted to standard spatial orientation, but not rescaled in size.
MRI Morphometric Analysis
Image analyses followed semi-automated procedures developed by the Center for Morphometric Analysis at MGH (44–46). Intracranial volumes were segmented on T2-TSE images, since cerebrospinal fluid has bright T2-TSE signal, allowing clear demarcation within the intracranial vault (meninges/dura). Gray-matter, white-matter, and ventricles were segmented on T1-weighted images using a computer-assisted approach (44). Gray-matter was then subdivided into cortical and subcortical components. Neocortex was subdivided further into parcellation units, involving a number of manual and computer-assisted operations (47). Cortical subcomponents of the reward-network were derived: dorsolateral-prefrontal (defined as the sum of the dorsolateral superior-frontal and middle-frontal gyri, approximating Brodmann’s cytoarchitechtonic areas 8, 9 and 46), insula, subcallosal, orbitofrontal, and cingulate cortices, parahippocampal gyrus, and temporal pole. Gray-matter subcortical structures in the reward-network included NAc (46), amygdala, hippocampus (44, 48), and ventral diencephalon (49), which according to our morphometric definition contains the hypothalamus, basal forebrain, and sublenticular extended amygdala (SLEA), as well as a large portion of ventral tegmentum (which is included in our ventral diencephalon region by convention although part of midbrain). For comparisons to reward-network regions, we included analyses of non-reward related frontal cortex (“frontal pole,” defined in each hemisphere as cortex anterior to a coronal plane at the rostral end of the anterior horizontal ramus of the Sylvian fissure (47)), sensory cortex (cuneal cortex), and subcortical (dorsal striatum) regions.
Segmentation and cortical parcellation were carried out by an experienced research assistant (S.K.) with training in neuroanatomy, supervised by our neuroanatomist (N.M.). Blindness of group assignment was maintained during analysis. High inter-rater and intra-rater reliability of these methods has been established (47–54).
Topological Analyses
Prior studies have indicated atrophic changes in amygdala and hippocampus in alcoholic subjects (21, 22, 55, 56). Therefore, to further elucidate the sub-regions of these structures potentially impacted by alcoholism, we performed topological analyses using methods described in a previous report (54). Skull-stripped T1-weighted scans were registered (FSL/FLIRT: see http://www.fmrib.ox.ac.uk/fsl) to a template scan of a 35-year-old normal male subject (distinct from either group). Hippocampus and amygdala probability for each group was calculated on a voxel-by-voxel basis with the aligned data. Isosurfaces for the 0.5-probability regions were created for each cohort. These surfaces were visualized in three-dimensional space to look for systematic group differences in topology of hippocampus and amygdala.
Volumetric and Correlation Analyses
Statistical analyses were performed using JMP software (version-5.0.1.2, SAS-Institute, Inc., Cary, NC). Regions were defined as raw volumes and as ratios to cerebrum size. Between-group comparisons were made on raw volumes using analysis-of-covariance (ANCOVA) controlling for age and total cerebral volume (for global brain volume measures, only age was covaried). We applied a multi-level data analysis approach. Firstly, global volumetric brain measures were assessed to determine if there were global differences in brain, gray-matter, white-matter, or cerebrospinal-fluid volumes between groups. Secondly, we assessed the reward-network as a whole (sum of all reward-network regions), to determine whether the reward-network specifically was affected in alcoholism. Thirdly, if total reward-network group differences were observed, we followed the overall reward-network analysis with post hoc assessments of differences within the reward-network by using ANCOVA of reward-related subregions. Thus, following the single total reward-network analysis, in the presence of positive findings, we probed which regions were most affected within the reward-network, knowing that overall differences existed. Therefore, this final post hoc analysis was exploratory, and multiple comparison corrections were not applied.
Within-group partial correlation analyses, covaried for age and total cerebrum volume, were applied to assess relationships between regional reward-network volumes with memory, IQ, and drinking-history. Distribution of drinking-history measures such as length-of-abstinence were highly skewed. Therefore, log values of drinking-history measures were used in correlation analyses. Only total reward-network volume and bilateral volumes of those subregions showing post hoc group differences were included in the correlation analyses to limit the number of comparisons.
Results
Subjects
Table 1 provides group comparisons on demographic and neuropsychological test measures. Groups did not differ significantly in age, Full-Scale or Verbal IQ, memory scores, or education, although AL subjects scored significantly lower on Performance IQ. Both groups were in the clinically normal range for depression and anxiety scores, although the AL group’s scores were higher than the NC group’s. The only significant group differences on neurobehavioral comparisons were decreased Performance IQ (especially Digit Symbol subtest scores) and higher depression scores in AL subjects.
Morphometric Analyses
Morphometric measures of global brain volume (Table 2) showed that intracranial volume and total cerebrum volume both were 4.5% larger in the AL group than in NC (p=0.14). While brain size and age were not significantly different between groups, we covaried for these variables in subsequent regional analyses to account for modestly larger brains and younger age in the AL group. Thus, reward-related regions were analyzed by covarying raw volumes for total cerebrum volume and age. Total reward-network (sum of all reward-regions-of-interest volumes bilaterally) showed a significant decrease in AL subjects (Table 2). As a second-level analysis, having identified overall reward-network volume decrease in AL, we performed post hoc analyses of reward-network subregions (Table 3). Within the reward-network, individual structures that demonstrated significant (p<0.05) volumetric decrease were right dorsolateral-prefrontal cortex, right anterior insular lobule and right NAc. Left amygdala showed a trend (p<0.07) toward decreased volume. However, assessed as a ratio to total cerebrum volume covaried for age, left amygdala was significantly decreased in AL subjects (p<0.05).
Table 2.
