Abstract
We have carried out an analysis of the expression of Prox1, a homeodomain transcription factor, during mouse inner ear development with particular emphasis on the auditory system. Prox1 is expressed in the otocyst beginning at embryonic day 11, in the developing vestibular sensory patches. Expression is down-regulated in maturing (myosin VIIA immunoreactive) vestibular hair cells and subsequently in the underlying support cell layer by E16.5. In the auditory sensory epithelium, Prox1 is initially expressed at embryonic day 14.5 in a narrow stripe of cells at the base of the cochlea. This stripe encompasses the full thickness of the sensory epithelium, including developing hair cells and support cells. Over the next several days, the stripe of expression extends to the apex, and as the sensory epithelium differentiates, Prox1 becomes restricted to a subset of support cells. Double labeling for Prox1 and cell-type specific markers revealed that the outer hair cells transiently express Prox1. After E18, Prox1 protein is no longer detectable in hair cells, but it continues to be expressed in support cells for the rest of embryogenesis and into the second postnatal week. During this time, Prox1 is not expressed in all support cell types in the organ of Corti, but is restricted to developing Deiters’ and pillar cells. The expression is maintained in these cells into the second week of postnatal life, at which time Prox1 is dynamically down-regulated. These studies form a baseline from which we can analyze the role of Prox1 in vertebrate sensory development.
Keywords: hair cells, auditory, organ of Corti, Deiters’ cells, pillar cells
INTRODUCTION
The organ of Corti is the sensory epithelium mediating auditory function in mammals. It resides in the bony cochlea in the inner ear. In the mature organ of Corti, sensory hair cells are arranged with nonsensory support cells in a highly stereotyped pattern. There are two specialized types of auditory hair cells, inner hair cells, which form a single row along the medial aspect, and outer hair cells, which form three rows more laterally. Hair cells are arranged tonotopically along the length of the organ of Corti, with those responding to high frequencies located at the base and those responding to low frequencies at the apex of the cochlea. Support cells, which are highly diverse morphologically, are interposed between hair cells. The inner hair cells are surrounded by inner phalangeal cells and border cells, while the outer hair cells are surrounded by Deiters’ cells. Interposed between the inner and outer hair cells are two rows of pillar cells, which surround a longitudinal fluid-filled space called the tunnel of Corti (Retzius, 1884; Held, 1926; Lim and Anniko, 1985). The vestibular sensory epithelia (utricle, saccule and cristae) also contain hair cells and support cells. There appears to be only a single type of support cell in the vestibular sensory epithelia. As in the cochlea, in the vestibular structures there are two different types of hair cells, termed type I and type II.
The epithelial components of the inner ear, including the organ of Corti, develop from the otic placode, a patch of lateral head ectoderm that becomes internalized during early development. In the organ of Corti, new cell production occurs in an apical-to-basal gradient (Ruben, 1967), while overt differentiation of both hair cells and support cells occurs in the opposite direction. In recent years, several important contributions to the understanding of vertebrate inner ear development have drawn on advances in the field of Drosophila neural development, since many of the molecular pathways underlying neurogenesis have been conserved (Myat et al., 1996; Adam et al., 1998; Haddon et al., 1998; Lanford et al., 1999; Shailam et al., 1999; Lanford et al., 2000; Zhang et al., 2000). While these studies have led to fundamental insights into the molecules required for the differentiation of hair cells, far less is known about the key regulators of support cell development. In the development of the Drosophila sensory organs, the neuron and the non-neuronal support cells are all derived from a common precursor, the sensory organ precursor (SOP). Several genes have been identified as being critical for development of the non-neuronal cells in Drosophila sensory organs through both genetic screens and, more recently, genomic approaches (Freeman et al., 2003). One of the genes important in Drosophila glial development is Prospero, a homeodomain transcription factor. Mutational analysis has shown that Prospero is necessary for glial cells in both developing CNS and PNS. Prospero is required to upregulate a glial-specific gene, gcm (glial cells missing) and induce glial cell differentiation (Freeman and Doe, 2001). Prospero also regulates glial cell proliferation in the fly peripheral nervous system (Griffiths and Hidalgo, 2004).
The vertebrate homolog to Prospero, Prox1, has been localized to the developing inner ear of zebrafish (Glasgow and Tomarev, 1998) and chickens (Stone et al., 2003). In the chick embryo, Prox1 is highly expressed in regions of the otocyst that will give rise to both the auditory and vestibular sensory epithelia and the statoacoustic ganglion (Stone et al., 2003). During development of the chick auditory epithelium, Prox1 protein is first identified in progenitor cells throughout sensorigenic and neurogenic regions of the early otocyst by stage 16 (Hamburger and Hamilton, 1992). Prox1 is expressed in immature hair cells and support cells, but it is down-regulated very quickly in differentiating hair cells. Prox1 expression is down-regulated over a more protracted period in support cells as they differentiate, starting with support cells in the center of the epithelium of the basilar papilla and spreading out toward the neural and abneural sides. Support cells along the neural side of the epithelium do not completely lose Prox1 immunoreactivity, but retain low levels into adulthood (Stone et al., 2003; Stone et al., 2004).
To determine whether Prox1 plays a role in the development of the support cells in the mammalian inner ear, we carried out an analysis of its developmental expression in mice. Mice have several advantages for this analysis, including a highly specialized set of support cells, in the organ of Corti, which can be differentiated from one another on the basis of morphology and position within the sensory epithelium. Moreover, these studies form a baseline from which we can analyze the effects of mutations in the neurogenic pathway to further define the role of Prox1 in vertebrate sensory development.
In this study, we characterize the temporal and spatial patterns of Prox1 protein expression in the developing mouse organ of Corti and the vestibular organs. Prox1 is expressed in the vestibular epithelia from E11 until E16.5. At embryonic day 14.5 (E14.5), we first detect Prox1 in the basal end of the developing cochlea in the precursors to hair cells and support cells. Over the next several days we see expression spread to the apex, and as the sensory epithelium differentiates, Prox1 becomes restricted to support cells. Prox1 is not expressed in all support cell types in the organ of Corti, but only in Deiters’ and developing pillar cells. The expression is maintained in these cells into the second week of postnatal life, at which time Prox1 is down-regulated and undetectable by postnatal day 20.
METHODS
Animals
Animals were housed in the Department of Comparative Medicine and all procedures were carried out in accordance with the guidelines of the animal care committee at the University of Washington. Embryos were obtained from timed pregnant matings of either Swiss-Webster or CBA mice purchased from Harlan (Indianapolis). We used the staging system of Theiler (1989) to accurately stage the embryos at the time of harvest (http://genex.hgu.mrc.ac.uk/Atlas/intro.html). For the postnatal animals, P0 is defined as the day of birth. Math1-GFP mice were obtained from Jane Johnson (University of Texas Southwestern); the generation and characterization of these mice has been previously described (Chen et al., 2002; Lumpkin et al., 2003).
Histology/Immunofluorescence
Embryos were harvested from timed pregnant Swiss Webster females at embryonic days 11, 12, 14, 14.5, 15, 15.5, 16.5 and 18.5. Whole heads were fixed for 2 hours at room temp in 4% paraformaldehyde. Inner ear tissues were also harvested from P0, P3, P4, P5, P6, P10, P12, P20 and adult mice (Swiss Webster and CBAs). At least three animals were examined at each time point. For P0 to P6 mice, entire heads were immersed in 4% paraformaldehyde for 2 hours at room temperature. For P10 animals, the temporal bone was removed rapidly, the stapes was removed, and a small opening was made in the apical turn of the cochlea prior to immersion of the temporal bone in fixative for 2 hours. For P12 through adult animals, an intralabyrinthine perfusion of fixative was performed prior to immersion of the temporal bone in fixative for 2 hours. For the embryos and some postnatal tissues, the tissue was washed in several changes of Phosphate Buffered Saline pH 7.4 (PBS) for over 1 hour, cryoprotected in successive changes of increasing sucrose concentrations, embedded in OCT and sectioned at 10 μm. Sections were collected on SuperFrost slides and air dryed for no more than 30 minutes. The plane of sectioning was oriented to produce midmodiolar sections through the cochlea. Other postnatal tissues that were to be prepared as surface preparations were washed 3 times in PBS, and segments of the organ (half turns) were carefully dissected from the cochlea. Utricles and saccules were isolated from the inner ear and otoconia were removed.
