Abstract
Rationale
Behavioral and anatomical data suggest that the ventral striatum, consisting of the nucleus accumbens and olfactory tubercle, is functionally heterogeneous. Cocaine and d-amphetamine appear to be more rewarding when administered into the medial olfactory tubercle or medial accumbens shell than into their lateral counterparts, including the accumbens core.
Objectives
We sought to determine whether rats self-administer the popular recreational drug (±)-3,4-methylenedioxymethamphetamine (MDMA) into ventrostriatal subregions and whether the medial olfactory tubercle and medial accumbens shell mediate MDMA's positive reinforcing effects more effectively than their lateral counterparts.
Results
Rats receiving 30 mM MDMA into the medial olfactory tubercle, medial accumbens shell, or accumbens core, but not the lateral tubercle or lateral shell, showed higher self-administration rates than rats receiving vehicle. The medial shell supported more vigorous self-administration of MDMA at higher concentrations than the core or medial olfactory tubercle. In addition, intra-medial shell MDMA self-administration was disrupted by co-administration of the D1 or D2 receptor antagonists SCH 23390 (1–3 mM) or raclopride (3–10 mM).
Conclusions
Our data suggest that the ventral striatum is functionally heterogeneous. The medial accumbens shell appears to be more important than other ventrostriatal subregions in mediating the positive reinforcing effects of MDMA via both D1- and D2-type receptors. Together with previous data, our data also suggest that unidentified actions of MDMA interfere with the positive reinforcing effects of dopamine in the medial olfactory tubercle.
Keywords: Intracranial self-administration, Reward, Reinforcement, Ecstasy, Dopamine, Nucleus accumbens, Core, Shell, D1 receptors, D2 receptors
Introduction
3,4-methylenedioxymethamphetamine (MDMA) is a primary component of ‘Ecstasy’ tablets, a popular recreational drug among young adults in many parts of the world (Parrott 2001, 2004; Pope et al. 2001). Like amphetamine and cocaine, MDMA administration increases extracellular dopamine concentration in the dorsal and ventral striatum (White et al. 1996; Yamamoto and Spanos 1988). In addition, MDMA administration acutely increases extracellular serotonin concentration in many brain regions (Green et al. 2003). MDMA's capacity to increase extracellular serotonin relative to dopamine appears to be greater than those of amphetamine or cocaine (Rothman et al. 2001). This pharmacological profile is reflected in its psycho-behavioral effects. For example, MDMA administration in humans is euphoric and hallucinogenic (Parrott 2001); the latter, in particular, is not a hallmark of amphetamine or cocaine.
The primary neuroanatomical substrate for the rewarding effects of psychomotor stimulants such as amphetamine and cocaine appears to be the dopamine neurons projecting to the ventral striatum from the A10 area, largely localized in the ventral tegmental area (Fibiger and Phillips 1986; Koob 1992; McBride et al. 1999; Pierce and Kumaresan 2006; Wise and Bozarth 1987). Based on intracranial self-administration and neuronal tracer data (Ikemoto 2007; Ikemoto and Wise 2004), it appears that the dopamine neurons projecting to the ventromedial striatum, including the medial olfactory tubercle and medial accumbens shell, from the medial A10 area primarily mediate the rewarding action of cocaine, amphetamine, and other drugs (Carlezon et al. 1995; Ikemoto 2003; Ikemoto et al. 1997, 2005; Rodd-Henricks et al. 2002). This circuitry appears to be involved in energizing ongoing approach and eliciting a positive affective state, leading to conditioned approach (Ikemoto 2007).
The purpose of this intracranial self-administration study is to determine whether MDMA, like amphetamine or cocaine, is self-administered into the medial shell and medial tubercle. Our data suggest that dopaminergic mechanisms within the medial accumbens shell play an important role in MDMA reward and that the medial olfactory tubercle is not as important in MDMA reward as cocaine or amphetamine reward.
Materials and methods
Animals
One hundred sixty-one male Wistar rats (Harlan, Dublin, VA, USA) weighing 250–350 g at the time of surgery were used. They were housed singly in a temperature- and humidity-controlled room on a reverse 12:12 light cycle (lights on at 9:00 PM). Food and water were available ad libitum except during testing. All procedures were approved by the Animal Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse, and were in accordance with the National Institutes of Health guidelines.
Surgery
Rats were anesthetized with sodium pentobarbital (31 mg/kg, i.p.) and chloral hydrate (142 mg/kg, i.p.) and implanted unilaterally with a guide cannula (24 gauge, Plastics One, VA, USA) that ended 1.0 mm above the medial and lateral olfactory tubercle, medial and lateral accumbens shell, and accumbens core. Efflux from central injections tends to follow a pressure gradient up the cannula shaft. Accordingly, the cannulae for medial shell and medial tubercle sites were inserted at a 20° angle from the right hemisphere through the midline into the left hemisphere to minimize diffusion of drug solution to the core or shell, respectively. The cannulae for core, lateral shell, and lateral tubercle sites were inserted vertically into the left hemisphere without any angle. Using a stereotaxic instrument, the following coordinates (in millimeters) were measured: 2.0 anterior to bregma, 1.9 lateral to the midline, and 7.6 ventral to the skull surface for the accumbens core; 2.0 anterior, 2.5 lateral, and 8.4 ventral for the lateral olfactory tubercle; 2.0 anterior, 2.3 lateral, and 7.7 ventral for the lateral accumbens shell; 2.2 anterior, 1.9 lateral, and 8.2 ventral for the medial olfactory tubercle; 2.0 anterior, 1.6 lateral, and 7.2 ventral for accumbens medial shell. All ventral measurements were made from the skull surface with the incisor bar set at approximately 3.3 mm below the interaural line. Each cannula was subsequently anchored to the skull by four stainless steel screws and dental acrylic. The surgery was followed by a minimum of 7 days of recuperation before the start of experimentation.