Morphometric measures of the total extended reward and oversight system (total reward network) expressed as raw volume (in cubic centimeters: cc), and as a ratio to total cerebrum volume (%). Raw volumes (cc) of the global brain regions are presented. Components of the reward network are shown in Table 3.
| Region |
Nonalcoholic Comparison Subjects (n=21) mean ± SD |
Subjects with Alcoholism (n=21) mean ± SD |
ANCOVA Group Effect(a) t-value |
|---|---|---|---|
| Total Reward Network Measures | |||
| Reward Ratio to Total Cerebrum Volume (%) | 12.2 ± 0.6 | 11.5 ± 1.0 | −2.4 * |
| Reward Raw Volume (cc) | 128.9 ± 13.6 | 127.6 ± 14.1 | −2.2 * |
| Global Measures (cc) | |||
| Intracranial Volume | 1456.9 ± 112.7 | 1491.1 ± 121.7 | 0.9 |
| Total Brain | 1210.1 ± 124.0 | 1264.3 ± 110.8 | 1.2 |
| Total Cerebrum | 1061.7 ± 111.2 | 1109.9 ± 99.4 | 1.2 |
| Total Cerebral Cortex | 524.9 ± 53.5 | 537.7 ± 64.1 | 0.4 |
| Total Cerebral White Matter | 456.1 ± 62.8 | 492.9 ± 65.0 | 1.6 |
| Total Ventricular System | 29.3 ± 16.4 | 27.0 ± 9.1 | −0.2 |
Abbreviation: SD - standard deviation.
p < 0.05
For Total Reward Network Measures, we show the t-value of the Group effect from the univariate ANCOVA tests on Raw Volumes, covaried for Total Cerebrum Volume (p < 0.001) and for Age (p = 0.4) and on Ratio to Total Cerebrum Volume, covaried for age; for the Global Measures, we show the t-value of the Group effect from the univariate ANCOVA tests on raw volumes, covaried for Age. There were significant Age effects (p < 0.05) for Total Brain, Cerebrum, Cortex (decreasing volume with age), and Ventricular System (increasing volume with age).
Table 3.
Volumes of regions within the extended reward and oversight system (reward regions) and non-reward control regions, expressed as a ratio to total cerebrum volume (%).
| Left Hemisphere | Right Hemisphere | |||||
|---|---|---|---|---|---|---|
| Region |
Nonalcoholic Comparison Subjects (n=21) mean ± SD |
Subjects with Alcoholism (n=21) mean ± SD |
ANCOVA Group Effect(a) t-value |
Nonalcoholic Comparison Subjects (n=21) mean ± SD |
Subjects with Alcoholism (n=21) mean ± SD |
ANCOVA Group Effect(a) t-value |
| Subcortical Reward Regions | ||||||
| Nucleus Accumbens | 0.054 ± 0.01 | 0.053 ± 0.01 | −0.5 | 0.054 ± 0.01 | 0.047 ± 0.01 | −2.5* |
| Amygdala | 0.15 ± 0.03 | 0.13 ± 0.02 | −1.8† | 0.14 ± 0.03 | 0.13 ± 0.02 | −1.1 |
| Hippocampus | 0.33 ± 0.04 | 0.31 ± 0.03 | −0.8 | 0.35 ± 0.04 | 0.33 ± 0.03 | −1.2 |
| Ventral Diencephalon | 0.44 ± 0.07 | 0.41 ± 0.05 | −0.9 | 0.43 ± 0.06 | 0.40 ± 0.05 | −1.1 |
| Cortical Reward Regions | ||||||
| Insula, Anterior | 0.42 ± 0.05 | 0.41 ± 0.05 | 0.0 | 0.41 ± 0.03 | 0.38 ± 0.04 | −2.2 * |
| DLPFC | 1.74 ± 0.31 | 1.59 ± 0.41 | −1.5 | 1.76 ± 0.30 | 1.57 ± 0.31 | −2.2 * |
| Insula, Posterior | 0.21 ± 0.04 | 0.19 ± 0.03 | −1.8 ‡ | 0.21 ± 0.02 | 0.21 ± 0.03 | −0.1 |
| Orbitofrontal Cortex | 0.44 ± 0.08 | 0.45 ± 0.12 | 0.8 | 0.46 ± 0.09 | 0.42 ± 0.08 | −1.5 |
| Cingulate Cortex | 0.99 ± 0.15 | 0.96 ± 0.19 | −0.4 | 1.04 ± 0.17 | 1.05 ± 0.15 | 0.6 |
| Subcallosal Cortex | 0.21 ± 0.03 | 0.20 ± 0.04 | −0.7 | 0.20 ± 0.03 | 0.20 ± 0.03 | −0.9 |
| Parahippocampal Gyrus | 0.37 ± 0.05 | 0.35 ± 0.07 | −0.5 | 0.35 ± 0.06 | 0.33 ± 0.07 | −0.5 |
| Temporal Pole | 0.72 ± 0.09 | 0.71 ± 0.12 | −0.3 | 0.69 ± 0.09 | 0.69 ± 0.09 | 0.3 |
| Non-Reward Control Regions | ||||||
| Dorsal Striatum | 0.93 ± 0.07 | 0.91 ± 0.09 | −0.7 | 0.96 ± 0.06 | 0.93 ± 0.08 | −1.3 |
| Frontal Pole | 3.65 ± 0.78 | 3.55 ± 0.53 | −0.3 | 3.85 ± 0.86 | 3.57 ± 0.58 | −0.8 |
| Cuneal Cortex (sensory) | 0.50 ± 0.09 | 0.51 ± 0.11 | 0.7 | 0.55 ± 0.09 | 0.58 ± 0.12 | 0.7 |
Abbreviations: SD - standard deviation; DLPFC - dorsolateral prefrontal cortex.
p < 0.05
p = 0.07, ratio covaried for age p < 0.05
p = 0.08, ratio covaried for age p = 0.2
We show the t-value of the Group effect from the univariate ANCOVA tests on raw volumes, covaried for Total Cerebrum Volume and for Age. The overall ANCOVA (including Group, Age, and Total Cerebrum Volume in the model) is significant for all regions except right amygdala, left ventral diencephalon, left orbitofrontal cortex, left parahippocampal gyrus, left cingulate cortex, and the right frontal pole; Age effects were non-significant except for the right accumbens and left amygdala; Total Cerebrum Volume was a significant covariate in all regions except the left orbitofrontal cortex and the parahippocampal gyrus bilaterally.
Non-reward reference regions included frontal pole as a frontal cortex control region, cuneal cortex as a sensory-cortex control region, and dorsal striatum as a subcortical control region. These regions did not differ between groups.