For immunofluorescence, sections were washed in PBS and sections or free-floating utricles, saccules and segments of the organ of Corti were then incubated with 10% normal goat serum in PBS/0.1%TritonX100 for 20-30 min and then incubated overnight at 4°C with primary antibody, diluted in PBS/0.1%TritonX100. The following day, the tissues were rinsed with PBS/0.1%TritonX100, incubated for 30-60 minutes (sections) or 90 minutes (half turns) with a fluorescent-conjugated secondary antibody, rinsed with PBS and coverslipped in Flouromount G (Southern Biotechnology Assoc., Inc.) (sections) or mounted in Vectashield antifade mounting medium containing the nuclear label, propidium iodide (Vector Laboratories) (half turns). Primary antibodies used were as follows: rabbit anti-Prox1 (Chemicon, AB5475) used at 1:1000 dilution; mouse anti-p27 (BD Transduction labs, 610241) used at 1:100 dilution after antigen retrieval with steaming 10mM NaCitrate pH 6.0; guinea pig anti-Myosin VIIA (gift from Stefan Heller, Harvard Medical School) used at 1:5000 dilution; mouse anti-S100A1 (Dako) used at 1:300. Secondary antibodies used were goat anti-mouse Alexa 594, goat anti-rabbit Alexa 594 and goat anti-guinea pig Alexa 488, all from Molecular Probes and used at 1:400 and goat anti-chicken Fluorescein from Aves Labs. Images of sections were captured on a Zeiss Axioplan microscope using a Photometrics CoolSnap CCD camera and Slidebook software. Organ of Corti half turns were examined as whole mounts with an MRC-1024 confocal laser scanning microscope (Bio-Rad), and images were collected by using Laser Sharp Version 2.1 (Bio-Rad). Confocal images were further processed using NIH Object Image (2.10), ImageJ (1.32) and Photoshop 7.0 (Adobe) software.
The Prox1 rabbit antisera used in these experiments was generated against the C-terminal 15 amino acids of the Prox1 protein (Bagri et al., 2002). This anti-serum gives the expected immunostaining in early lens, and we have used the immunizing peptide to demonstrate that the pattern we see in the inner ear is specific (see supplementary Figure S1). Comparable results were seen with antiserum generated to a larger portion of the Prox1 protein obtained from Stanislav Tomarev (NEI). The p27 antibody used in this study was generated against mouse Kip1 and has been shown by western blot to give a specific band of 27kD (BD Transduction labs, 610241, package insert). The S100A1 from Dako was generated against human S100A1 and has also been shown to be specific by western blot performed by the manufacturer and the staining with this anti-sera has been described in mouse inner ear Woods et al., 2004: Sage et al.2005) Polyclonal antibodies to murine myosin VIIA (gift from Stefan Heller, Stanford University) were generated in guinea pigs (Covance) by injecting affinity-purified hexahistidine-tagged myosin VIIA tail protein (amino acids 845-994), expressed in SF9 insect cells using the Gibco/BRL pFastBac baculovirus expression system. This antiserum recognizes hair cells and shows identical staining patterns to other rabbit myosin VIIA antibodies (Hasson et al., 1997; Sahly et al., 1997).
In situ hybridization
Tissue was dissected in Hank’s buffered saline solution pH 7.3 (HBSS) or PBS and fixed in a modified Carnoy’s for 2-4 hours at room temperature. The samples were then dehydrated and washed in 100% ethanol overnight at 4°C. The tissue was embedded in paraffin and 6-8 μm sections were collected. Digoxigenin labeled Prox1 probe, NT 41-2210, (cDNA was a gift from Stanislav Tomarev) was prepared and the hybridization carried out according to Etchevers et al (Etchevers et al., 2001). The in situ product was visualized using anti-digoxigenin alkaline phosphatase conjugated secondary antibody (Roche Biochemicals).
RESULTS
Prox1 expression in vestibular epithelia
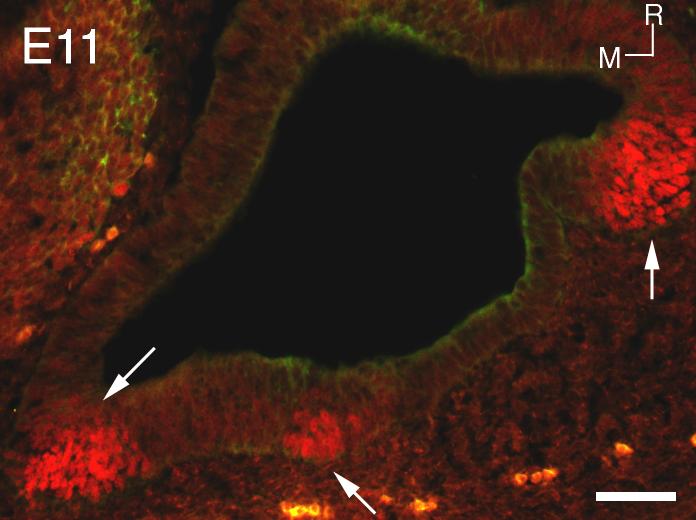
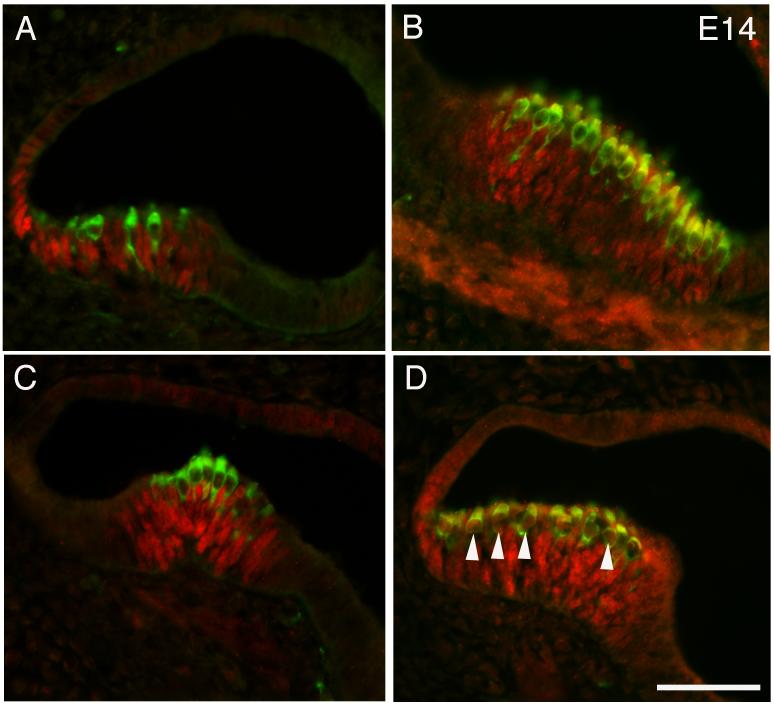
Previous studies in non-mammalian vertebrates have shown that Prox1 is expressed in the inner ear as early as the otic vesicle stage, and eventually becomes confined to the sensory epithelia and the statoacoustic ganglia (Glasgow and Tomarev, 1998; Stone et al., 2003). Therefore, we analyzed mouse embryos from embryonic day 11 for Prox1 immunoreactivity. In the section shown, three distinct regions of Prox1 expression can be seen at this age in the developing otocyst (Fig. 1, arrows). Figure 2A shows in situ hybridization with a Prox1 probe at E14. Labeling can be seen in the developing utricle (arrow) and the three cristae of the semicircular canals (arrowheads). At E14, we also see robust immunoreactivity for Prox1 in the developing vestibular structures. Figure 3 A, B and C represents images from saccule, utricle and a crista respectively. It is interesting that the immunoreactivity seems to be waning in the utricle relative to the other structures. This observation may reflect the earlier development of this organ (Ruben, 1967). Figure 3D shows another section through an E14 saccule in which hair cells that express both Myosin VIIA and Prox1 can clearly be seen. Prox1 is down-regulated first in the developing hair cells and subsequently in support cells. We see no Prox1 labeling in the utricle after E16.5. Postnatal saccule and utricle samples were also examined for expression of Prox1 but no expression was detected.
Figure 1.
