Abstract
Although the oral route has traditionally been used for THC administration during the perinatal period in the rat, most studies administering THC during the postnatal period utilize intraperitoneal (ip) administration. In an effort to utilize the same route of administration in both prenatal and postnatal studies, we administered Δ-9-tetrahydrocannabinol (THC) in sesame oil by gavage during postnatal days 22-40 (equivalent to childhood to early adolescence) to male and female rats. We quantified behavior 40 minutes after administration in the Accuscan activity monitor on days 22, 29 and 40. In addition, we examined active and passive avoidance behaviors in treated adults. Acutely, THC had subtle effects on activity when measured during the period of drug administration and minimal effects on avoidance behaviors examined up to 140 days of age.
Since several groups have found that lower doses of THC administered intraperitoneally to periadolescent rats do produce behavioral alterations, we suspect that the inordinately slow absorption of the drug via the oral route may be responsible for the paucity of significant findings in our study. Therefore, oral administration of THC, particularly under conditions of mild food deprivation, may lead to sub-psychoactive concentrations of the drug within the brain.
Keywords: neurobehavioral toxicity, pre and peri-adolescence, cannabinoids, marijuana
1. Introduction
Marijuana is the most widely abused illegal substance in the world today. It is widely recognized that cognitive function is altered in adolescents and adults using marijuana, as well as children whose mothers consumed marijuana during pregnancy [6;13;19;20;22]. However, not all the domains of cognitive function are equally impaired by marijuana exposure. Tasks requiring planning, integration, analysis and synthesis in addition to basic visuoperceptual abilities are negatively impacted by prenatal marijuana exposure [7]. Tasks involving spatial and nonspatial working memory and alterations in locomotor activity have been shown to be affected by THC administration in adolescent and adult rats and mice [2;15;22]. Two studies have reported that adolescents are more sensitive to the effects of THC including locomotion than adults [2;22]. However, adults have been reported to be more sensitive to the anxiogenic, aversive and locomotor effects of THC than adolescents [17]. Stiglick and coworkers [19;20] reported that long term (3 months) intubations of THC at 20mg/kg or cannabis extract facilitated active avoidance behaviors when measured during administration and some of the effects persisted for months following drug administration (see also [15]). An open question is whether short term THC exposure during early adolescence would result in alterations in avoidance behavior in adulthood.
Therefore, the goal of this study was to determine whether periadolescent THC administration altered activity during the dosing period and had long term effects on avoidance behaviors as adults. To this end, we dosed preadolescent pups with THC in sesame oil via oral intubation, the traditional route of administration used in prenatal studies [10] and collected locomotor activity responses during the dosing period as well as performance on measures of active and passive avoidance in adulthood.
2. Methods
2.1 Subjects
Cohorts of Sprague-Dawley (VAF, Charles-River; Wilmington, ME) male and female rat pups arrived in the lab on postnatal day 1 and were housed with a surrogate dam in a reverse light cycle vivarium (lights off at 11:00) with temperature controlled to 20–22 °C. until postnatal day 21 at which point they were housed 2/cage in same-sex groupings. Each cohort consisted of 12 pups which were semi-randomly (controlling for sex) assigned to receive THC at 0, 1 or 5mg/kg in sesame oil (Fisher Scientific) at a volume of 10ml/kg. THC (Δ-9-tetrahydrocannabinol) was a generous gift of National Institute of Drug Abuse through Research Triangle Institute, Research Triangle Park, North Carolina. Within each cohort, 2 males and 2 females received either Vehicle, 1 or 5mg/kg THC. These doses were selected based on studies in pregnant rats [11]. Eight cohorts of 12 rats each were generated and the subjects in this study were randomly drawn from this pool. On postnatal day 22, daily gastric intubations were begun in selected subjects 20 min after the lights went out. Dosing was discontinued on day 40. All procedures were pre-approved by the SUNY Institutional Animal Care and Use Committee (IACUC).
2.2 Equipment
Activity was measured in the Accuscan activity chambers on days 22, 29 and 40. On the day of activity recording, rats were placed individually in a Plexiglas box (42 × 42 × 30 cm with no bedding) equipped with a Versamax activity monitor (VMRXYZ16; Accuscan Instruments, Columbus, OH) to record locomotor activity. Total distance traveled in cm was measured for 1 h.