Topological Analyses
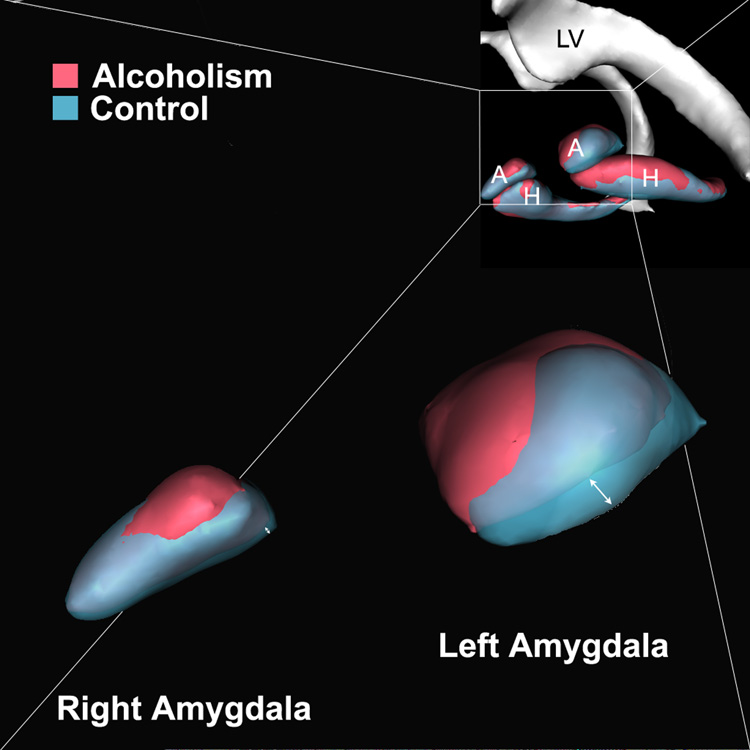
Topological analyses of amygdala and hippocampus showed group differences in topology. Decreased volume in AL was localized in basolateral-central nuclear groups of amygdala and subicular region (Figure 4). This was principally noted in left amygdala, which showed reduced volume in AL.
Figure 4.
The hippocampus and amygdala shown as three-dimensional isosurfaces. The average shape of the hippocampus and amygdala of control subjects is coregistered and superimposed on the average shape of these structures in the subjects with alcoholism. This figure indicates that the volumetric reductions are bilateral, but more pronounced in the left basolateral amygdala (see arrow) as well as bilaterally at the subiculum. These data suggest a topological specificity to volume reductions in amygdala-hippocampal structures in alcoholism. Abbreviations: A – amygdala, H – hippocampus, LV – lateral ventricle.
Correlations associating Morphometry with Memory, IQ, Age, and Drinking-History
In AL subjects, age was significantly correlated with increasing length-of-abstinence (r=0.59; p=0.005), and had trends toward correlation with increasing years-of-heavy-drinking (r=0.42; p=0.06), and decreasing QFI (r=−0.36; p=0.11). Cerebral cortex volume had positive partial correlation, covarying for age, with length-of-abstinence (r=0.48; p=0.03), and negative partial correlation with years-of-heavy-drinking (r=−0.49; p=0.03). Both groups displayed decreasing brain volume and increasing ventricular volume with increasing age. However, these age effects were significant in AL subjects (brain: r=−0.43; p=0.05, ventricles: r=0.56; p=0.01) but not in NC (brain: r=−0.29; p=0.2, ventricles: r=0.24; p=0.3).
Total reward-network volume, and specific reward-regions showing post hoc morphometric differences (dorsolateral-prefrontal cortex, anterior insula, NAc, and amygdala), were included bilaterally in correlation analyses with memory, IQ, and drinking-history. Since the AL group averaged 3.3 years younger with 4.5% larger brains than the NC group, and since aging impacts morphometry and cognition, partial correlations were applied covarying for age and total cerebrum volume. We restricted analyses to these regions and behavior/demographic measures to limit the number of comparisons.
While groups were equivalent on Full-Scale IQ, and memory test scores, groups differed with respect to correlations between memory scores and volumes of reward circuit regions. In AL subjects, but not in NC subjects, total reward-network volume correlated positively with Working Memory scores, and total amygdala volume correlated with General Memory scores. NAc and anterior insula volumes increased with length-of-sobriety in AL subjects, demonstrating morphometric improvement with length-of-abstinence. IQ measures did not show significant correlations with reward-network measures in either group.
Discussion
The extended reward and oversight system consists of a network of cortical and subcortical regions that mediate the effects of positive and negative reinforcement (reward and aversion). By virtue of its cortical and subcortical centers and its multiple interconnections (57, 58), the reward-network is central to such functions as sensory processing, stimulus-reward associations and memory, and determination of mood (58, 59, 60). This system is strongly involved in executive functions and decision making (61), inhibition of perseverative behaviors (14), and initiating drug and alcohol abuse or relapse (1). In the present study, we used segmentation-based MRI morphometry to measure volumetric brain alterations in abstinent long-term alcoholic men. We tested the hypothesis that the reward-network is altered volumetrically in alcoholism. We observed that total reward-network volume was significantly reduced in alcoholic men compared to nonalcoholic controls. Reward-regions affected included right dorsolateral-prefrontal cortex, right anterior insula, right NAc, and left amygdala. Total reward-network volume correlated positively with Working Memory scores, while amygdala volume correlated with General Memory. Furthermore, NAc and anterior insula volumes improved in alcoholic subjects with increasing length-of-abstinence, suggesting some potential recovery of structural deficits. Deficits were specific to the reward-network; global brain and gray-matter measures did not differ between groups, nor did cortical (frontal pole, cuneal cortex), or subcortical (dorsal striatum) control regions.
Prior structural neuroimaging studies in alcoholism have focused principally on global atrophic changes in cerebral cortex, white-matter, and cerebellum, plus local effects in hippocampus (62), demonstrating volume reduction (21–23, 63). Smaller right amygdalae have been reported in relatives of alcoholics (56). Neuropathological observations demonstrated alcoholism-related neuronal loss in prefrontal association-cortex, hypothalamus, and cerebellum (64, 65). Frontal dysfunction in alcoholism has been demonstrated by neuropsychological investigations (66–71) and by metabolic (20), cerebral blood flow (72, 73), and functional MRI (74) studies. Brain structural changes become more prominent with aging (17, 21, 35), and alcoholism can exaggerate age-related volumetric reductions (75, 76).