Section through the otocyst at E11 labeled with Prox1 antibody (red). The arrows point to three regions of Prox1 immunoreactivity. R = rostral, M = medial. Scale bar = 50μm
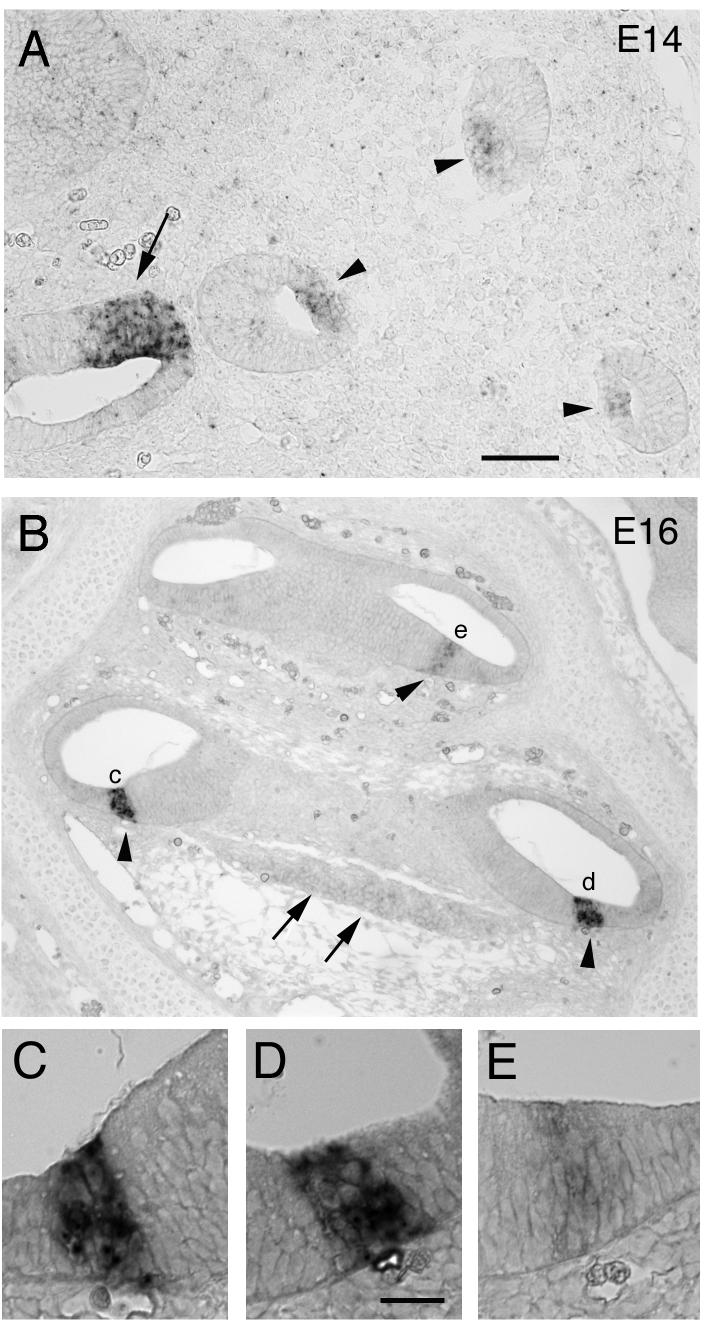
Figure 2.
In situ hybridization with a probe to mProx1. (A) A section through the otocyst at E14 showing the developing vestibular structures which all express Prox1, arrow points to developing utricle and arrowheads to developing cristae of the semicircular canals. (B) A section of an E16 cochlea, arrowheads indicate the developing organ of Corti and arrows the spiral ganglion. (C, D, E) Higher magnification images of the staining in the developing organ of Corti. There is more intense labeling in the base (C, D) relative to the apex (E) at this stage in development. The scale bar in A = 50 μm and applies also to B and the scale bar in D = 20 μm and applies to C-E.
Figure 3.
Sections through the otocyst of an E14 embryo showing Prox1 (red) and Myosin VIIA (green) immunoreactivity in the developing vestibular organs. (A) A section through the developing saccule. (B) A section through the developing utricle. (C) A crista from one of the semicircular canals. (D) A section through the developing saccule that shows some double-labeled hair cells (arrowheads). Scale bar = 50 μm
Prox1 onset in auditory epithelium
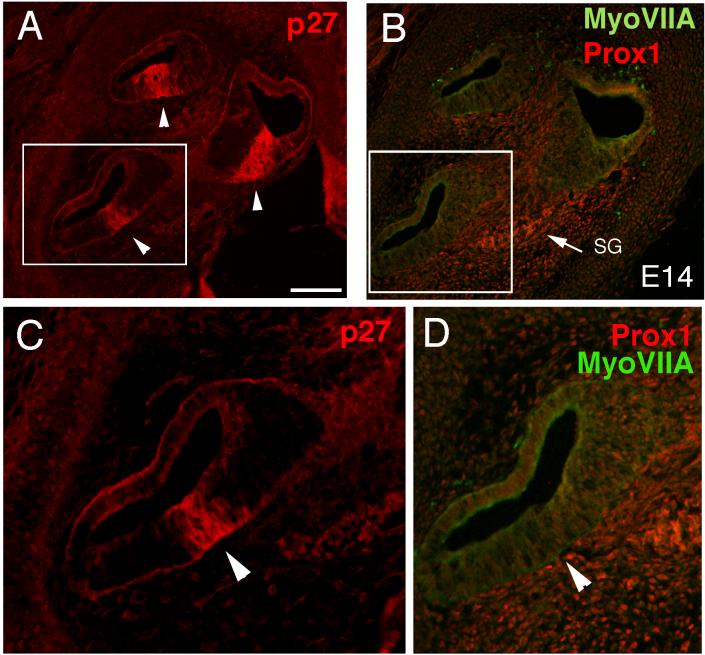
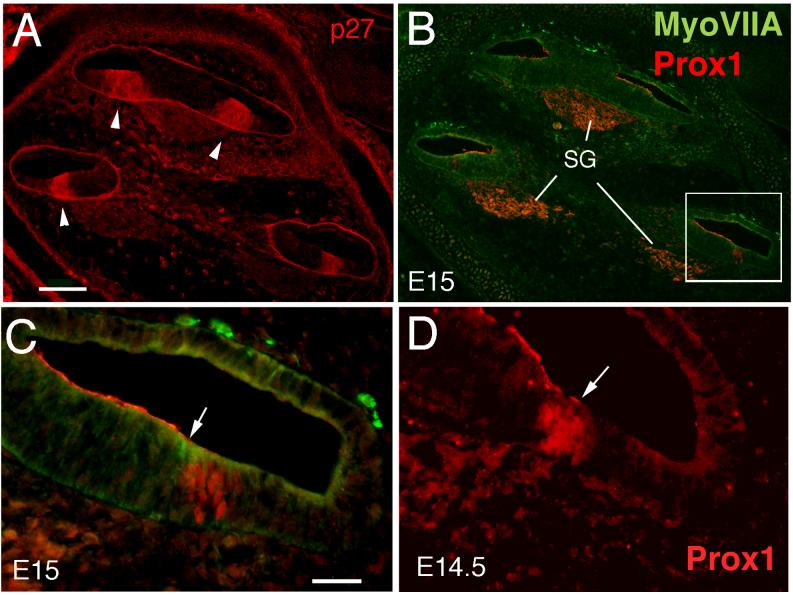
At E14, the zone of non-proliferating cells (ZNPC: Chen and Segil, 1999) in the cochlea is defined by p27 kip1 labeling (Fig. 4A,C). This domain of early postmitotic cells prefigures the location of the auditory sensory epithelium. At E14, Prox1 is not detectable in the auditory sensory epithelium (Fig. 4B,D), but is expressed in the developing spiral ganglion at this time. We detected Prox1 expression by in situ (Fig. 2B arrows) and immunofluorescence (Fig. 4B, arrow) in the spiral ganglion; however, this expression is much weaker than that seen in the developing sensory epithelia and does not appear to be nuclear. The labeling in the spiral ganglion remained non-nuclear through all ages examined. We were first able to detect Prox1 immunoreactivity in the mouse auditory epithelium at E14.5, in the most basal region of the developing organ of Corti (Fig. 5D). Prox1 labeling was nuclear and spanned the depth of the epithelium. The Prox1 immunoreactive cells constitute a subset of the cells within the ZNPC (Fig. 5A-C). Since p27 kip1 expression coincides with the withdrawal of the sensory precursors from the cell cycle (Chen and Segil, 1999), we conclude that Prox1 immunoreactivity is first detected in the mouse cochlea shortly after the cells have completed their last round of division.
Figure 4.
Sections through the cochlea of an E14 mouse labeled with antibodies against p27 (A, C), Myosin VIIA and Prox1 (B, D). The area in the box in A is shown at higher magnification in C, while the area in the box in B is shown at higher magnification in D. The arrowheads in A and C point out the zone of non-proliferating cells (ZNPC) expressing p27. The arrow in B points out the spiral ganglion, SG, which shows Prox1 immunoreactivity at this age. (D) shows the lack of labeling for either Prox1 or Myosin VIIA at E14 (arrowhead). In panels A and B the apex is located at the top of the figure. Scale bar = 100μm
Figure 5.
Sections through the cochlea of E15 (A, B, C) and E14.5 mouse embryos labeled with antibodies against p27 (A), Myosin VIIA and Prox1 (B, C), and Prox1 (D). (A) The ZNPCs can be clearly identified with p27 labeling (arrowheads). (B) An adjacent section showing the Myosin VIIA and Prox1 immunoreactivity. The cells of the SG are strongly labeled for Prox1, while a subset of cells within the ZNPC at the basal end of the cochlea label for both Myosin VIIA and Prox1. (C) The double-labeling in the region in the box in B is shown at higher magnification. The arrow points out the Myosin VIIA (green) labeled hair cells, while immediately adjacent to them are Prox1 labeled nuclei (red). (D) Prox1 immunoreactive cells at E14.5, at the base of the cochlea (arrow). In panels A and B, the apex is located at the top of the figure. Scale bar = 100μm in A and B and 25μm in C and D.