Drugs
(±)-MDMA HCl (Research Triangle Institute, Research Triangle Park, NC, USA), d-amphetamine HCl, the D1 receptor antagonist R(+)-SCH 23390, and the D2 receptor antagonist S(−)-raclopride (+)-tartrate salt (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in artificial cerebro-spinal fluid consisting of (in millimolars): 148 NaCl, 2.7 KCl, 1.2 CaCl2, and 0.85 MgCl2, pH adjusted to 6.5–7.5.
Self-administration apparatus and procedures
Each rat was placed individually in the operant conditioning chamber (30×22×24 cm; Med Associates, St. Albans, VT, USA) equipped with a lever (45 mm wide× 2 mm thick, protruding 20 mm from the wall) below a cue light. An injection cannula was inserted and secured into the guide cannula, which was connected by polyethylene tubing to a micropump consisting of a drug reservoir and step motor (Ikemoto and Sharpe 2001), which hanged a few millimeters above the rat's head. A lever-press triggered the step motor to turn its shaft in six incremental steps (9° per step) over 5 s, driving its threaded shaft into the drug reservoir and, in turn, pushing a 78-nl volume out of the reservoir into the brain. A lever-press also triggered a 5-s tone and extinction of light cue and the retraction of the lever timeout signals that lasted for 15 s. Following this 15-s timeout period, the lever and cue light were both reinstated. Sessions lasted 90 min or until the rats received a total of 60 infusions. The maximum number of infusions was set to minimize the possibility of tissue damage. Sessions were separated by 24 h. A 90-min habituation session took place a day before the procedures described below.
Effects of MDMA as function of site and concentration
Each rat received the following infusions into one of the five ventral striatal regions: vehicle in sessions 1 and 6, 30 mM MDMA in sessions 2–5 (acquisition phase), and 10, 30, and 100 mM MDMA, respectively in sessions 7–9. These concentrations of MDMA were initially used because 30 and 100 mM were found to be effective for d-amphetamine self-administration in our previous experiments (Ikemoto et al. 2005). One group of rats, however, received vehicle infusions throughout all nine sessions. The vehicle control group consisted of rats with guide cannulae targeting the aforementioned five brain regions. Because infusion rates from each region that received vehicle did not differ significantly, they were pooled into a single group.
Behavioral and pharmacological selectivity
Operant conditioning chambers were identical to the ones described above except that they were equipped with two retractable levers mounted on a wall, separated by 12 cm. A response on the “active” lever triggered a 78-nl infusion, turned on a cue light above the lever over 5 s, and retracted both levers for 10 s. A response on the “inactive” lever retracted both levers for 10 s without an infusion or light signal. The assignment of left and right levers for active and inactive functions was counterbalanced among subjects and remained the same for each rat throughout the experiment. Rats (N=8) received the following infusions into the medial shell: vehicle in sessions 1, 4, and 7 and 100 mM MDMA in sessions 2 and 3.
The effects of dopamine receptor antagonists SCH 23390 (1 mM, a D1 antagonist) and raclopride (3 mM, a D2 antagonist) were evaluated in sessions 5 and 6 and sessions 8 and 9. Rats received either MDMA (100 mM) or a mixture of MDMA (100 mM) plus one of the antagonists over two consecutive sessions. The order of testing the two treatments and the two antagonists was counterbalanced among the subjects.
Effects of high MDMA concentrations
Using the single-lever procedure described above, each rat received infusions into either the medial tubercle, medial shell, or core: vehicle in session 1, 500 mM MDMA in sessions 2 and 3, and 1,000 mM MDMA in sessions 4 and 5. They also received 100 mM d-amphetamine in sessions 6 and 7.
Effects of dopamine receptor antagonists on high MDMA
Using the single-lever procedure described above, groups of ten and eight rats were used to evaluate the effects of 1 and 3 mM SCH 23390, respectively. Rats received vehicle infusions in session 1, 1,000 mM MDMA in sessions 2 and 3 (acquisition phase), and vehicle in session 4. One half of the group received 1,000 mM MDMA in session 5 and the mixture of 1,000 mM MDMA and SCH 23390 (1 or 3 mM) in session 6. The other half received the same treatments in reverse order.
A group of eight rats was used to examine the effects of 3 and 10 mM raclopride. After receiving vehicle infusions in sessions 1 and 4 and 1,000 mM MDMA in sessions 2 and 3, one half of the group received 1,000 mM MDMA in session 5, the mixture of 1,000 mM MDMA and 3 mM raclopride in session 6, vehicle in session 7, 1,000 mM MDMA in session 8, and the mixture of 1,000 mM MDMA and 10 mM raclopride in session 9. The other half received the same treatments but they received the treatments of sessions 5 and 6 and those of sessions 8 and 9 in reverse order.
Histology
At the end of the experiment, the rats were deeply anesthetized with sodium pentobarbital (31 mg/kg, i.p.) and chloral hydrate (142 mg/kg, i.p.) and decapitated. Their brains were removed and placed in 10% formalin for a minimum of 2 days prior to sectioning on a cryostat. Frozen coronal sections near the cannula tip were cut at a 40-μm thickness, mounted on gelatinized glass slides, and stained with cresyl violet. The placements of injection cannulae were verified by microscopic examination.