Regarding overall brain volumes, studies comparing alcoholics and nonalcoholic controls have yielded variable results. One factor contributing to this variability is length-of-abstinence. In one study (77), alcoholics showed improvement in cortical gray-matter, sulcal, and lateral ventricular volumes early in abstinence (up to a month), as well as improvement in third ventricular volume with continued abstinence (up to a year). A recent report noted baseline atrophy in abstinent alcoholics, which improved with eight months abstinence (78). Another study followed alcoholics and controls over a five-year period (79): Age-related changes were observed in both groups, but alcoholics showed a greater rate of gray-matter volume loss than controls, a result similar to the age-related brain changes observed in the current study. However, measures of ventricular enlargement in alcoholics who maintained sobriety were comparable to those of controls, and the authors concluded that continued alcohol abuse results in progressive brain tissue volume shrinkage. In the present study, the length-of-abstinence of the AL group was 5.9 years (±10.4 years), and this may account for the similarity of the AL and NC groups in overall brain volume. Furthermore, we observed negative correlation between cerebral cortex volume and years-of-heavy-drinking, while length-of-abstinence correlated positively with volume in cerebral cortex and in some of the affected reward subregions (NAc, anterior insula), thereby confirming both the deleterious cerebral effects of chronic alcoholism, and the potential for improvement in brain structural deficits with abstinence.
Frontolimbic Relationships within the Reward-network
Dorsolateral-prefrontal cortex is richly interconnected with many reward-network structures (82, 83) enabling it to assess reward-aversion information, enhance rewarded behaviors, and modify the probability of subsequent responses (4). Moreover, the role of dorsolateral-prefrontal cortex in oversight of limbic-paralimbic centers within the reward circuitry is crucial for normal cognitive and emotional functioning (80–82). Furthermore, there is extensive connectivity among the amygdala and adjacent structures within the basal forebrain and NAc, structures considered to be part of the same neural system (84–87). These centers are involved in establishing associations between stimulus cues and reward, and for evaluating effectiveness of reinforcing stimuli. Largely through these associations, sensory inputs get transformed into powerful motivational and emotional representations (83). Therefore, structural alterations in dorsolateral-prefrontal cortex, anterior insula, NAc, and amygdala would disrupt the reward processing stream and disorganize these integrative functions.
The reward-network may be an integral part of the neurobiology of drug addiction in general, and alcohol dependence in particular (88). Structural malfunction in this system may increase risk for drug-seeking behaviors and Reward Deficiency Syndrome (1, 89). These abnormalities may reflect a genetically influenced alteration in alcoholism (90). Recent neuroimaging positron emission tomography (PET) reports have identified higher levels of dopamine D2 receptors in ventral striatum (NAc) in nonalcoholic members of alcoholic families, suggesting that higher levels of dopamine in the reward system may have a protective effect (91). Striatal D2 receptor availability in nonalcoholic family members correlated with positive emotionality, and with metabolism in orbitofrontal, anterior cingulate, and prefrontal cortex, demonstrating a connection between behavior, subcortical D2 neuroreceptors, and cortical function in reward-network regions. A related PET study found that alcohol craving in detoxified alcoholic men correlated with lower levels of striatal dopamine receptors, and corresponded to a greater relapse risk on follow-up (92). This circuitry may be crucial to alterations in hedonic set points related to development of drug use and dependence (88, 93, 94).
Processing of emotions engages differentially the two hemispheres, with negative emotional processing predominantly in right hemisphere and positive emotional processing primarily in the left hemisphere (35, 95, 96). In the present report, the localization of right dorsolateral-prefrontal cortex, right anterior insula, and right NAc deficits supports the right hemisphere and frontal hypotheses of deficits in alcoholism (16, 35). Currently what is known, however, regarding cerebral dominance and lateralization of the reward function is limited. Structural brain asymmetry and lateralization of functional dominance may result from molecular regulation, neural connections, and plasticity (97). Although genetic factors connecting cerebral asymmetry and functional dominance are supported, molecular correlates of cerebral asymmetry have yet to be identified (98, 99).
Topological Analyses in Amygdala and Hippocampus
Our topological analysis of hippocampus and amygdala indicated volumetric decrease in alcoholics was localized in the basolateral nuclear group of left amygdala and the subiculum. Remarkably, connections of amygdala with other structures within the reward-network such as dorsolateral-prefrontal cortex, anterior insula, hippocampus, and NAc are through its basolateral nuclear group. Whereas the central nucleus is associated with autonomic behavior modulating the general motivational influence of reward-related events, the basolateral nuclear group is thought to be part of the frontotemporal association system mediating outcome-specific incentive processes (100, 101). In the present study, AL subjects displayed correlation between Working Memory and reward-network volume, and between amygdala volumes and General Memory. Left amygdala volumetric decrease may account for deficits in performance related to analytical aspects of memory, as well as verbal memory (14, 102). Hippocampal and amygdala volume reductions were previously reported in alcoholics (22, 55, 56), and reduced amygdala volume was present in adolescents of high-risk alcoholism families, indicating a possible neurodevelopmental component (56). However, no prior studies evaluated particular sub-portions of these structures.
Limitations
Firstly, we studied only men, whereas effects of alcoholism may be even more devastating in women (103, 104). However, we selected men in order to avoid interactions of gender effects. In an ongoing companion study, we are examining women alcoholic subjects and controls to determine whether these effects are seen in alcoholic women as well. Secondly, we did not include highly sensitive behavioral or functional MRI measures of brain asymmetry in the present study; these findings will be detailed in a separate report. Thirdly, we did not selectively recruit our AL subjects from families with a high prevalence of alcoholism, and the volumetric abnormalities we observed may be better defined in alcoholics with a strong positive family history. Fourthly, we applied a multi-level analysis approach to identify whether the reward-network overall was affected in alcoholism, followed by post hoc analyses of reward-subregions. We did not apply multiple comparison corrections, since these post hoc regional analyses were performed to explore which reward-related subregions were most influential in the presence of an overall reward-network group effect. However, our findings should be replicated in a larger sample. In addition, the effect sizes we observed were modest, and while the overall reward-network demonstrated group differences, only a few regions within the network displayed significant effects. Finally, the morphometric analysis framework samples relatively large regions; small structures such as ventral tegmentum or SLEA may have regional differences that are undetectable with these methods. In addition, we do not have a parcellation unit defined for ventral putamen, even though this region may be related to NAc. However, we chose neuroanatomically specific and highly reliable regional parcellation methods over other, more automated methods, in order to assess specific morphometry of reward-network regions a priori. There are advantages and disadvantages of the methods employed in the present study compared with more automated methods such as FreeSurfer (105, 106) or voxel-based morphometry (107). FreeSurfer was validated using the methods described herein (105, 106). Voxel-based morphometry is not well-suited to testing hypotheses regarding an a priori, specifically defined, extensive neuroanatomical network. The advantages of FreeSurfer or voxel-based morphometry are automation, speed, and low-cost. However, automated methods are more prone to co-registration artifacts and atlasing misalignment (108). Although automated methods are preferable for some studies, and are less time-consuming, our methods are the gold-standard for anatomical accuracy, and they ensure precision in measuring regional neuroanotomic networks.