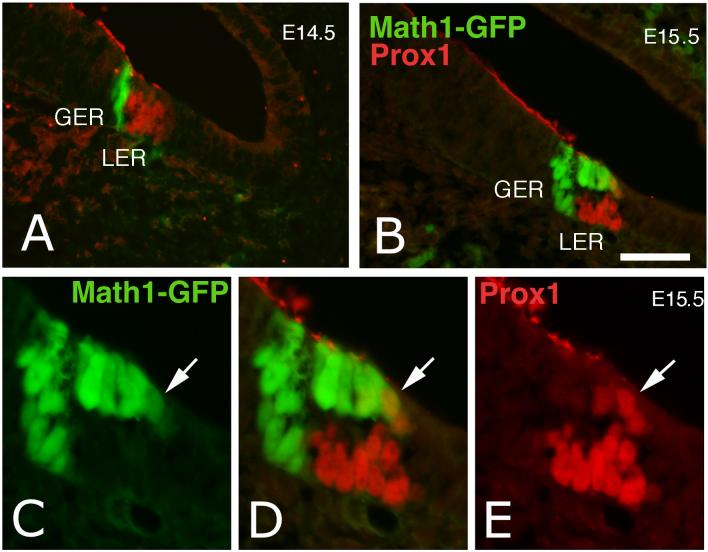
To determine which cell types were expressing Prox1 at early embryonic stages, we analyzed sections for expression of two hair cell markers, Myosin VIIA immunoreactivity and a GFP transgene under the control of the Math1 promoter (Helms et al., 2000; Lumpkin et al., 2003)(Math1-GFP). We also used S100A1 to label the support cells and inner hair cells (Woods et al., 2004). In E14.5 mice (Fig. 6), the earliest Math1-GFP expressing cells do not express Prox1 (Fig. 6A). At this stage of early cochlear development it is not clear if all the Math1 positive cells go on to differentiate into hair cells, or if some become support cells. At E15, Myosin VIIA is beginning to be detected in the basal region of the cochlea. The nuclei of the hair cells that express the highest levels of Myosin VIIA immunoreactivity do not express Prox1 (arrowhead, Figure 7C). These data suggest that the earliest generated hair cells do not express Prox1. Twelve hours later, at E15.5, more Math1-GFP positive cells are present in the basal region of the developing organ, and Prox1 immunoreactive cells are now present primarily in the support cell layer of the epithelium (Fig. 6 B, E). We did not find cells that expressed both Prox1 immunoreactivity and the Math1-GFP transgene in the greater epithelial ridge (GER), the region of the epithelium that gives rise to the inner hair cells (Eggston and Wolff, 1947; Lim and Rueda, 1992). By contrast, we found many examples of Prox1 immunoreactive nuclei in the Math1-GFP expressing hair cells in the lesser epithelial ridge (LER), the area thought to give rise to the outer hair cells (Eggston and Wolff, 1947; Lim and Rueda, 1992). The outer hair cells that express the highest levels of Prox1 are typically found along the lateral edge (Fig. 6D, arrow). In the early embryonic sections it appears that there are more Prox1 positive cells than ultimately will contribute to the organ of Corti at this level. This is presumably because the cochlea grows after the cells have stopped dividing by a process thought to include convergent extension (Chen and Segil, 1999; McKenzie et al., 2004).
Figure 6.
Sections through the cochlea of E14.5 and E15.5 mouse embryos that express GFP under the control of the Math1 promoter. (A) The Math1-GFP labeled inner hair cells appear at E14.5 (green), and are located immediately adjacent to the Prox1 immunoreactive cells (red) at this stage. (B) The Math1-GFP hair cells are now present in both the LER and the GER at E15.5; Prox1 immunoreactive cells are present primarily in the support cell layer of the LER, though several Math1 expressing hair cells are also immunoreactive for Prox1. (C-E) Higher magnification of the organ of Corti shown in B, to better show the double-labeling (arrow) of Prox1 (E) and GFP (C). LER: lesser epithelial ridge, GER: greater epithelial ridge. Scale bar = 20 μm.
Figure 7.
Sections through the cochlea of E16 mouse embryos showing labeling of p27 (A, D), Myosin VIIA and Prox1 (B, C). The micrograph shown in C is a higher magnification view of the region in the box in B. The section shown in B is an adjacent section to that shown in A. (B) The spiral ganglion (SG) cells express Prox1 at this stage, as do many of the cells in the LER. (C) The inner hair cells (green, arrowhead) express Myosin VIIA at this stage, but are not labeled with the Prox1 antibody (red). (D) A section of E16 mouse cochlea showing that only a subset of the p27 positive cells (red) also label for Prox1 (green). LER: lesser epithelial ridge, GER: greater epithelial ridge. Scale bar = 100 μm in B and 20 μm in D.
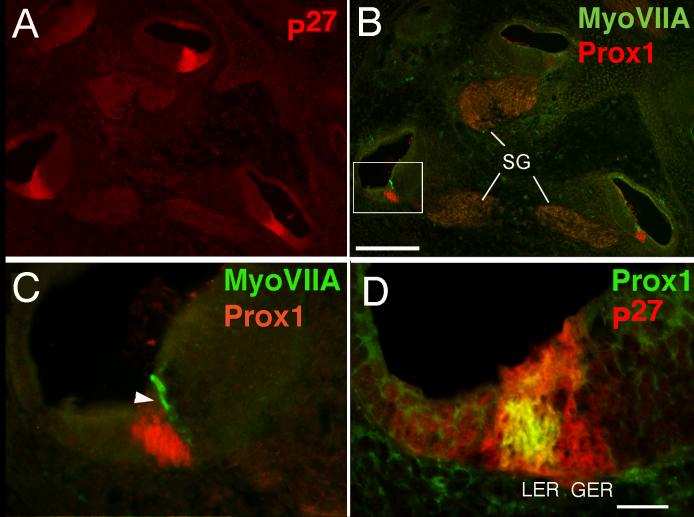
Late embryonic stages
As described above, Prox1 is initially expressed in the basal end of the cochlea at E14.5. As development proceeds, expression of Prox1 proceeds apically in the p27 kip1 domain. By E16 (Fig. 7), Prox1 can be detected in the apical region of the developing cochlea (Fig. 7B). At E16, Myosin VIIA is clearly expressed in developing inner hair cells (Fig. 7C, arrowhead), and some of the outer hair cells in the basal end of the cochlea. Prox1 expressing cells lie adjacent to the Myosin VIIA positive hair cells, but Prox1 is not expressed in the inner hair cells. An occasional hair cell shows double labeling, but these are exclusively in the outer hair cell region (Fig. 6 C-E). This finding was verified by confocal analysis of double-labeled nuclei (data not shown). As expected, we find that the Prox1 immunoreactivity lags slightly behind the RNA expression. At E16 there is robust expression of the RNA as detected by in situ hybridization in the base of the cochlea (see arrowheads Fig. 2B,C) but the expression is much weaker in the more apical turns where Prox1 immunoreactivity is just detectable (Fig. 2E). The Prox1 immunoreactivity resides in a subset of the p27 kip1 positive cells (Fig. 7D).