Passive avoidance and Active avoidance tests were carried out in a Coulburn Shuttle Box with two equal compartments divided by white Plexiglas and equipped with shockers and an activity monitor.
2.3 Procedures
Activity
On postnatal day 22, 29 and 40, subjects were dosed and returned to the home cage. Forty min. later, they were placed in the activity chamber and activity was recorded for 60 min. At the end of the hour, subjects were immediately returned to the home cage.
Passive Avoidance
Passive Avoidance was tested when the animals were 132 days old - 90 days following the last dose of THC. On the first day of testing, animals were placed in the shuttle box and following a 60 sec blackout period, received a single 180 sec habituation trial, in which the compartment light that the animal was in turned on. During habituation, animals were able to move freely into the dark side of the chamber without receiving any shock. On the second day, rats were placed back in the chamber and received 3 trials of 180 sec test with an inter-trial blackout time of 60 sec. During each trial, a 0.5 mA foot shock was delivered if the animal entered into the dark (non-illuminated) compartment of the chamber and the shock was terminated when the rat crossed back into the illuminated compartment. On the third day, animals received a single trial (180 sec) during which no shock was delivered upon crossing into the dark side of the chamber. The latency to cross on the 3rd day was considered retention of the conditioned stimulus (CS) and was used as the dependent variable. If the animals crossed quickly into the dark compartment, this indicated a deficiency in retaining the aversive memory.
Active Avoidance
Active Avoidance was tested when the animals were 140 days old – 100 days after the last dose of THC. Animals were put in the left side of the shuttle box at the beginning of the trial. The test began automatically when the animal was detected. After a 60 sec habituation period in which both chambers were dark, a sound (90 db) and a light in the same compartment the animal was in turned on. A 0.5 mA foot shock was delivered if the animal did not cross into the other compartment within 5 sec after the onset of the sound and light stimuli. However, the shock expired 5 sec later even if the animal didn’t move. The trial was repeated 30 sec after either the occurrence of a crossing or the expiration of shock time for 20 replications. The identical test was run for three consecutive days. The number of avoidances and / or the percentage of avoidances each day were used for analysis. Higher scores in either number or percentage avoidances suggest better working memory and faster learning.
2.4 Data Analyses
Mixed linear models as described in Applied Mixed Models in Medicine [1] were used for all analyses using SAS 9.1.3 software (SAS Institute, Cary, NC). Cohort was introduced as a random factor for all analyses while sex, treatment, and day (if applicable) were considered fixed factors. Post hoc pairwise comparisons were conducted as appropriate. Satterthwaite adjustments to the denominator degrees of freedom were applied. P values equal or below 0.05 were considered significant.
3. Results
3.1 Body weight
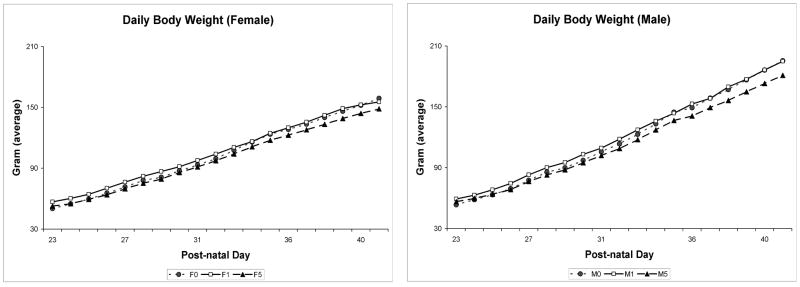
Growth curves for male and female rats (figure 1) indicate that there are no significant drug effects for body weight during the period of drug administration or at the time of assessment of avoidance behaviors.
Figure 1.
Body weight (g) curves for male and female adolescent rats dosed with THC at 0, 1 or 5mg/kg/day in sesame oil during postnatal days 22-40.