Conclusions
Abstinent long-term chronic alcoholics have volumetric deficits in the brain’s extended reward and oversight system. Deficits were most pronounced in right dorsolateral-prefrontal cortex, right anterior insula, right NAc, and left amygdala. The present study differs from prior investigations in two principal aspects. First, structures related to processing reward information were considered as an interconnected and interrelated system, which was treated as a unique group of regions in statistical analyses. Second, correlations associating reward-network morphometry with behavioral tests and drinking-history were performed. Memory correlated positively with reward-network volume, amygdala in particular, while length-of-abstinence correlated with increased volumes in NAc and anterior insula. The circuitry overseeing reward and aversion is fundamental for normal emotional functioning and its malfunction. The finding that the reward system is altered in alcoholism and is correlated with memory and drinking-history argues for a condition that either predisposes individuals to alcohol dependence, perhaps as a result of a genetic deficit in reward circuitry, and/or that long-term alcoholism damages parts of the brain involved in reward processing, and may lead to a cycle of accelerating dependence on alcohol.
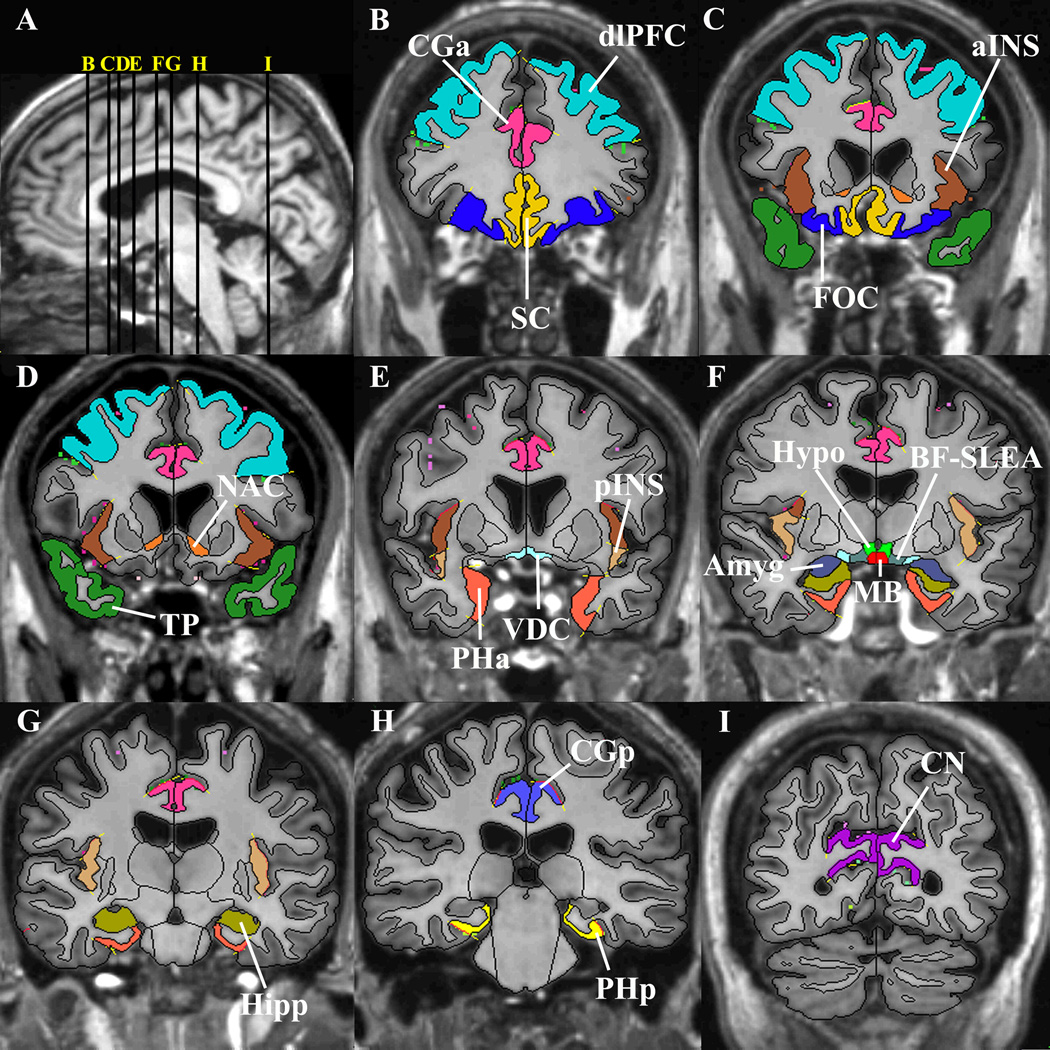
Figure 2.
Segmentation method of the cortical and subcortical structures composing the reward system, shown in T1-weighted magnetic resonance images (see 44, 47). Image A shows the midsagittal section on which vertical lines indicate the locations of representative coronal slices of images B-I. Abbreviations: aINS – anterior insular lobule, Amyg – amygdala, BF-SLEA – basal forebrain/sublenticular extended amygdala, CGa – anterior cingulate cortex, CGp – posterior cingulate gyrus, CN – cuneal cortex (a cortical control region not included in the reward-network), dlPFC – dorsolateral-prefrontal cortex, FOC –orbitofrontal cortex, Hipp – hippocampus, Hypo – hypothalamus, MB – mammilary body, NAC – nucleus accumbens area, PHa – anterior parahippocampal gyrus, PHp – posterior parahippocampal gyrus, pINS – posterior insular lobule, SC – subcallosal cortex, TP – temporal pole, VDC – ventral diencephalon.