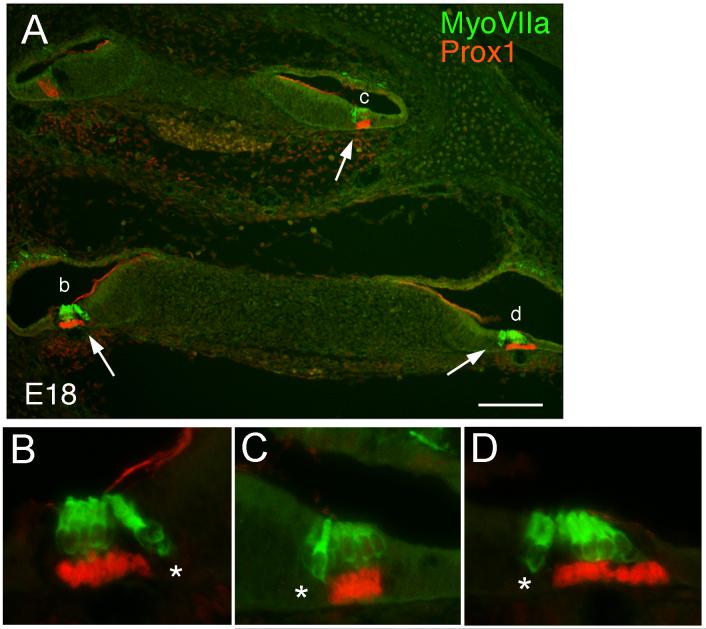
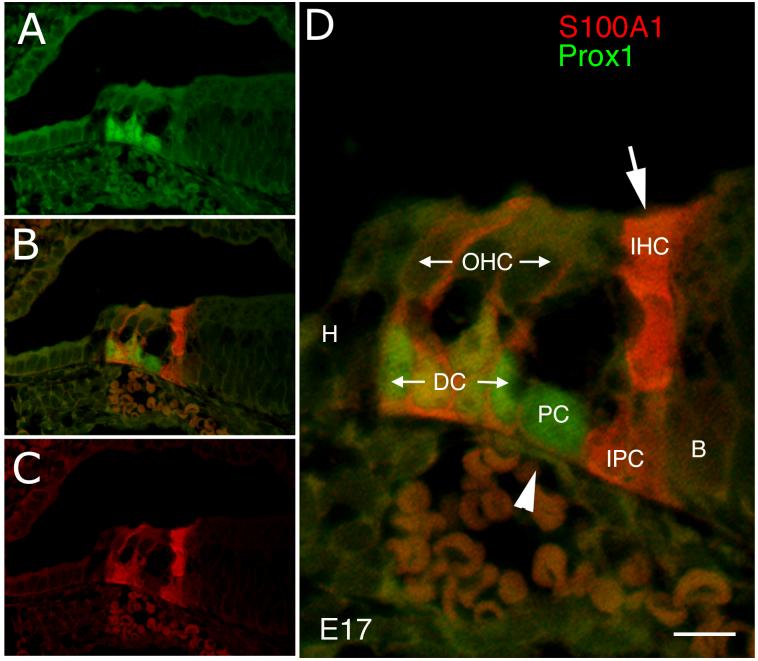
At E18, the morphological distinction between the support cells and the hair cells is clear throughout the cochlea. The hair cell nuclei are consistently unlabeled with the Prox1 antibody, whereas the support cells underlying the outer hair cells express high levels of the protein (Fig. 8). It is interesting that the support cells underlying the inner hair cells do not express Prox1 (asterisks, Fig. 8 B-D), distinguishing these two adjacent populations of support cells. To directly determine whether Prox1 is expressed in the support cells, we co-labeled sections from E17 embryos with antibodies against Prox1 and S100A1. Figure 9 shows an example of this labeling. Antibodies against S100A1 label the Deiters’ cells, inner hair cells, and inner phalangeal cells (red). Prox1 immunoreactivity is located in the nuclei of the Deiters’ cells (co-labeled with S100A1), and the pillar cells (not labeled with S100A1; Fig. 9D, arrowhead), but not the inner hair cells, and inner phalangeal cells (Fig. 9D, arrow). Prox1 is also absent from the outer hair cells, Hensen’s cells and border cells.
Figure 8.
Sections through the cochlea of E18 mouse embryos showing labeling of Myosin VIIA (green) and Prox1 (red). (A) Low power micrograph showing the entire cochlea. (B-D) Higher magnification views of the regions indicated by the arrows in A. Prox1 labels the nuclei of the Deiters’ and pillar cells but not the inner phalangeal cell (asterisks). Scale bar = 100μm
Figure 9.
Confocal micrograph of a section through the apical cochlea of an embryonic day 17 mouse labeled for S100A1 (red) and Prox1 (green). (A; B; C) Prox1, merged and S100A1 labeling, respectively. (D) Higher magnification view of B. Arrow points to the S100A1 labeling of the inner hair cell, while the arrowhead points to the pillar cell nuclei, which are positive for Prox1 and negative for S100A1. H: Hensen cell, DC: Deiters’ cell, PC: pillar cell, IHC: inner hair cell, OHC: outer hair cell, IPC: inner phalangeal cell and B: border cell. Scale bar = 10μm
Postnatal development
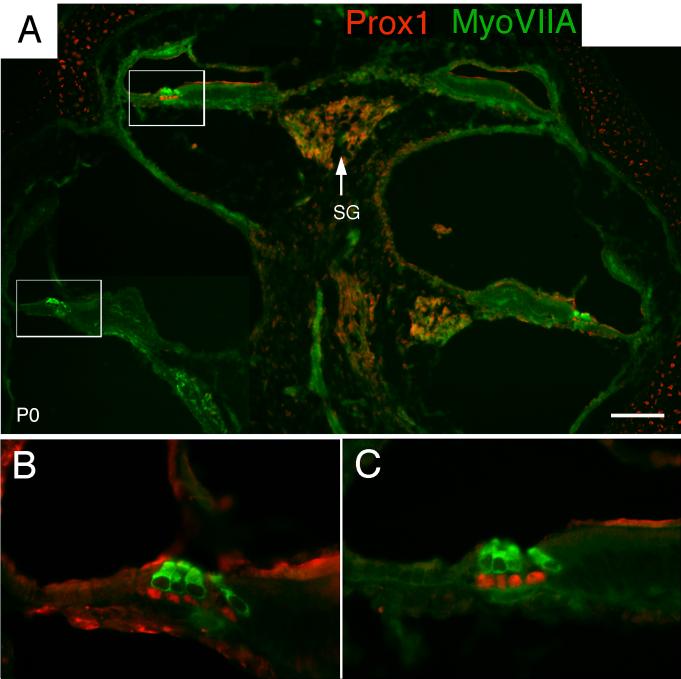
After birth, Prox1 continues to be expressed in Deiters’ and pillar cell nuclei. At this point, the different support cells are easily distinguished by their position relative to the inner and outer hair cells and the basilar membrane (Fig. 10). Co-labeling with Myosin VIIA and Prox1 antibodies at postnatal day 0 (P0) demonstrate that there is no overlap between the cells expressing these markers at this stage. The two pillar cells and the three Deiters’ cells express Prox1, while the inner phalangeal cells, border cells and Hensen’s cells do not. Thus, Prox1 immunoreactivity clearly distinguishes between the different classes of support cells in the organ of Corti. Immunostaining of the spiral ganglion is still seen at this age (Fig. 10A). The staining in the cartilage and at the lumenal surface of the epithelia is assumed to be background, as this same pattern is seen with many antibodies.
Figure 10.
Sections through the cochlea of postnatal day 0 mouse showing labeling of Myosin VIIA (green) and Prox1 (red). (A) Low power micrograph showing the entire cochlea. (B, C) Higher magnification views of the regions indicated by the boxes in A. Prox1 labels the nuclei of the Deiters’ and pillar cells but not the hair cells. SG = spiral ganglion. Scale bar = 100 μm
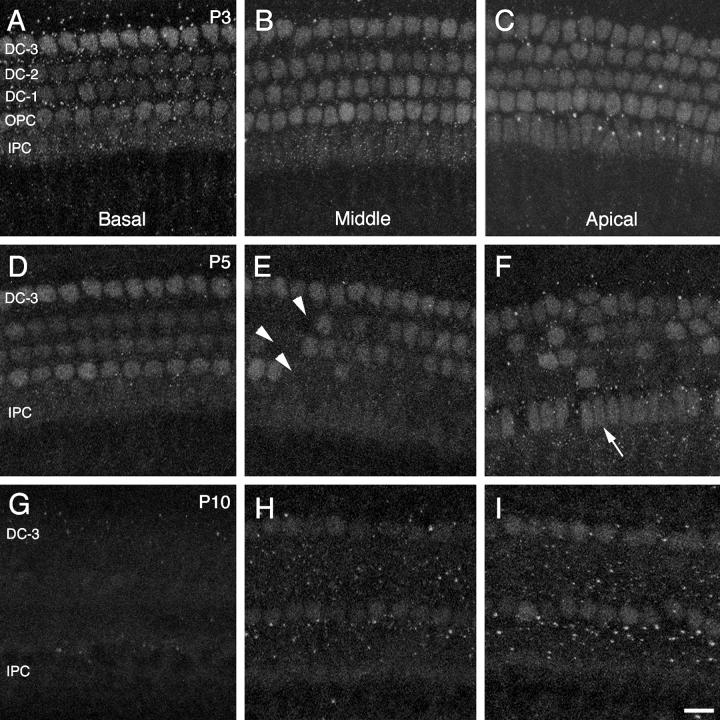
Prox1 expression is down-regulated in support cell nuclei between birth and P20. In Swiss Webster mice at P3, the basal region of the organ of Corti shows a reduction in Prox1 nuclear labeling in inner pillar cells and in the first and second rows of Deiters’ cells, relative to the outer pillar cells and third row of Deiters’ cells (Fig. 11A). In the middle region, which is developmentally less advanced than the basal end, Prox1 labeling only appears reduced in the inner pillar cell nuclei (Fig. 11B). In the apical region, the least mature region, all rows of support cell nuclei appear clearly labeled (Fig. 11C). At P5, the basal region of the organ of Corti shows labeling similar to that observed at P3 (Fig. 11D); however, in the middle and apical regions, Prox1 immunoreactivity is clearly reduced in subsets of support cells, particularly those in the first and second rows of Deiters’ cells and outer pillar cells (Fig. 11E). An interesting difference between the middle and apical regions is that in the apex, more inner pillar cell nuclei retain strong Prox1 labeling than in the middle region (Fig. 11F).
Figure 11.