Statistical analyses
Rates of infusions during the MDMA acquisition were analyzed using a 6×5 mixed design analysis of variance (ANOVA) with region (five regions plus vehicle control) as a between-subjects factor and session (1–5) as a within-subjects factor, followed by a Newman–Keuls post hoc test. Similarly, rates of infusions during the varying concentration sessions were analyzed using a 6×4 mixed design ANOVA with region and concentration (0, 10, 30, and 100 mM MDMA), followed by a Newman–Keuls post hoc test. Two-lever discrimination effects were analyzed by a 3×2 within-subjects design ANOVA on responses with treatment (sessions 1–3) and lever (active and inactive), followed by a Newman–Keuls test. For the effects of SCH 23390 and raclopride, one-way within-subjects design ANOVAs were performed on infusion rates with treatment (vehicle, MDMA, and the mixture of MDMA and antagonist), followed by Newman–Keuls post hoc tests. The effects of high MDMA concentrations and d-amphetamine were analyzed on infusion rates using a 3×7 mixed design ANOVA with region (medial olfactory tubercle, medial accumbens shell, and accumbens core) as a between-subjects factor and session (1–7) as a within-subjects factor, followed by a Newman–Keuls post hoc test.
Results
Effects of MDMA as function of site and concentration
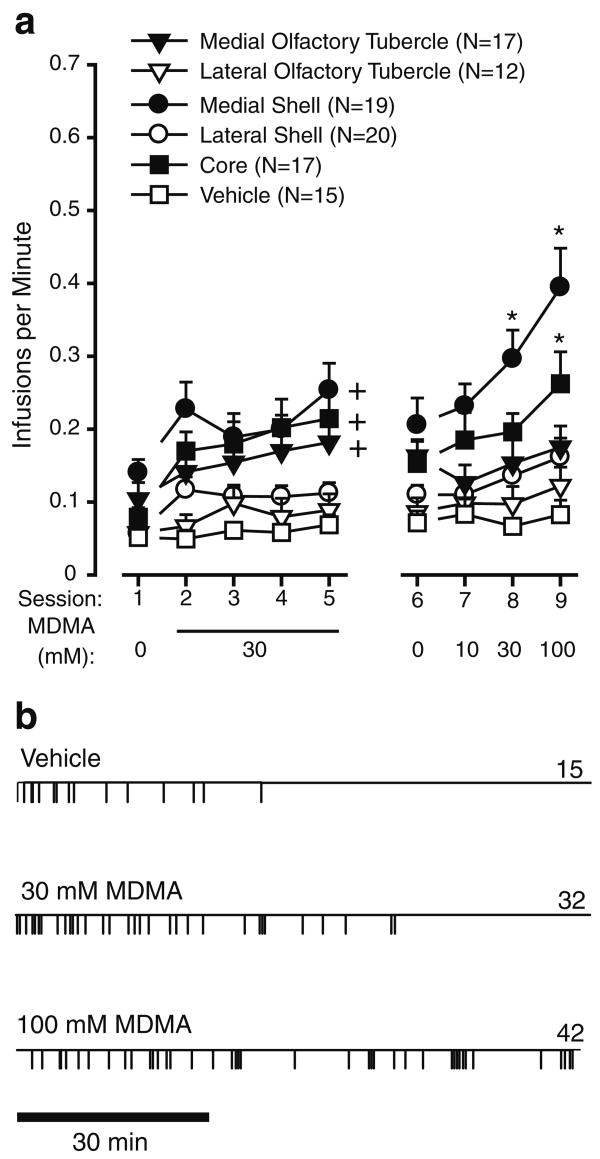
Figure 1 shows representative placements of injection cannulae for five regions: medial and lateral olfactory tubercle, medial and lateral accumbens shell, and accumbens core. Rats reliably increased infusion rates when they received 30 mM MDMA into the medial olfactory tubercle, medial accumbens shell, and accumbens core. This observation is confirmed by a significant main region effect, F(5,94)=9.88, P<0.001, followed by a Newman–Keuls test during the acquisition phase, sessions 1–5 (Fig. 2a). Similarly, a two-way ANOVA with brain region and MDMA concentration (sessions 6–9) revealed a significant interaction between region and concentration, F(15,282)=2.19, P<0.01. Self-administration rates appeared to increase as a function of concentration for the medial shell and, less strongly, for the core. Intra-medial shell injections of 100 mM MDMA appeared to support self-administration throughout the session (Fig. 2b), whereas 10 or 30 mM MDMA did not, suggesting that low concentrations (10 or 30 mM) were not as rewarding as the higher one. Intra-core administration of 100 mM MDMA significantly increased infusion rates but failed to maintain them throughout the session (data not shown). All concentrations administered into the medial tubercle, lateral tubercle, or lateral shell failed to increase self-administration rates.
Fig. 1.