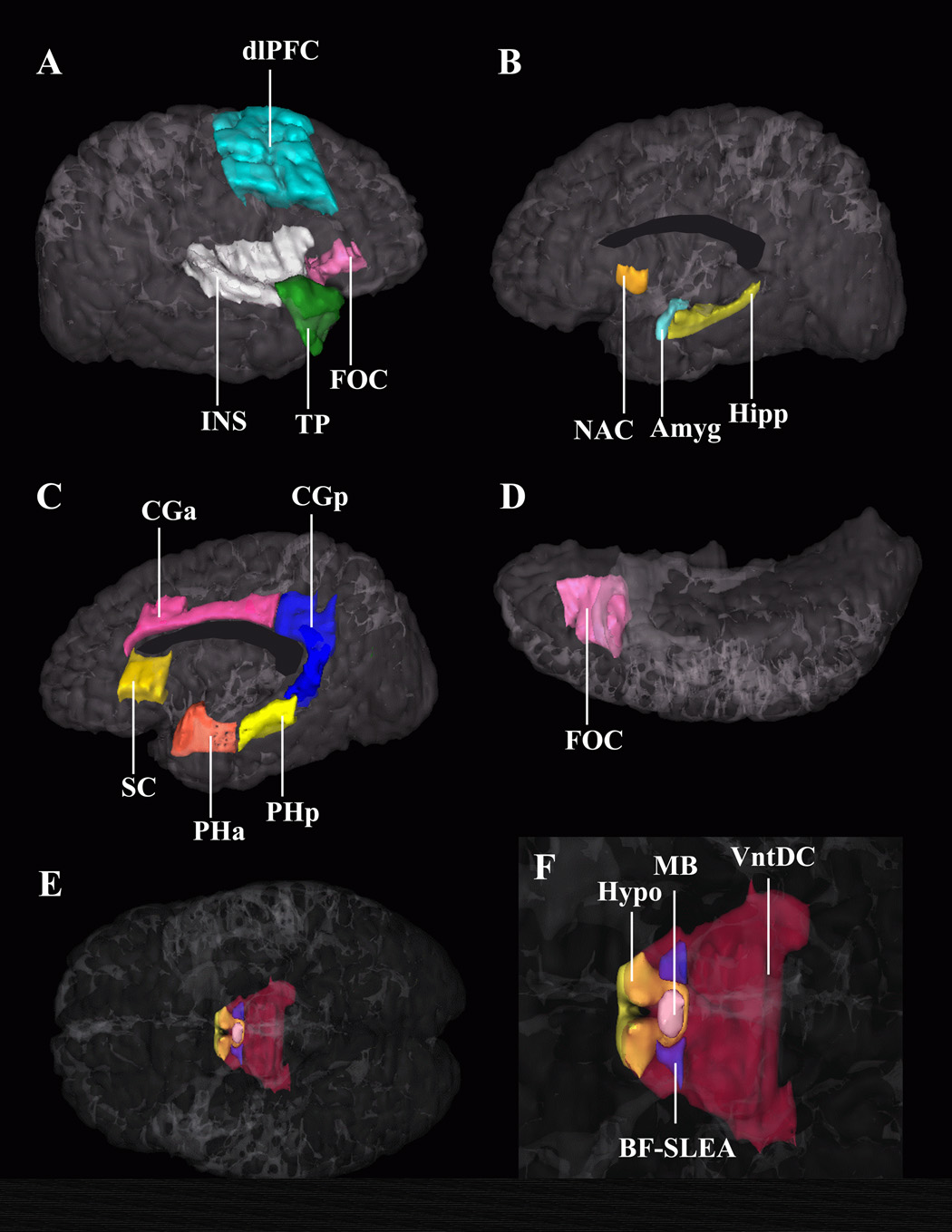
Figure 3.
Three-dimensional representation of the cortical and subcortical structures composing the reward system in the human brain. Image A shows a lateral view of the right hemisphere. Images B and C show a medial view of the right hemisphere; image D is an inferior view; images E and F are ventral views of both hemispheres showing the hypothalamus, mammilary bodies, SLEA, and ventral diencephalon. The latter structures are shown in image F in a zoomed view. Abbreviations: Amyg – amygdala, BF-SLEA – basal forebrain/sublenticular extended amygdala, CGa – anterior cingulate cortex, CGp – posterior cingulate gyrus, dlPFC – dorsolateral-prefrontal cortex, FOC –orbitofrontal cortex, Hipp – hippocampus, Hypo – hypothalamus, INS – insula, MB – mammilary body, NAC – nucleus accumbens area, PHa – anterior parahippocampal gyrus, PHp – posterior parahippocampal gyrus, SC – subcallosal cortex, TP – temporal pole, VDC – ventral diencephalon.
Table 4.
Partial correlations (Pr) between behavioral data and volumes of subcomponents of the reward circuitry, controlled for effects of Age and Total Cerebrum Volume (only regions with significant Group effects were included in correlation analyses). No significant correlations were observed for IQ or for other drinking history measures.