Confocal images of whole-mounted organ of Corti pieces from basal (A, D, G), middle (B, E, H), and apical (C, F, I) regions of the cochlea at P3 (A-C), P5 (D-F), and P10 (G-H). All panels show Prox1 immunolabeling in the support cell nuclear layer of the epithelium. Approximately 10 1-μm slices from Z series stacks were brightest-point projected to capture the support cell nuclei in each image. IPC (inner pillar cells), OPC (outer pillar cells), DC-1, DC-2, and DC-3 (first through third rows of Deiters’ cells). The arrowheads in E point to regions lacking Prox1 immunoreactivity in the first and second rows of Deiters’ cells and in the outer pillar cells. The arrow in F points to IPC nuclear labeling. Scale bar = 15μm.
By P10, approximately the onset of hearing (Ehret, 1976; Kraus and Aulbach-Kraus, 1981), Prox1 expression is not detectable in the basal region (Fig. 11G), and it is highly attenuated in the middle and apical regions, being detectable only in the outer pillar cell and third row Deiters’ cell nuclei (Fig. 11H,I). No signal could be detected in any region of the organ of Corti at P20 or in the adult mice (data not shown). We found that, at early postnatal stages of development (P0-P5), patterns of Prox1 expression are similar in Swiss Webster and CBA mouse strains. However, while Prox1 labeling is lost from the organ of Corti by P20 in Swiss Webster mice, it is retained in the middle and apical regions of CBA mice at 8 weeks, in the outer pillar cells and third row of outer Deiters’ cells (data not shown).
DISCUSSION
Many important regulators of neural development are conserved in vertebrates and invertebrates (Chitnis, 1999). In the inner ear, several key transcription factors and signaling molecules, first identified in Drosophila neurogenesis, have been shown to have critical functions in hair cell development (Bermingham et al., 1999; Kageyama and Ohtsuka, 1999; Zheng et al., 2000; Zine et al., 2001; Bryant et al., 2002). The Prox1 gene, a homolog of the Drosophila Prospero divergent homeodomain transcription factor, is expressed in the developing inner ear of vertebrates, but its detailed expression in mouse cochlea was not previously known. In this report, we have described the expression of Prox1 in the developing mouse inner ear.
Prox1 is expressed in the otocyst at E11 in what we presume are the developing vestibular structures. Since at E12 most of the cells in the sensory epithelia of the developing vestibular system have not yet exited the cell cycle (Ruben, 1967) it is likely that Prox1 is expressed in dividing cells. We find that Prox1 expression continues until E16.5 in the vestibular structures. After this time, Prox1 is no longer expressed in the vestibular system. In the vestibular system Prox1 is expressed in the precursors of hair cells and support cells, but it is down-regulated in the hair cells after they start to express Myosin VIIA. This downregulation is not as rapid as we see in the cochlea, such that it is relatively easy to see cells double labeled for Prox1 and Myosin VIIA in the vestibular structures. It is also interesting to note that Prox1 is on early in the development of the vestibular structures when most of the cells are still cycling whereas in the auditory epithelium we only detect Prox1 after the cells have already exited the cell cycle.
We have made several observations that implicate the Prox1 gene in the development of the organ of Corti in mice. We have found that Prox1 is first expressed in the developing organ of Corti by embryonic day 14.5. Prox1 is initially expressed in the LER, the prosensory region that gives rise to Deiters’, pillars and the outer hair cells; however, as the outer hair cells differentiate, they rapidly down-regulate the expression of Prox1. After this initial stage of expression, Prox1 continues to be expressed in two types of support cells, the Deiters’ cells and the pillar cells. As the support cells further differentiate Prox1 immunolabeling is down-regulated in the nuclei of these cells, such that by P20, no nuclei in the organ of Corti retain Prox1. The loss of Prox1 expression from these cells coincides with the opening of the tunnel of Corti, which occurs in mice between postnatal day 7 and 10, with a base to apex progression. We have also found that Prox1 is expressed in the sensory ganglion, from at least embryonic day 14 until the second postnatal week, and may persist into adulthood.
A comparison of Prox1 expression in the developing mouse inner ear with that of the chick and the fish highlights some interesting similarities across vertebrate development. In the embryonic chick, Prox1 is expressed in the statoacoustic ganglion (Stone et al, 2003), as it is in mouse. In the chick, Prox1 is expressed earlier in the developing auditory sensory epithelium than in the mouse, since co-labeling of embryonic chick sensory epithelium with Prox1 antibodies and mitotic markers reveals Prox1 immunoreactivity in precursor cells that are still mitotically active (Stone et al., 2003; Stone et al., 2004). By contrast, in the mouse organ of Corti, Prox1 is expressed at least a day after p27kip1, which is upregulated in cells of the ZNPC at their terminal mitosis (Chen and Segil, 1999). In the vestibular system in the mouse, Prox1 is expressed early, prior to cell cycle withdrawal of the cells in the sensory patches. In both the chick and the mouse, Prox1 is down-regulated in the hair cells as they differentiate, and expression remains in a subclass of the support cells. While in the mouse, Prox1 is expressed in the Deiters’ cells and the pillar cells, in the chick, on the first posthatch day, Prox1 is expressed only in the support cells along the neural edge.
Another interesting feature of Prox1 expression is the fact that it is an early marker of Deiters’ and pillar cell differentiation. Several other support cell-specific proteins are known to be expressed in the mouse inner ear during embryonic development. S100A1, p75 (NGFR) and Fgfr3 are all expressed in various types of support cells in the developing organ of Corti. S100A1 is expressed in nearly all the cells in the ZNPC at E14.5 (data not shown); at later stages of development, S100A1 becomes restricted to the phalangeal cells and the Deiters’ cells, though the inner hair cells are also labeled with S100A1 antibodies (Woods et al., 2004; Sage et al., 2005). The neurotrophin co-receptor, p75 (NGFR), is expressed by the developing pillars and Deiters’ cells at E15 (Mueller et al., 2002) and becomes restricted to pillar cells at later embryonic stages (Pirvola et al., 1994; Schecterson and Bothwell, 1994; Knipper et al., 1996; Knipper et al., 1999). Fgfr3 is also expressed in developing pillar and Deiters’ cells (Pirvola, 1998; Pickles, 2001; Mueller et al., 2002) and is critical for pillar cell development (Colvin et al., 1996). The earliest pillar cell marker appears to be Sprouty 1, which is expressed in a two cell wide region of the developing organ of Corti at E14.5 and continues to be expressed in pillar cells through P5 (Shim et al., 2005). Sprouty 2 another member of the family, is expressed in a much wider region of the developing epithelium at E14.5, but at E16.5 becomes restricted in its expression to pillar, Deiters’ and Claudius cells (Shim et al., 2005). Components of the Notch signaling pathway are also expressed in the support cells during development of the organ of Corti. Specifically, Notch1 and Hes5 are expressed in most, if not all, cells of the organ of Corti at E15, but by E18, the expression of both Hes5 and Notch1 becomes restricted to pillar and Deiters’ cells (Lanford et al., 1999; Zine et al., 2000; Zine et al., 2001). This pattern of expression is most similar to what we have observed for Prox1 expression and suggests that there may be a link between these pathways.
What is the function of Prox1 in the developing inner ear?
Prox1/Prospero can act either as a transcriptional activator (Lengler et al., 2001; Cui et al., 2004) or as a repressor (Kauffmann et al., 1996; Cook et al., 2003). The function of Prox1 is best understood in lens development. Prox1 is expressed in the early lens placode, and is up-regulated in the elongating lens fiber cells (Tomarev et al., 1996; Duncan et al., 2002). In Prox1 deficient mice, the lens fiber cells do not elongate, due in part to a reduction in crystallin expression (Wigle et al., 1999). Prox1 activates several of the different crystallin promoters, and acts synergistically with another lens transcription factor, Maf, to activate transcription of ß1 and ß2 crystallins (Lengler et al., 2001; Duncan et al., 2002; Cui et al., 2004). In the Drosophila eye, Prospero is expressed in the R7 photoreceptor and the presumptive non-neuronal lens-like cone cells (Cook et al, 2003). In the photoreceptors, Prospero functions to regulate the R7 vs. R8 fate by repressing R8 specific rhodopsin (Cook et al., 2003). While the role of Prospero in the cone cells is not clear, recent evidence indicates that the promoters of crystallins are conserved from Drosophila to mice (Blanco et al., 2005), and it may be that Prospero and Prox1 have conserved functions in regulation of lens-specific genes.
The differentiation of the lens epithelial cells into lens fiber cells is marked by dramatic changes in cell shape. The support cells that express Prox1 in the inner ear are the pillar cells and Deiters’ cells. Both of these cell types undergo dramatic cell shape changes during their differentiation (Spicer and Schulte, 1993; Ito et al., 1995). This is particularly evident for the pillar cells, which line the tunnel of Corti and provide the rigid support for this structure. The nuclei of these cells move apart from each other during differentiation to form the base of the triangle structure of the tunnel. One possible function of Prox1 might be to regulate the very extended morphology of the pillar cells, much like it does for lens fiber elongation.