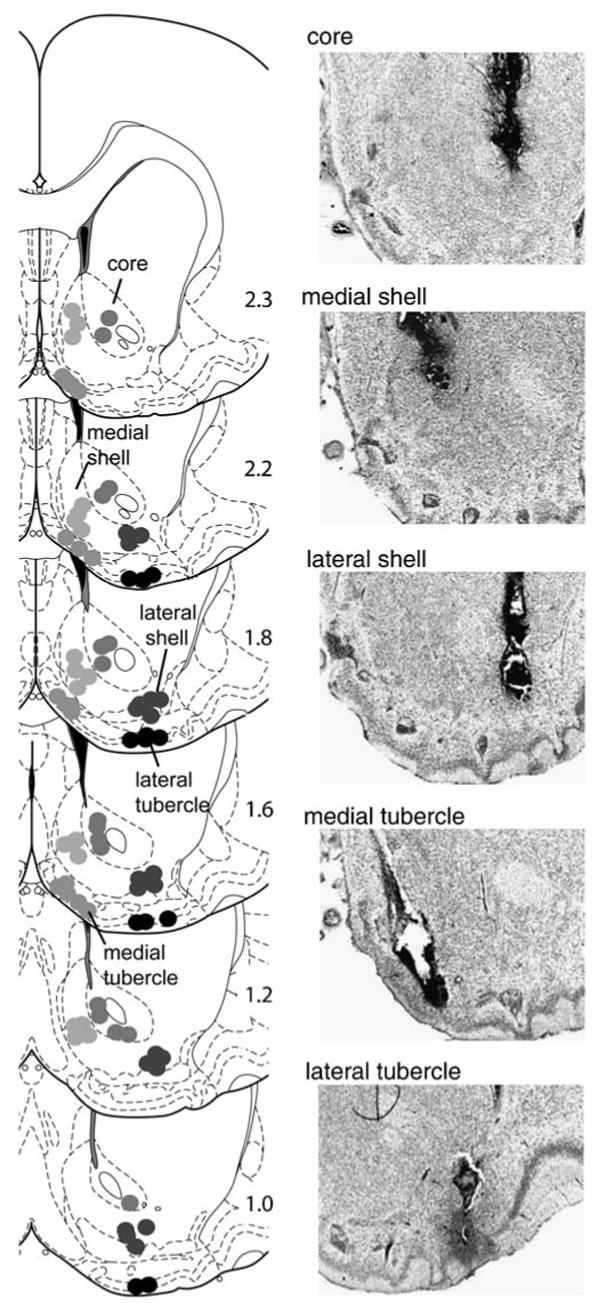
Injection cannula tip placements. The drawings of coronal sections (Paxinos and Watson 2005) on the left depict the location of injection cannula tips for the accumbens core (N=17), medial accumbens shell (N=19), lateral accumbens shell (N=20), medial olfactory tubercle (N=17), and lateral olfactory tubercle (N=12). The number on the left of each drawing indicates the distance (in millimeters) anteriorly from bregma. Photomicrographs of Nissl-stained coronal sections on the right show typical cannula placements for respective regions
Fig. 2.
Intracranial self-administration of MDMA into the ventral striatum. a Mean (±SEM) infusion rates are shown as function of session and region. Plus signs, P<0.05, region group, with session collapsed together, significantly different from vehicle group. Asterisks, P<0.05, concentration significantly different from its vehicle. b Event records from a representative rat receiving infusions into the medial accumbens shell. Each vertical line on the horizontal line indicates the time of an infusion. The number on the right of each event record represents total infusions for that session
Behavioral and pharmacological selectivity
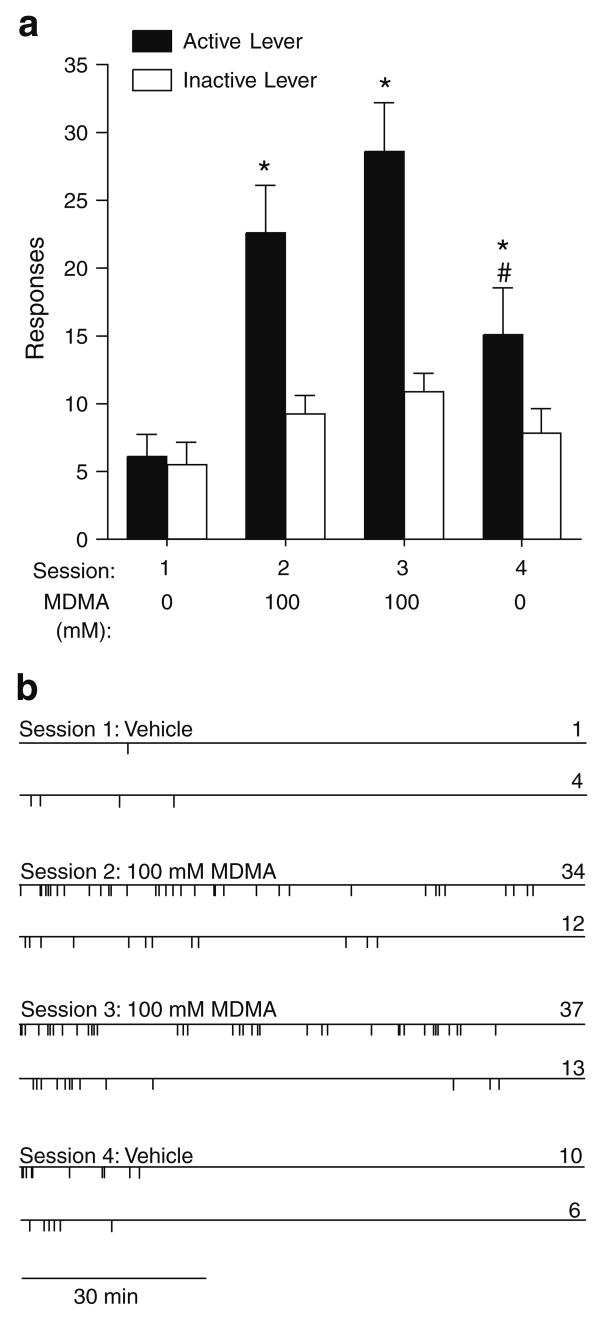
We examined whether intra-medial shell injections of MDMA merely elicited general arousal resulting in increased lever-pressing without positive reinforcing effects. When rats had to discriminate between two levers for vehicle infusions in session 1, they did not respond on the active lever more than the inactive lever (Fig. 3). On the other hand, the rats responded on the active lever more than the inactive lever when they received 100 mM MDMA into the medial shell upon active, but not inactive, lever-pressing in sessions 2 and 3. When the rats received vehicle again in session 4, rats did respond on the active lever more than the inactive lever even though their response levels were lower than when they had received MDMA. These observations are confirmed by a significant interaction between session and lever, F(3,21)= 15.91, P<0.0001, followed by a Newman–Keuls test.