3.2 Activity
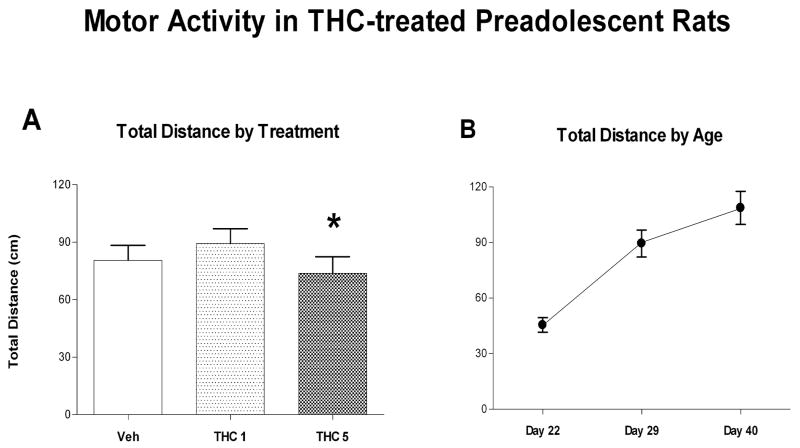
Activity data were recorded on: control (Veh) (8♀; 7♂), low dose (THC-1) (8♀; 7♂) and high dose (THC-5) (8♀; 7♂) subjects. Total distance traveled was collapsed into a single score for each trial, log transformed and analyzed using a Mixed Linear Model in SAS (figure 2A). Results show that Treatment produced a significant main effect [F(2,114)=3.2; p=0.0446] but there were no main effects for sex or interactions between sex and treatment. Post hoc pairwise comparisons indicated that activity in the vehicle control group was not significantly different from that in the 1mg/kg group and the 5mg/kg group. However, activity in the 1mg/kg group was significantly greater than that in the 5 mg/kg group [Tukey adjusted p=0.035]. When activity of all groups was analyzed by age, age produced a significant effect [F(2,114)=34.82; p<0.001] but there were no significant interactions with age (Figure 2B) Activity increased with increasing age. Cohort was not significant [Pr Z =0.139].
Figure 2.
Behavior in the Accuscan activity chamber collected 40 min after oral intubation with THC. A shows total activity collapsed across the 3 test ages and sex. * indicates a significant difference between activity recorded in rats in the THC 1mg/kg and 5mg/kg groups. B shows activity at each age collapsed across sex and treatment groups. There was a significant increase in activity with age. Mean ± sem.
3.3 - Passive Avoidance
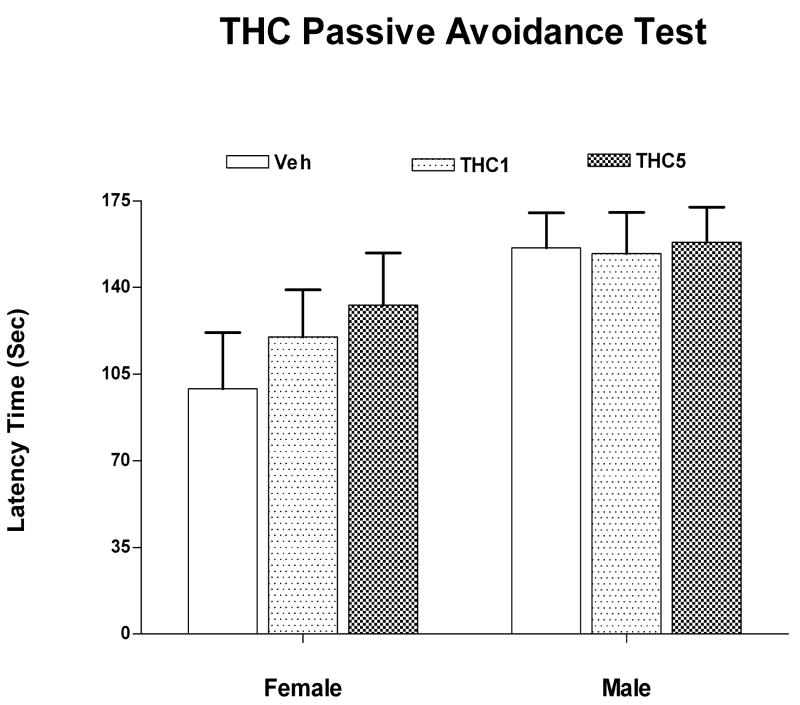
Passive avoidance data in adult rats (132-135 days of age) were collected from 12 females in each group and between 6 and 13 males in each dose group. Data were analyzed for retention of the CS on day 3 using a Mixed Linear Model. There were no significant effects of the THC treatment, sex or interactions (figure 3). Cohort was not significant [PrZ=0.069].
Figure 3.
shows the latency in sec to cross to the shocked side of the test chamber on the third test day in the passive avoidance test. There were no significant treatment or sex effects of the preadolescent THC administration on passive avoidance behavior measured at 132 days of age. Mean ± sem.