|
Nonalcoholic Comparison Subjects n=20 |
Subjects with Alcoholism n=21 |
||||
|---|---|---|---|---|---|
| Region | Test | Pr | p | Pr | p |
| Total Reward | Length of Sobriety | - | - | 0.26 | 0.3 |
| Total Amygdala | Length of Sobriety | - | - | −0.32 | 0.2 |
| Total Anterior Insula | Length of Sobriety | - | - | 0.60 | 0.007 |
| Total Dorsolateral Prefrontal Cortex | Length of Sobriety | - | - | 0.11 | 0.7 |
| Total Nucleus Accumbens | Length of Sobriety | 0.47 | 0.04 | ||
| Total Reward | General Memory | −0.01 | 0.9 | −0.32 | 0.2 |
| Total Amygdala | General Memory | −0.07 | 0.8 | 0.61 | 0.006 |
| Total Anterior Insula | General Memory | −0.50 | 0.03 | −0.37 | 0.12 |
| Total Dorsolateral Prefrontal Cortex | General Memory | 0.06 | 0.8 | −0.28 | 0.2 |
| Total Nucleus Accumbens | General Memory | −0.11 | 0.7 | −0.22 | 0.4 |
| Total Reward | Working Memory | −0.03 | 0.9 | 0.57 | 0.01 |
| Total Amygdala | Working Memory | 0.13 | 0.6 | 0.21 | 0.4 |
| Total Anterior Insula | Working Memory | −0.25 | 0.3 | 0.27 | 0.3 |
| Total Dorsolateral Prefrontal Cortex | Working Memory | −0.23 | 0.4 | 0.34 | 0.2 |
| Total Nucleus Accumbens | Working Memory | 0.10 | 0.7 | 0.06 | 0.8 |
Financial Disclosures and Acknowledgements
None of the authors reported having any biomedical financial interest or potential conflicts of interest. This work was supported in part by grants from: the National Association for Research in Schizophrenia and Depression (NARSAD) and the National Institutes of Health National Center for Complementary and Alternative Medicine (NCAM) to Dr. Nikos Makris; National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants R37-AA07112 and K05-AA00219, and the Medical Research Service of the U.S Department of Veterans Affairs to Dr. Marlene Oscar-Berman; NIAAA K01-AA13402 to Dr. Ksenija Marinkovic; the Fairway Trust to Dr. David Kennedy, and by the National Center for Research Resources (P41RR14075) and the Mental Illness and Neuroscience Discovery (MIND) Institute. We thank Diane Merritt for assistance in recruiting the research participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;132:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- 2.Oscar-Berman M, Bowirrat A. Genetic influences in emotional dysfunction and alcoholism-related brain damage. Neuropsychiatr Dis Treat. 2005;1:211–229. [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 4.Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 6.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeDoux JE, Farb CR, Romanski LM. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci Lett. 1991;134:139–144. doi: 10.1016/0304-3940(91)90526-y. [DOI] [PubMed] [Google Scholar]
- 8.Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. discussion 116–118. [DOI] [PubMed] [Google Scholar]
- 9.McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- 10.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 11.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 12.Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 13.Price JL. Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci. 1999;877:383–396. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer A, Braver TS, Reynolds JR, Burgess GC, Yarkoni T, Gray JR. Individual differences in amygdala activity predict response speed during working memory. J Neurosci. 2006;26:10120–10128. doi: 10.1523/JNEUROSCI.2567-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breiter HC, Gasic GP, Makris N. Imaging the neural system for motivated behavior and their dysfunction in neuropsychiatric illness. In: Deisboeck TS, Kresh JY, editors. Complex Systems Science in Biomedicine. New York: Springer Inc.; 2006. pp. 763–810. [Google Scholar]
- 16.Oscar-Berman M, Marinkovic K. Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: relation to ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- 19.Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- 20.Szabo Z, Owonikoko T, Peyrot M, Varga J, Mathews WB, Ravert HT, et al. Positron emission tomography imaging of the serotonin transporter in subjects with a history of alcoholism. Biol Psychiatry. 2004;55:766–771. doi: 10.1016/j.biopsych.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 22.Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- 23.Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, Tiihonen J. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res. 2000;109:177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- 24.Hyman SE, Nestler EJ. The molecular foundations of psychiatry. Washington, DC: American Psychiatric Press; 1993. [Google Scholar]
- 25.Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11:230–238. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- 26.Oldfield R. The assessment and analysis of handedness. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 27.Robins L, Helzer J, Cottler L, Goldring E. NIMH Diagnostic Interview Schedule: Version III Revised (DIS-III-R) St. Louis, MO: Washington University; 1989. [Google Scholar]
- 28.APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 29.Cahalan V, Cisin I, Crossley HM. American drinking practices: A national study of drinking behavior and attitudes, Report 6. New Brunswick, NJ: Rutgers Center for Alcohol Studies; 1969. [Google Scholar]
- 30.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 31.Wechsler D. Wechsler Memory Scale-III. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 32.Hamilton MA. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- 34.Zuckerman M, Lubin B. Multiple Affect Adjective Check List. San Diego, CA: Educational and Industrial Testing Service; 1965. [Google Scholar]
- 35.Oscar-Berman M, Schendan HE. Asymmetries of brain function in alcoholism: Relationship to aging. In: Obler L, Connor LT, editors. Neurobehavior of Language and Cognition: Studies of Normal Aging and Brain Damage. New York: Kluwer Academic Publishers; 2000. pp. 213–240. [Google Scholar]
- 36.Ellis RJ, Oscar-Berman M. Alcoholism, aging, and functional cerebral asymmetries. Psychol Bull. 1989;106:128–147. doi: 10.1037/0033-2909.106.1.128. [DOI] [PubMed] [Google Scholar]
- 37.Rourke SB, Loberg T. The neurobehavioral correlates of alcoholism. In: Grant I, Nixon SJ, editors. Neuropsychological Assessment of Neuropsychiatric Disorders, 2nd ed. New York: Oxford University Press; 1996. pp. 423–485. [Google Scholar]
- 38.US Army. Army Individual Test Battery. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General's Office; 1944. [Google Scholar]
- 39.Heaton R, Chelune G, Talley J, Kay G, Curtis G. Wisconsin Card Sorting Test: Computer Version 4. Lutz, Florida: Psychological Assessment Resources, Inc.; 1993. [Google Scholar]
- 40.Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- 41.Grant DA, Berg EA. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- 42.Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City, IA: University of Iowa; 1976. [Google Scholar]
- 43.Kennedy DN, Filipek PA, Caviness VS., Jr Anatomic segmentation and volumetric calculations in nuclear magnetic resonance imaging. IEEE Transactions on Medical Imaging. 1989;8:1–7. doi: 10.1109/42.20356. [DOI] [PubMed] [Google Scholar]
- 44.Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- 45.Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- 46.Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI-Based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage. 1999;9:18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- 47.Caviness VS, Jr, Meyer JW, Makris N, Kennedy DN. MRI-based topographic parcellation of the human neocortex: An anatomically specified method with estimate of reliability. J Cogn Neurosci. 1996;8:566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- 48.Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Toomey R, et al. Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biol Psychiatry. 1999;46:941–954. doi: 10.1016/s0006-3223(99)00075-x. [DOI] [PubMed] [Google Scholar]