Alternatively, the expression of Prox1 in the hair/support cell lineage may reflect its functions in other regions of the nervous system. In the vertebrate CNS, Prox1 has also been shown to be involved in several stages of development. In the retina, Prox1 is required for the development of a particular class of interneuron, the horizontal cell (Dyer et al., 2003). In the ventral forebrain, Prox1 and Mash1 are transiently expressed in a subclass of progenitors, after they no longer express markers of neural stem cells (Torii et al., 1999). Thus, the function of Prox1 in the inner ear might relate to the state of differentiation of the stem/progenitor cell. The retention of Prox1 expression in a subset of support cells in the chicken basilar papilla suggests that this epithelium, unlike the mammalian counterpart, retains some immature precursor/progenitor-like cells. It suggests some support cells are poised at an early state of differentiation, which may reflect a pluripotency, including the ability to generate hair cells and support cells by cell division (Corwin and Cotanche, 1988; Ryals and Rubel, 1988) and to generate new hair cells by direct transdifferentiation (Roberson et al., 1996, 2004). The dynamics of Prox1 expression that accompanies hair cell regeneration in the chick is consistent with this possibility (Stone et al., 2004).
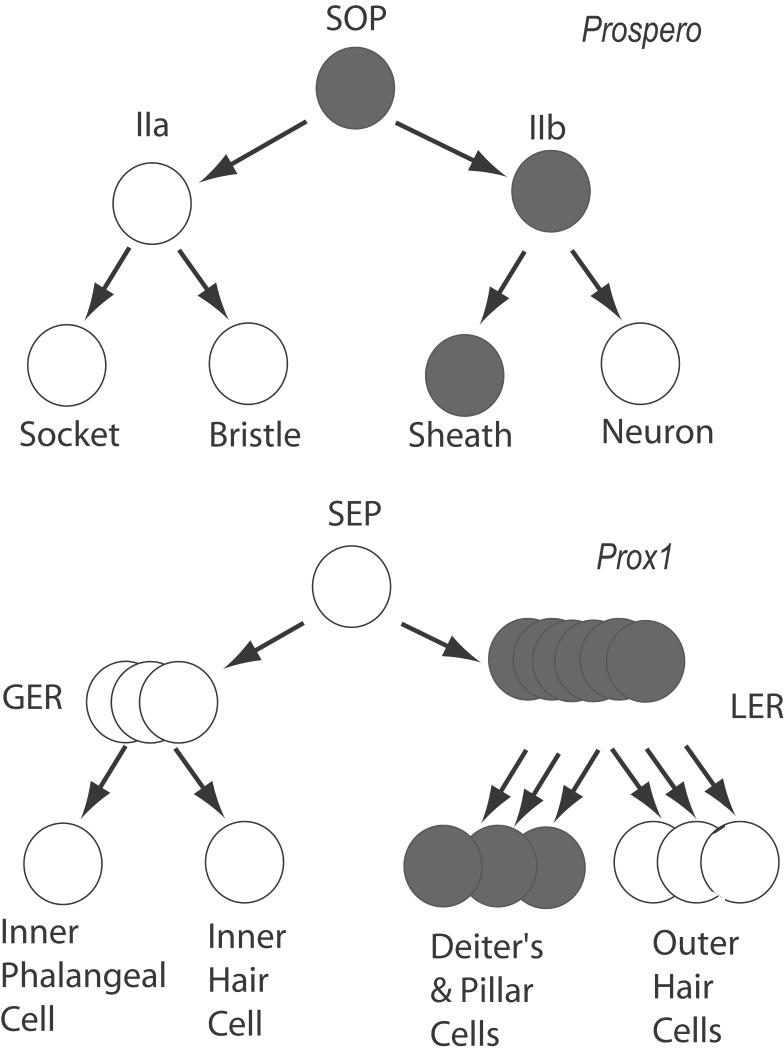
A comparison of Prox1 expression in the inner ear with that of Prospero in the developing fly external sensory organ lineage, also suggests some common functions in sensory cell development (Fig. 12). The external sensory organs of the fly are generated from two successive cell divisions of the sensory organ precursor, or SOP. The first division of the SOP generates the II-a cell and the II-b cell (Freeman and Doe, 2001). These cells divide again to produce the socket and bristle cells from the II-a and the sheath and neuronal cells from II-b. Prospero is initially expressed in the SOP, is then segregated to the II-b cell and is transiently expressed in the neuron produced by the II-b cell and persists in the glial/sheath cell (Freeman and Doe, 2001). An analogous expression pattern of Prox1 in the organ of Corti is suggested by our studies. Prox1 is expressed in the precursors to the outer hair cells and their associated support cells in the LER, but does not appear to be expressed in the precursors to inner hair cells or phalangeal cells in the GER. Once the hair cells and support cells are clearly differentiated from one another, Prox1 expression continues in the Deiters’ and pillar cells. While the expression patterns between the SOP and the inner ear thus show an interesting similarity, one clear difference between the two systems is that the expression of Prox1 is not directly tied to cell division in the organ of Corti, as is Prospero in the SOP.
Figure 12.
Diagram showing the relationship between prospero (grey) expression and the development of the external sensory organ cells in Drosophila (top) and that of Prox1 (grey) and the developing cochlear cells (bottom). SOP = sensory organ precursor; SEP = hypothetical sensory epithelial precursor in the inner ear; GER = greater epithelial ridge; LER = lesser epithelial ridge.
Prospero is required in both the embryonic and adult SOP lineages, to produce the neuron/glial producing II-b cell. Prospero mutants lose the ability to generate the neuron/glial lineage and instead generate bristle and socket cells. Occasionally a neuron is produced by a Prospero-deficient SOP, but glial sheath cells are never observed. Likewise, experimental over-expression of Prospero in the SOP lineage results in an overproduction of glial sheath cells (Manning and Doe, 1999). Thus, Prospero seems to be important at several successive stages of sensory organ development, but perhaps the most relevant to the present study is the critical requirement in the non-neuronal cell of the II-b lineage. By analogy with the Drosophila, we would predict that loss of Prox1 in the inner ear would result in the failure of Deiters’ and pillar cells to differentiate from the LER, and possibly an overproduction of those cells derived from the GER, the inner hair cells and the phalangeal cells. Future work will be necessary to determine whether Prox1 serves this analogous role.
Supplementary Material
Figure S1. This figure illustrates the specificity of the Prox1 antiserum. Panel A shows Prox1 staining in E14 lens. Panel B shows Prox1 staining (red) in the organ of Corti at E18 and panel C shows that the Prox1 staining is completely eliminated by incubation with the peptide (50μg/ml) against which the antiserum was raised. Staining with the MyosinVIIA antibody (green) is not affected by the addition of the peptide.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support of Dr. Edwin Rubel and the Virginia Merrill Bloedel Hearing Research Center. We wish to thank Dale Cunningham for help with histology and Stephanie Smith, Jialin Shang for technical assistance and Glen MacDonald for help with microscopy and imaging. We thank the following for generously providing reagents, Dr. Stanislav Tomerev for Prox1 cDNA clone and for the original Prox1 antisera, Dr. Stefan Heller for the Myosin VIIA antisera and Dr. Jane Johnson for the Math1-GFP mice. We thank Dr. Thomas Reh for critically reading the manuscript and for endless discussions during the course of this work.
Funding from NIH DC005953 (OBMcD), DC003944 (ECO), DC003696 (JSS), DC006437 (CH), NIDCD P30 DC004661, NICHHD P30 HD002274, NASA:NAG-2-1415 (JSS) and VMBHRC Hearing Regeneration Initiative.