3.4 - Active Avoidance
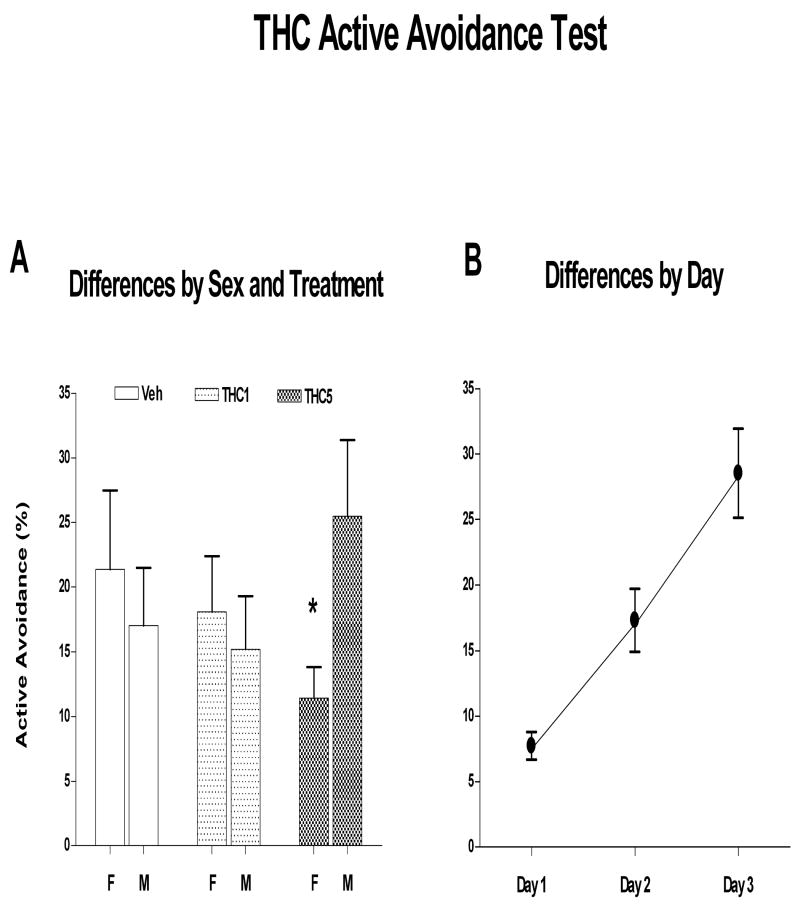
Active avoidance data in adult rats (140 -143 days of age) were collected from 12 females each group and between 8 and 14 males in each dose group. Data were analyzed using a Generalized mixed linear model in SAS such that the number of trials avoided out of a total of 20 trials on each of three days was the dependent variable. While there were no main effects, there was a significant interaction between sex and treatment [F(2,62)=4.19, p=0.020] (figure 4A). Simple effect analysis indicated that there were no effects of treatment within males or females. However, when the data were sliced by treatment, there was a significant difference between males and females that received THC at 5mg/kg [F(1,62)=5.01) p=0.029]. Time (days) was highly significant [F(2,60)=34.41; p<0.001] indicating that all subjects improved performance over the course of testing (figure 4B) but none of the other variables interacted with time. Cohort was not significant [PrZ=0.139].
Figure 4.
shows effects of preadolescent THC on % avoided in the active avoidance test measured at 140 days of age. A illustrates the significant sex by treatment interaction where males receiving 5 mg/kg THC did better across all test days than females receiving the same dose. B shows that all groups improve performance across the 3 test days. Mean ± sem.
4. Discussion
The results of this experiment suggest that oral administration of THC during the preadolescent period has subtle effects on activity during the period of drug administration and on avoidance behaviors in adult life. Since Stiglick and coworkers had utilized a similar route of administration and identified enhanced avoidance behavior in the cannabinoid-treated rats [21], the length of the treatment must have been too short or the dose too low in our study to obtain a similar result. Alternatively, the preadolescent brain may not be as sensitive as post adolescents or mature brains to the effects of THC. Recent studies by Cha et al and Wiley et al [2;22]however, suggested that adolescents are more sensitive than adults although the opposite has also been reported [17]. Perhaps, if we had tested larger oral doses for longer periods of time, we may have identified greater effects.