Fig. 3.
Self-administration of intra-medial shell MDMA with two levers. a Mean (±SEM) lever-presses (N=8) are shown as function of session/treatment and lever. Asterisks, P<0.01, active lever-presses significantly greater than its inactive lever-presses and from active lever-presses of vehicle. Number sign, P<0.01, active lever-presses significantly lower than active lever-presses in session 2 or 3. Inactive lever-presses in sessions 2, 3, and 4 were not significantly different from inactive lever-presses in session 1. b Event records from a representative rat receiving infusions into the medial accumbens shell. Each vertical line on the horizontal line indicates the time of a response. For each session, upper and lower horizontal lines indicate timelines for active and inactive lever-presses, respectively. The number on the right of each line represents total responses for that lever
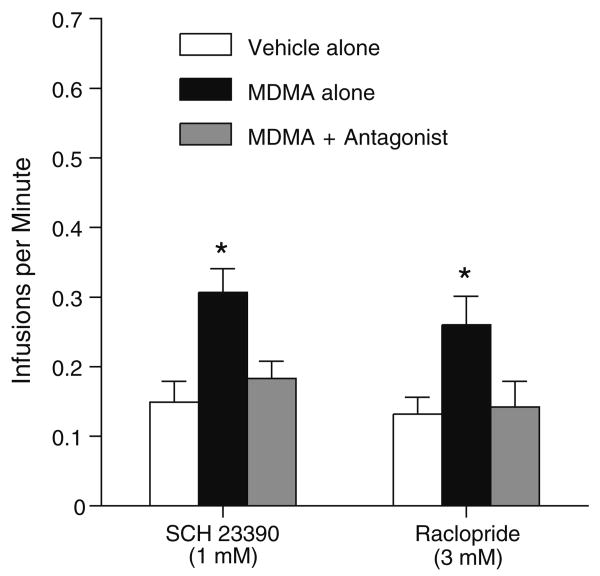
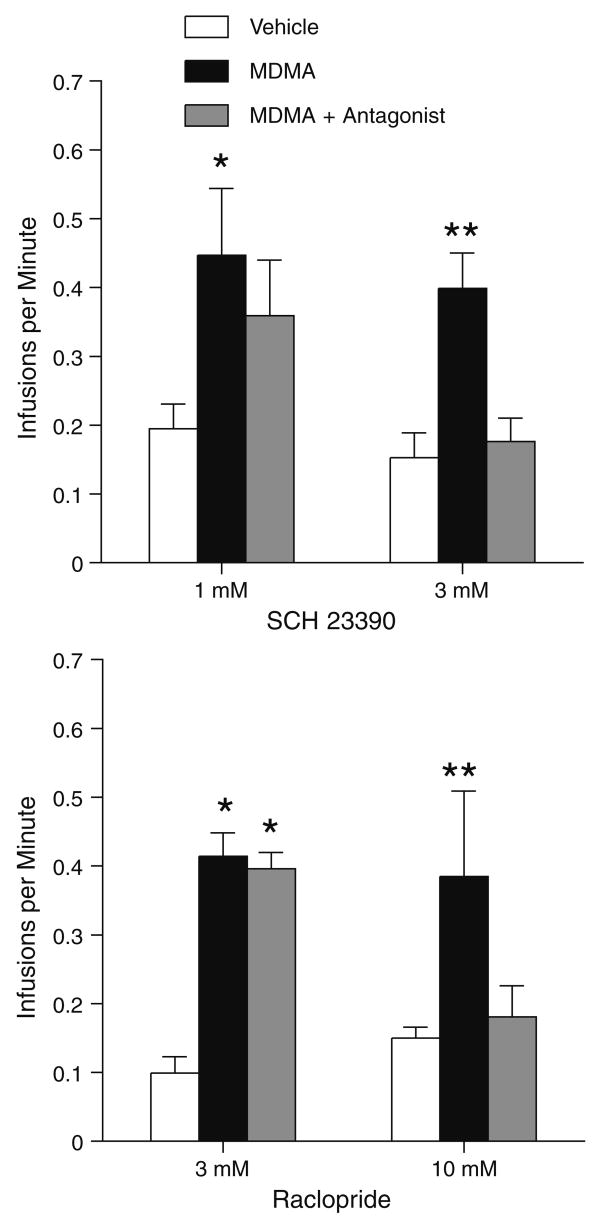
The reinforcing effects of intra-medial shell injections of MDMA appear to be mediated by both D1 and D2 receptors. When the rats received the mixture of MDMA and SCH 23390 (1 mM) or raclopride (3 mM), they significantly reduced self-administration rates, F(2,14)= 12.86, P<0.001 and F(2,14)=9.65, P<0.005, respectively (Fig. 4).
Fig. 4.