LITERATURE CITED
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense- organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Blanco J, Girard F, Kamachi Y, Kondoh H, Gehring WJ. Functional analysis of the chicken delta1-crystallin enhancer activity in Drosophila reveals remarkable evolutionary conservation between chicken and fly. Development. 2005;132:1895–1905. doi: 10.1242/dev.01738. [DOI] [PubMed] [Google Scholar]
- Bryant J, Goodyear RJ, Richardson GP. Sensory organ development in the inner ear: molecular and cellular mechanisms. Br Med Bull. 2002;63:39–57. doi: 10.1093/bmb/63.1.39. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chitnis AB. Control of neurogenesis--lessons from frogs, fish and flies. Curr Opin Neurobiol. 1999;9:18–25. doi: 10.1016/s0959-4388(99)80003-8. [DOI] [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Cook T, Pichaud F, Sonneville R, Papatsenko D, Desplan C. Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Dev Cell. 2003;4:853–864. doi: 10.1016/s1534-5807(03)00156-4. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1771–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cui W, Tomarev SI, Piatigorsky J, Chepelinsky AB, Duncan MK. Mafs, Prox1, and Pax6 can regulate chicken betaB1-crystallin gene expression. J Biol Chem. 2004;279:11088–11095. doi: 10.1074/jbc.M312414200. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Cui W, Oh DJ, Tomarev SI. Prox1 is differentially localized during lens development. Mech Dev. 2002;112:195–198. doi: 10.1016/s0925-4773(01)00645-1. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Livesey FJ, Cepko CL, Oliver G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet. 2003;34:53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- Eggston AA, Wolff D. Embryology of the ear. In: Histopathology of the ear, nose, and throat. Williams and Wilkins Co.; Baltimore: 1947. pp. 37–64. [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J Am Audiol Soc. 1976;1:179–184. [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Doe CQ. Asymmetric Prospero localization is required to generate mixed neuronal/glial lineages in the Drosophila CNS. Development. 2001;128:4103–4112. doi: 10.1242/dev.128.20.4103. [DOI] [PubMed] [Google Scholar]
- Glasgow E, Tomarev SI. Restricted expression of the homeobox gene prox 1 in developing zebrafish. Mech Dev. 1998;76:175–178. doi: 10.1016/s0925-4773(98)00121-x. [DOI] [PubMed] [Google Scholar]
- Griffiths RL, Hidalgo A. Prospero maintains the mitotic potential of glial precursors enabling them to respond to neurons. Embo J. 2004;23:2440–2450. doi: 10.1038/sj.emboj.7600258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125:4637–4644. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG, Mooseker MS, Corey DP. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol. 1997;137:1287–1307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held H, Bethe . Handbuch der normalen und pathologischen Physiologie. 11/1. Springer; Berlin: 1926. Die cochlea der säuger und der vögel, ihre entwicklung und ihr bau; pp. 467–541. [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Ito M, Spicer SS, Schulte BA. Cytological changes related to maturation of the organ of Corti and opening of Corti’s tunnel. Hear Res. 1995;88:107–123. doi: 10.1016/0378-5955(95)00106-e. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T. The Notch-Hes pathway in mammalian neural development. Cell Res. 1999;9:179–188. doi: 10.1038/sj.cr.7290016. [DOI] [PubMed] [Google Scholar]
- Kauffmann RC, Li S, Gallagher PA, Zhang J, Carthew RW. Ras1 signaling and transcriptional competence in the R7 cell of Drosophila. Genes Dev. 1996;10:2167–2178. doi: 10.1101/gad.10.17.2167. [DOI] [PubMed] [Google Scholar]
- Knipper M, Zimmermann U, Rohbock K, Kopschall I, Zenner HP. Expression of neurotrophin receptor trkB in rat cochlear hair cells at time of rearrangement of innervation. Cell Tissue Res. 1996;283:339–353. doi: 10.1007/s004410050545. [DOI] [PubMed] [Google Scholar]
- Knipper M, Gestwa L, Ten Cate WJ, Lautermann J, Brugger H, Maier H, Zimmermann U, Rohbock K, Kopschall I, Wiechers B, Zenner HP. Distinct thyroid hormone-dependent expression of TrKB and p75NGFR in nonneuronal cells during the critical TH-dependent period of the cochlea. J Neurobiol. 1999;38:338–356. [PubMed] [Google Scholar]; Kraus HJ, Aulbach-Kraus K. Morphological changes in the cochlea of the mouse after the onset of hearing. Hear Res. 1981;4:89–102. doi: 10.1016/0378-5955(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton CR, Gridley T, Kelley MW. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J Assoc Res Otolaryngol. 2000;1:161–171. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengler J, Krausz E, Tomarev S, Prescott A, Quinlan RA, Graw J. Antagonistic action of Six3 and Prox1 at the gamma-crystallin promoter. Nucleic Acids Res. 2001;29:515–526. doi: 10.1093/nar/29.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DJ, Anniko M. Developmental morphology of the mouse inner ear. A scanning electron microscopic observation. Acta Otolaryngol Suppl. 1985;422:1–69. scanning electron microscopic observation. Acta Otolaryngol Suppl 422:1-69. [PubMed] [Google Scholar]
- Lim DJ, Rueda J. Structural development of the cochlea. In: Romand, editor. Development of auditory and vestibular systems 2. Elsevier; 1992. pp. 33–58. [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3:389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Manning L, Doe CQ. Prospero distinguishes sibling cell fate without asymmetric localization in the Drosophila adult external sense organ lineage. Development. 1999;126:2063–2071. doi: 10.1242/dev.126.10.2063. [DOI] [PubMed] [Google Scholar]
- McKenzie E, Krupin A, Kelley MW. Cellular growth and rearrangement during the development of the mammalian organ of Corti. Dev Dyn. 2004;229:802–812. doi: 10.1002/dvdy.10500. [DOI] [PubMed] [Google Scholar]
- Mueller KL, Jacques BE, Kelley MW. Fibroblast growth factor signaling regulates pillar cell development in the organ of corti. J Neurosci. 2002;22:9368–9377. doi: 10.1523/JNEUROSCI.22-21-09368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myat A, Henrique D, Ish-Horowicz D, Lewis J. A chick homologue of Serrate and its relationship with Notch and Delta homologues during central neurogenesis. Dev Biol. 1996;174:233–247. doi: 10.1006/dbio.1996.0069. [DOI] [PubMed] [Google Scholar]
- Pickles JO. The expression of fibroblast growth factors and their receptors in the embryonic and neonatal mouse inner ear. Hear Res. 2001;155:54–62. doi: 10.1016/s0378-5955(01)00247-7. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Arumae U, Moshnyakov M, Palgi J, Saarma M, Ylikoski J. Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hear Res. 1994;75:131–144. doi: 10.1016/0378-5955(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Pirvola UL, Virkkala J, Fuxe J, Petterssson R, Ylikoski J. FGF-8 and its high affinity receptors are expressed in the mammalian and chick cochlea. Assoc. Res. Otolaryngol. Abstracts. 1998;21:167. X.Q. [Google Scholar]
- Retzius G. Das Gehörorgan der Säugethiere. Vol. 2. Centraltryckeriet; Stockholm: 1884. [Google Scholar]
- Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Audit Neurosci. 1996;2:195–205. [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78:461–471. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol Suppl. 1967;220:1–44. [PubMed] [Google Scholar]
- Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang D-S, Garcia-Anoveros J, Hinds PW, Corwin JT, Corey DP, Chen Z-Y. Proliferation of Functional Hair Cells in Vivo in the Absence of the Retinoblastoma Protein. Science. 2005;307:1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- Sahly I, El-Amraoui A, Abitbol M, Petit C, Dufier JL. Expression of myosin VIIA during mouse embryogenesis. Anat Embryol (Berl) 1997;196:159–170. doi: 10.1007/s004290050088. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Neurotrophin and neurotrophin receptor mRNA expression in developing inner ear. Hear Res. 1994;73:92–100. doi: 10.1016/0378-5955(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Shailam R, Lanford PJ, Dolinsky CM, Norton CR, Gridley T, Kelley MW. Expression of proneural and neurogenic genes in the embryonic mammalian vestibular system. J Neurocytol. 1999;28:809–819. doi: 10.1023/a:1007009803095. [DOI] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Cytologic structures unique to Deiters cells of the cochlea. Anat Rec. 1993;237:421–430. doi: 10.1002/ar.1092370316. [DOI] [PubMed] [Google Scholar]
- Stone JS, Shang JL, Tomarev S. Expression of Prox1 defines regions of the avian otocyst that give rise to sensory or neural cells. J Comp Neurol. 2003;460:487–502. doi: 10.1002/cne.10662. [DOI] [PubMed] [Google Scholar]
- Stone JS, Shang JL, Tomarev S. cProx1 immunoreactivity distinguishes progenitor cells and predicts hair cell fate during avian hair cell regeneration. Dev Dyn. 2004;230:597–614. doi: 10.1002/dvdy.20087. [DOI] [PubMed] [Google Scholar]
- Tomarev SI, Sundin O, Banerjee-Basu S, Duncan MK, Yang JM, Piatigorsky J. Chicken homeobox gene Prox 1 related to Drosophila prospero is expressed in the developing lens and retina. Dev Dyn. 1996;206:354–367. doi: 10.1002/(SICI)1097-0177(199608)206:4<354::AID-AJA2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Torii M, Matsuzaki F, Osumi N, Kaibuchi K, Nakamura S, Casarosa S, Guillemot F, Nakafuku M. Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development. 1999;126:443–456. doi: 10.1242/dev.126.3.443. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Zhang N, Martin GV, Kelley MW, Gridley T. A mutation in the Lunatic fringe gene suppresses the effects of a Jagged2 mutation on inner hair cell development in the cochlea. Curr Biol. 2000;10:659–662. doi: 10.1016/s0960-9822(00)00522-4. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 2000;127:4551–4560. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- Zine A, Van De Water TR, de Ribaupierre F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development. 2000;127:3373–3383. doi: 10.1242/dev.127.15.3373. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. This figure illustrates the specificity of the Prox1 antiserum. Panel A shows Prox1 staining in E14 lens. Panel B shows Prox1 staining (red) in the organ of Corti at E18 and panel C shows that the Prox1 staining is completely eliminated by incubation with the peptide (50μg/ml) against which the antiserum was raised. Staining with the MyosinVIIA antibody (green) is not affected by the addition of the peptide.