The dose and route of administration we selected were based on many, many pre- and postnatal studies showing effects of THC on offspring (see Navarro for review)[11]. However, most studies involving postnatal administration give the drug ip and we have found that only 2mg/kg THC depresses locomotor activity in preadolescent rats [9]. Schramm-Sapyta et al [17], administering similar ip doses, found a suggestion of an increase in locomotion in adolescents. Since the decrease in locomotion that we found was only in comparison with the slight increase found in the 1mg/kg THC group (not the control group), we must conclude that under the conditions of our experiment, oral THC in adolescents produces very subtle effects on locomotion. An additional caveat is that we administered the drug at the beginning of the dark phase, minutes after the rats became active. Since rats do not feed in the light, our rats were essentially fasted at the time of drug administration. Pryor et al. [14] reported that oral administration of THC in sesame oil to fed rats produced peak plasma THC levels 2-3 hours after administration and 8 hours after administration to fasted rats. Therefore, while we did not intentionally fast the rats prior to drug administration, since they were housed under reverse light cycle conditions and the THC was administered within minutes of the lights going out, our rats were effectively fasted prior to drug administration. Perhaps, we might have observed a more robust locomotor depressing effect of the THC if we had assessed the subjects 6-8 hr after dosing.
Both active and passive avoidance behaviors measured in adulthood were relatively unaffected by the THC exposure. Our major finding was that 5mg/kg THC produced a sex difference in active avoidance behavior where the 5mg/kg males performed significantly better than the females when the data are collapsed across test days (Figure 4). Escapes and shock duration showed similar patterns. Since avoidance behavior develops differently in males and females during the period of drug administration [3], we would expect to see sex differences in response to cannabinoid administration. Also, since the cannabinoid receptor concentration in brain increases rapidly during the dosing period [16], we would expect to see disruption of behaviors relying on hippocampus. Since Stiglick and co workers [19-21] studied only male rats and found that chronic cannabinoid treatment improved, albeit minimally in some studies, active avoidance behavior, our results are consistent with theirs. They, however, administered larger doses than we did. Certainly, one possible explanation for the subtle effects on both locomotor activity and avoidance behavior in our study is the hypothetically slow absorption of THC from the gut due to the fasted condition of the rats.
Clearly, THC distribution curves are very different after intraperitoneal (ip), oral (po) or subcutaneous (sc) routes of administration: that is, absorption continues during the elimination phase particularly for the po and sc routes of administration. These constant low blood levels may be insufficient to produce psychoactive changes in the brain. On the other hand, administration of only .15mg/kg THC intravenously during pregnancy has been reported to alter drug taking behavior and neurochemistry in adulthood [18]. Clearly route of administration is an important factor to consider in developmental studies of drug toxicity. However in humans, synthetic THC administered orally has been reported to precipitate psychosis and cannabis use along with food and drink can undergo rapid absorption [5]. The oral route can be an effective route of administration for cannabinoids and can result in similar subjective effects as produced by smoking marijuana [8;12]. Since various cannabinoid preparations are now available to treat nausea and vomiting, loss of appetite in cancer and AIDS, etc., consideration should be given to the conditions, such as the time following feeding, which may substantially increase or decrease the psychoactive effects of the drugs.
Our study found no effect of THC administration during adolescence on body weight. While marijuana has been found to increase appetite primarily in humans, preclinical studies usually find either decreases or no change in body weight depending on the dose administered [4;8;19]. Since our dose was relatively low, it is not surprising that there was no effect on body weight.
5. Conclusions
Oral THC at 1 and 5 mg/kg administered to fasted subjects during preadolescence has subtle effects on activity during the period of drug administration and on avoidance behaviors in adulthood. Presumably the slow absorption of oral THC in fasted rats minimizes the psychoactive effects, particularly, in comparison with other routes such as ip. When developing an animal model to study the effects of smoking marijuana, use of a route of administration which results in rapid increases in plasma drug levels is preferable.