Effects of dopamine receptor antagonists on self-administration of 100 mM MDMA into the medial accumbens shell. Data are mean (±SEM) infusion rates. Asterisks, P<0.01, significantly different from vehicle and MDMA+antagonist values
Effects of high MDMA concentrations
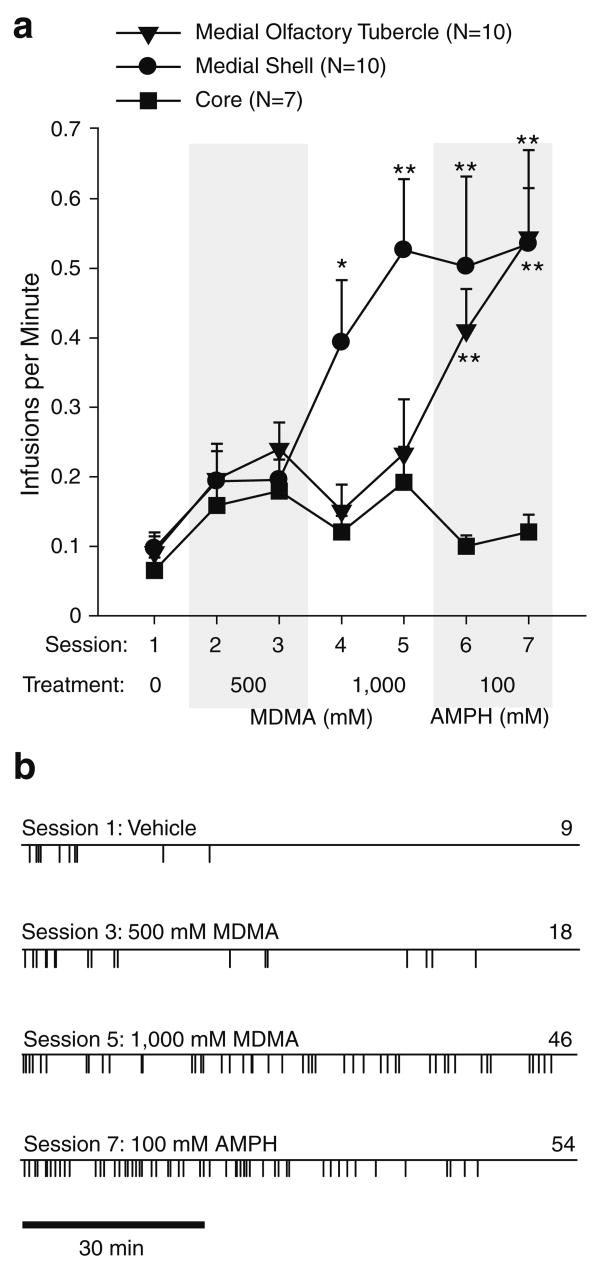
Since previous studies show that rats readily learn to self-administer cocaine or amphetamine into the medial olfactory tubercle (Ikemoto 2003; Ikemoto et al. 2005), it was intriguing that the rats did not readily learn to self-administer MDMA into the medial tubercle in the above experiments. We therefore examined the effects of high concentrations of MDMA. For a given concentration of d-amphetamine, a higher concentration of MDMA may be required to achieve the same level of extracellular DA release produced by d-amphetamine in the ventral striatum (Kankaanpaa et al. 1998). In this experiment, the effects of 500 and 1,000 mM MDMA were evaluated on self-administration. The rats tended to increase self-administration rates when they received 500 mM MDMA although the effects were statistically unreliable. The rats receiving 1,000 mM MDMA into the medial shell increased self-administration rates significantly, whereas the rats receiving the same concentration into the medial tubercle or core did not (Fig. 5a). The medial shell tended to support constant rates of self-administration throughout the session, whereas the medial tubercle or core did not sustain self-administration throughout the session (Fig. 5b), suggesting that intra-medial tubercle or core injections of MDMA are not as rewarding as intra-medial shell injections. When 1,000 mM MDMA was replaced with 100 mM d-amphetamine, the medial tubercle supported vigorous self-administration, the medial shell maintained similar self-administration, and the core again failed to support self-administration. These observations are confirmed by a significant region-by-session interaction, F(12,144)=3.79, P<0.0001, followed by a Newman–Keuls test.
Fig. 5.
Self-administration of high doses of MDMA and d-amphetamine (AMPH). a Mean (±SEM) infusion rates are shown as function of session and region. Asterisk, P<0.05; double asterisks, P<0.01, session value significantly different from its vehicle-session value. b Event records from representative rats receiving infusions into the medial accumbens shell. Each vertical line on the horizontal line indicates the time of an infusion. The number on the right of each event record represents total infusions for that session
Effects of dopamine receptor antagonists on high MDMA concentrations
We examined whether the reinforcing effects of the high MDMA concentration (1,000 mM) were also dependent on dopamine receptors. The rats quickly learned to self-administer 1,000 mM MDMA into the medial shell in sessions 2 and 3 (data not shown), replicating the findings above. When a mixture of MDMA plus 1 mM SCH 23390 or 3 mM raclopride was substituted for MDMA, self-administration rates did not significantly decrease (Fig. 6). These observations are confirmed by Newman–Keuls tests following significant main treatment effects, F(2,18)=4.07, P<0.05 and F(2,14)=63.78, P<0.0001 for 1 mM SCH 23390 and 3 mM raclopride experiments, respectively. On the other hand, when a mixture of MDMA plus 3 mM SCH 23390 or 10 mM raclopride was substituted for MDMA, self-administration rates significantly decreased (Fig. 6). These observations are confirmed by Newman–Keuls tests following significant main treatment effects, F(2,14)=12.36, P<0.001 and F(2,14)=3.82, P<0.05 for 3 mM SCH 23390 and 10 mM raclopride experiments, respectively.
Fig. 6.
Effects of dopamine receptor antagonists on self-administration of 1,000 mM MDMA into the medial accumbens shell. Data are mean (±SEM) infusion rates. Asterisks, P<0.05, significantly different from vehicle values. Double asterisks, P<0.05, significantly different from vehicle and MDMA+antagonist values
Discussion
We found that rats quickly learn to self-administer MDMA directly into the ventral striatum and that ventrostriatal self-administration of MDMA depended on the subregion as well as its concentration. The medial accumbens shell appears to be most responsible for supporting MDMA self-administration on the basis of relationships we discovered between MDMA concentration and self-administration rate and response patterns over the course of the self-administration session. In the following sub-sections, we provide interpretations of our findings on the effects of MDMA in the accumbens shell, core, and olfactory tubercle. We focus our analysis on concentration–rate relationships which are important not only for determining the most effective subregion for MDMA self-administration but also detecting drug diffusion and interpreting the effects of dopamine receptor antagonists.