- 49.Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, et al. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- 51.Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 52.De Fosse L, Hodge SM, Makris N, Kennedy DN, Caviness VS, Jr, McGrath L, et al. Language-association cortex asymmetry in autism and specific language impairment. Ann Neurol. 2004;56:757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- 53.Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Lange N, Bakardjiev A, et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- 54.Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, et al. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 55.Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, et al. Hippocampus volume loss due to chronic heavy drinking. Alcohol Clin Exp Res. 2006;30:1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 56.Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- 57.Fuster JM. Cortex and Mind. New York: Oxford University Press; 2003. [Google Scholar]
- 58.Fuster JM. The cognit: a network model of cortical representation. Int J Psychophysiol. 2006;60:125–132. doi: 10.1016/j.ijpsycho.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 59.Fuster JM. The prefrontal cortex. Anatomy, physiology, and neuropsychology of the frontal lobe. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 60.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Mountcastle VB, Plum F, editors. Handbook of Physiology: The Nervous System, Vol V: Higher Functions of the Brain. Bethesda, MD: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- 61.Nixon SJ, Parsons OA. Application of the Tridimensional Personality Questionnaire to a population of alcoholics and other substance abusers. Alcohol Clin Exp Res. 1990;14:513–517. doi: 10.1111/j.1530-0277.1990.tb01190.x. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan EV. Neuropsychological vulnerability to alcoholism: evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's neuroscience and behavioral research (Monograph No 34) Bethesda, MD: National Institute of Alcohol Abuse and Alcoholism; 2000. pp. 473–508. [Google Scholar]
- 63.Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- 64.Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- 65.Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:951–961. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- 66.Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, et al. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A. Factors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizophrenia and in chronic alcoholism. Psychiatry Res. 1993;46:175–199. doi: 10.1016/0165-1781(93)90019-d. [DOI] [PubMed] [Google Scholar]
- 68.Ratti MT, Bo P, Giardini A, Soragna D. Chronic alcoholism and the frontal lobe: which executive functions are impaired? Acta Neurol Scand. 2002;105:276–281. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- 69.Uekermann J, Daum I, Schlebusch P, Wiebel B, Trenckmann U. Depression and cognitive functioning in alcoholism. Addiction. 2003;98:1521–1529. doi: 10.1046/j.1360-0443.2003.00526.x. [DOI] [PubMed] [Google Scholar]
- 70.Uekermann J, Daum I, Schlebusch P, Trenckmann U. Processing of affective stimuli in alcoholism. Cortex. 2005;41:189–194. doi: 10.1016/s0010-9452(08)70893-1. [DOI] [PubMed] [Google Scholar]
- 71.Oscar-Berman M. Brain. In: Craighead WE, Nemeroff CB, editors. The Concise Encyclopedia of Psychology and Behaviour Science. New York: Wiley; 2004. pp. 135–137. [Google Scholar]
- 72.Demir B, Ulug B, Lay Ergun E, Erbas B. Regional cerebral blood flow and neuropsychological functioning in early and late onset alcoholism. Psychiatry Res. 2002;115:115–125. doi: 10.1016/s0925-4927(02)00071-9. [DOI] [PubMed] [Google Scholar]
- 73.Noel X, Sferrazza R, Van Der Linden M, Paternot J, Verhas M, Hanak C, et al. Contribution of frontal cerebral blood flow measured by (99m)Tc-Bicisate spect and executive function deficits to predicting treatment outcome in alcohol-dependent patients. Alcohol Alcohol. 2002;37:347–354. doi: 10.1093/alcalc/37.4.347. [DOI] [PubMed] [Google Scholar]
- 74.Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, Lim KO, Begleiter H. A functional MRI study of visual oddball: evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage. 2004;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 75.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 76.Kubota M, Nakazaki S, Hirai S, Saeki N, Yamaura A, Kusaka T. Alcohol consumption and frontal lobe shrinkage: study of 1432 non-alcoholic subjects. J Neurol Neurosurg Psychiatry. 2001;71:104–106. doi: 10.1136/jnnp.71.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 78.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- 80.Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 81.Petrides M, Pandya DN. Association pathways of the prefrontal cortex and functional observations. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. pp. 31–50. [Google Scholar]
- 82.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- 83.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 84.Johnston JB. Further contribution to the study of the evolution of the forebrain. J Comp Neurol. 1923;35:337–481. [Google Scholar]
- 85.De Olmos JS. A cupric-silver method for impregnation of terminal axon degeneration and its further use in staining granular argyrophilic neurons. Brain Behav Evol. 1969;2:213–237. [Google Scholar]
- 86.De Olmos JS, Ingram WR. The projection field of the stria terminalis in the rat brain. An experimental study. J Comp Neurol. 1972;146:303–334. doi: 10.1002/cne.901460303. [DOI] [PubMed] [Google Scholar]
- 87.De Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- 88.Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci. 1999;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- 89.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- 91.Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 92.Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, et al. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- 93.Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- 94.Koob GF. Neurobiological mechanisms in cocaine and opiate dependence. Res Publ Assoc Res Nerv Ment Dis. 1992;70:79–92. [PubMed] [Google Scholar]
- 95.Adolphs R, Jansari A, Tranel D. Hemispheric perception of emotional valence from facial expressions. Neuropsychology. 2001;15:516–524. [PubMed] [Google Scholar]
- 96.Hugdahl K, Davidson RJ, editors. The Asymmetrical Brain. Cambridge, MA: The MIT Press; 2004. [Google Scholar]
- 97.Anokhin AP, Heath AC, Myers E. Genetic and environmental influences on frontal EEG asymmetry: a twin study. Biol Psychol. 2006;71:289–295. doi: 10.1016/j.biopsycho.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006;7:655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- 99.Sun T, Patoine C, Abu-Khalil A, Visvader J, Sum E, Cherry TJ, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308:1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Swanson LW. The amygdala and its place in the cerebral hemisphere. Ann N Y Acad Sci. 2003;985:174–184. doi: 10.1111/j.1749-6632.2003.tb07081.x. [DOI] [PubMed] [Google Scholar]
- 101.Corbit LH, Balleinex BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aggleton JP. The amygdala: a functional analysis. 2nd ed. Oxford: Oxford University Press; 2000. [Google Scholar]
- 103.Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- 104.Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25:236–245. [PubMed] [Google Scholar]
- 105.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 106.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 107.Barros-Loscertales A, Meseguer V, Sanjuan A, Belloch V, Parcet MA, Torrubia R, Avila C. Striatum gray matter reduction in males with an overactive behavioral activation system. Eur J Neurosci. 2006;24:2071–2074. doi: 10.1111/j.1460-9568.2006.05084.x. [DOI] [PubMed] [Google Scholar]
- 108.Devlin JT, Poldrack RA. In praise of tedious anatomy. Neuroimage. 2007;37:1033–1041. doi: 10.1016/j.neuroimage.2006.09.055. discussion 1050–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]