Acknowledgments
Supported by NIDA grant DA019348. The expert technical assistance of April Jackson, Lucille Grullon, and Onika Murray is gratefully appreciated. The statistical analyses of Dr. Jeremy Weedon and the manuscript preparation by Dothlyn Dunkley, MS are also gratefully appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Brown H, Prescott R. Applied Mixed Models in Medicine. John Wiley & Son; 1999. [Google Scholar]
- 2.Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83:448–455. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Cimadevilla JM, Fenton AA, Bures J. Transient sex differences in the between-session but not the within session memory underlying an active place avoidance task in weanling rats. Behav Neurosci. 2001;115:695–703. doi: 10.1037//0735-7044.115.3.695. [DOI] [PubMed] [Google Scholar]
- 4.Ellgren M. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2006:1–9. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- 5.Favrat B, Menetrey A, Augsburger M, Rothuizen LE, Appenzeller M, Buclin T, Pin M, Mangin P, Giroud C. Two cases of “cannabis acute psychosis” following the administration of oral cannabis. BMC Psychiatry. 2005;5:17. doi: 10.1186/1471-244X-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried PA. Behavioral outcomes in preschool and school-age children exposed prenatally to marijuana: a review and speculative interpretation. NIDA Res Monogr. 1996;164:242–260. [PubMed] [Google Scholar]
- 7.Fried PA, Smith AM. A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol. 2001;23:1–11. doi: 10.1016/s0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 8.Hart C, Ward A, Haney M, Comer S, Foltin R, Fischman M. Comparison of smoked marijuana and oral Ä9-tetrahydrocannabinol in humans. Psychopharmacology. 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- 9.Harte LC, Dow-Edwards D. Sex differences in the locomotion-depressing effects of Tetrahydrocannabinol during adolescence. College Prob Drug Depend. 2007:57. abstract. [Google Scholar]
- 10.Molina-Holgado F, Amaro A, Gonzalez MI, Alvarez FJ, Leret ML. Effect of maternal delta 9-tetrahydrocannabinol on developing serotonergic system. Eur J Pharmacol. 1996;316:39–42. doi: 10.1016/s0014-2999(96)00753-4. [DOI] [PubMed] [Google Scholar]
- 11.Navarro M, Rubio P, de Fonseca FR. Behavioural consequences of maternal exposure to natural cannabinoids in rats. Psychopharmacology (Berl) 1995;122:1–14. doi: 10.1007/BF02246436. [DOI] [PubMed] [Google Scholar]
- 12.Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma levels of delta 9-tetrahydrocannabinol after intravenous, oral, and smoke administration. NIDA Res Monogr. 1981;34:250–256. [PubMed] [Google Scholar]
- 13.Pope HG, Jr, Gruber AJ, Hudson JI, Cochane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 14.Pryor GT, Hussain S, Mitoma C. Influence of fasting on the absorption and effects of delta9-tetrahydrocannabinol after oral administration in sesame oil. Pharmacol Biochem Behav. 1977;6:331–241. doi: 10.1016/0091-3057(77)90033-8. [DOI] [PubMed] [Google Scholar]
- 15.Robichaud RC, Hefner MA, Anderson JE, Goldberg ME. Effects of delta9-tetrahydrocannabinol (THC) on several rodent learning paradigms. Pharmacology. 1973;10:1–11. doi: 10.1159/000136415. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez de Fonseca R, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology. 2007;191:867–877. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- 18.Spano MS, Ellgren M, Wang X, Hurd YL. Prenatal Cannabis Exposure Increases Heroin Seeking with Allostatic Changes in Limbic Enkephalin Systems in Adulthood. Biol Psychiatry. 2007;61:554–563. doi: 10.1016/j.biopsych.2006.03.073. [DOI] [PubMed] [Google Scholar]
- 19.Stiglick A, Kalant H. Behavioral effects of prolonged administration of delta 9-tetrahydrocannabinol in the rat. Psychopharmacology (Berl) 1983;80:325–330. doi: 10.1007/BF00432114. [DOI] [PubMed] [Google Scholar]
- 20.Stiglick A, Kalant H. Residual effects of chronic cannabis treatment on behavior in mature rats. Psychopharmacology. 1985;85:436–439. doi: 10.1007/BF00429660. [DOI] [PubMed] [Google Scholar]
- 21.Stiglick A, Llewellyn M, Kalant H. Residual effects of prolonged chronic cannabis treatment on shuttle-box avoidance in the rat. Psychopharmacology. 1984;84:476–479. doi: 10.1007/BF00431452. [DOI] [PubMed] [Google Scholar]
- 22.Wiley JL, O’connell MM, Tokarz ME, Wright MJ., Jr Pharmacological effects of acute and repeated administration of Delta (9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320:1097–1105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]