Medial accumbens shell
Our observations suggest that 100 and 1,000 mM were more effective in maintaining self-administration than the lower concentrations. The data from our first experiment suggest that self-administration rates increased as MDMA concentrations increased from 10 to 100 mM. In subsequent experiments, the 1,000 mM concentration was found to be as effective as, if not more effective than, 100 mM since it supported self-administration at mean rates of 0.4 infusions per minute or higher. In addition, 10 or 30 mM MDMA did not typically support self-administration throughout the 90-min session, whereas 100 or 1,000 mM did.
The effects of 500 mM on self-administration rates were not as great as expected. The available data do not allow us to conclude that the effects of 500 mM on self-administration rates were less than those of 100 mM because the effects of these two concentrations were not subjected to systematic comparisons. Although we did not detect a significant effect of the 500 mM concentration with the statistical analysis described in the “Results” section, we should note that mean self-administration rates doubled with 500 mM MDMA from vehicle values and that the effect of 500 mM was highly significant (analysis not shown) when an ANOVA was conducted on rates just for the first three sessions with vehicle and 500 mM MDMA.
Behavioral selectivity: motor vs. motivation
In the two-lever discrimination test, rats responded more on the lever that delivered MDMA into the medial shell than the lever that did not, suggesting that MDMA self-administration did not result from incidental lever-pressing due to un-aimed somatomotor activity elicited by the drug. In addition, when rats received vehicle in substitution for MDMA in session 4 after two consecutive MDMA sessions, rats still responded more on the active lever than the inactive lever. Given that these rats did not respond on the active lever more than the inactive lever in session 1 with vehicle infusions, increased active lever-presses over inactive lever-presses in session 4 most likely indicate conditioned approach. These data support the hypothesis that increased dopamine concentration in the medial shell elicits a positive affective state, leading to conditioned approach (Ikemoto 2007), given that intra-medial shell injections of MDMA elevated extracellular dopamine concentration, a notion that is supported by antagonist experiments.
Roles of D1- and D2-type receptors in MDMA reinforcement
Intra-medial shell self-administration of MDMA appears to be mediated by D1 and D2 receptors. Intra-medial shell co-administration of the D1 receptor antagonist SCH 23390 or the D2 receptor antagonist raclopride with MDMA decreased self-administration rates. Self-administration of 100 mM MDMA was decreased by co-administration of either 1 mM SCH 23390 or 3 mM raclopride. Interestingly, higher concentrations of these antagonists (3 mM SCH 23390 and 10 mM raclopride) were needed to decrease self-administration of 1,000 mM MDMA, suggesting that administration of the 1,000 mM concentration led to greater dopamine transmission than 100 mM. Our data are consistent with previous data that a low dose of systemic SCH 23390 disrupts intravenous MDMA self-administration (Daniela et al. 2004) and other data suggesting that dopamine transmission is important for rewarding effects of drugs of abuse. The blockade of dopamine receptors or the loss of dopaminergic terminals within the medial shell, but not the core, disrupts conditioned place preference induced by systemic amphetamine, cocaine, nicotine, or morphine (Fenu et al. 2006; Sellings and Clarke 2003; Sellings et al. 2006b; Spina et al. 2006). In addition, the blockade of either D1 or D2 receptors within the medial shell disrupts self-administration of systemic cocaine (Bari and Pierce 2005). Because rats readily learn to self-administer the mixture of D1 and D2 receptor agonists, but not the D1 or D2 agonist alone, into the medial accumbens shell (Ikemoto et al. 1997), both D1 and D2 receptors appear to be involved in psychomotor stimulant rewards in such a way that transmission at D1-type receptors works cooperatively with that at D2-type receptors to mediate positive reinforcement.
Accumbens core
Our present findings suggest that the core can mediate positive reinforcement of drugs. The intra-core MDMA group reliably increased self-administration rates in comparison to vehicle injection group. However, rates of core self-administration were low. The 100 mM concentration supported the highest rates (about 0.3 mean infusions per minute) among the concentrations we evaluated. The effects of intra-core injections on self-administration cannot be explained by a simple hypothesis of medial shell diffusion because we would have observed rate increases as concentrations increased. The small self-administration effects of intra-core injections of MDMA compared to those of intra-shell injections may be explained by differences in dopamine function between these subregions, a theoretical point discussed below.
The present finding raises a reasonable possibility that dopamine transmission in the core supports self-administration of psychomotor stimulants. Previous studies (Carlezon et al. 1995; Ikemoto 2003; Rodd-Henricks et al. 2002) do not provide adequate data to determine whether rats self-administer drugs into the core at all because they were designed to examine the question of which is more important between subregions rather than whether intra-core injections mediate positive reinforcement at all. Therefore, the latter question should be examined with properly designed experiments.
Medial olfactory tubercle
It is most likely that MDMA exerts unique pharmacological actions in the medial olfactory tubercle and antagonizes the positive reinforcing effects mediated by its dopaminergic actions. Previous data suggest that the medial tubercle plays an important role in psychomotor stimulants' positive reinforcing effects via dopaminergic mechanisms. Rats learn to self-administer cocaine into the medial tubercle at lower concentrations and higher rates than into the medial shell or other ventrostriatal sites (Ikemoto 2003). The reinforcing effects of intra-medial tubercle injections of cocaine are diminished by co-administration of either D1 or D2 receptor antagonists, suggesting dopamine mediation of reinforcement. The medial tubercle supports amphetamine self-administration as vigorously as the medial shell (Ikemoto et al. 2005). In addition, selective lesions of dopaminergic terminals within the medial olfactory tubercle disrupt conditioned place preference induced by systemic methylphenidate more effectively than those within the shell or core (Sellings et al. 2006a). On the basis of these previous data plus the present finding that the accumbens shell supported MDMA self-administration via dopaminergic mechanisms, it was surprising to observe that intra-medial tubercle injections of MDMA had only marginal effects on self-administration. High concentrations up to 1,000 mM did not make intra-medial tubercle MDMA more effective in supporting self-administration. Because our medial tubercle rats quickly learned to self-administer d-amphetamine, replicating our previous work (Ikemoto et al. 2005), MDMA administration into the medial tubercle appears to exert an antagonizing effect over the positive reinforcing effects of its dopaminergic actions.
Implications for ventrostriatal organization, dopamine reward circuitry, and systemic MDMA self-administration
On the basis of projection connectivity and behavioral findings, some of which are reviewed above, heightened dopamine concentration in the ventromedial striatum (consisting of the medial accumbens shell and medial olfactory tubercle) is hypothesized to energize ongoing approach and elicit a positive affective state, which leads to conditioned approach (Ikemoto 2007). These functional properties of ventromediostriatal dopamine are also thought to be primarily responsible for the acquisition and maintenance of psychomotor stimulant self-administration into the ventromedial striatum. This hypothesis is generally supported by present findings. Most notably, the medial shell supported dopamine-mediated MDMA self-administration while the lateral shell did not support self-administration at any concentration that we examined. These findings are consistent with the idea that the accumbens shell is functionally heterogeneous and with the finding that the medial portion of the accumbens shell is more important for amphetamine reward than the lateral portion (Ikemoto et al. 2005). It should be noted, however, that the functional heterogeneity between the medial and lateral shell may not be obtained for all functions mediated by the accumbens shell. A recent study (Bossert et al. 2007) found that the blockade of dopamine receptors in either the medial or lateral accumbens shell (but not in the core) disrupts the reinstatement of heroin seeking induced by contextual stimuli. These observations suggest a complex functional organization within the accumbens shell.
Core dopamine's unique functional role may explain the marginal effect of intra-core MDMA on self-administration. Accumbens core dopamine may be involved in selection of conditioned responses (for a review, see section 4.7.2 of Ikemoto 2007). This means that the impact of core dopamine on self-administration is both quantitatively and qualitatively different from that of ventromediostriatal dopamine. Increased ventromediostriatal dopamine may energize responding at any incentive stimuli while increased core dopamine may energize conditioned responding. Although it is unclear how exactly these functional differences translate into the acquisition and maintenance of intracranial self-administration, the accumbens core most likely plays an important role in systemic self-administration of MDMA in a different manner than the ventromedial striatum, as found for systemic cocaine self-administration (Ito et al. 2004).
The lack of effects on self-administration of intra-medial tubercle injections of MDMA is not consistent with the ventromediostriatal dopamine reward hypothesis described above. Additional investigations are necessary to examine what actions of MDMA within the medial tubercle may have interfered with positive reinforcing effects mediated by its actions on dopaminergic system. MDMA's capacity to interfere with the positive reinforcing actions of dopamine in the medial olfactory tubercle may explain weak reinforcement of systemic MDMA in comparison with amphetamine or cocaine, observations documented in systemic self-administration models (De La Garza et al. 2007). In addition, MDMA administration may interfere with reinforcement processes mediated by other regions that were not investigated in the present study.
Conclusions
Our data suggest that the ventral striatum is functionally heterogeneous. The medial accumbens shell appears to be more important for positive reinforcing effects of MDMA than other ventrostriatal subregions. Together with previous data, our data also suggest that unidentified actions of MDMA interfere with positive reinforcing effects mediated by dopamine in the medial olfactory tubercle.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
References
- Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology. 1995;122:194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Daniela E, Brennan K, Gittings D, Hely L, Schenk S. Effect of SCH 23390 on (+/−)-3,4-methylenedioxymethamphetamine hyperactivity and self-administration in rats. Pharmacol Biochem Behav. 2004;77:745–750. doi: 10.1016/j.pbb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Fabrizio KR, Gupta A. Relevance of rodent models of intravenous MDMA self-administration to human MDMA consumption patterns. Psychopharmacology (Berl) 2007;189:425–434. doi: 10.1007/s00213-005-0255-5. [DOI] [PubMed] [Google Scholar]
- Fenu S, Spina L, Rivas E, Longoni R, Di Chiara G. Morphine-conditioned single-trial place preference: role of nucleus accumbens shell dopamine receptors in acquisition, but not expression. Psychopharmacology (Berl) 2006;187:143–153. doi: 10.1007/s00213-006-0415-2. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Phillips AG. Reward, motivation, cognition: psychobiology of mesotelencephalic dopamine systems. In: Mountcastle VB, Bloom FE, Geiger SR, editors. Handbook of physiology: vol 4. The nervous system. American Physiological Society; Bethesda: 1986. pp. 647–675. [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci. 2003;23:9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Sharpe LG. A head-attachable device for injecting nanoliter volumes of drug solutions into brain sites of freely moving rats. J Neurosci Methods. 2001;110:135–140. doi: 10.1016/s0165-0270(01)00428-9. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47:190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell and olfactory tubercle valid. J Neurosci. 2005;25:5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Lillsunde P, Seppala T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacol Biochem Behav. 1998;59:1003–1009. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Human psychopharmacology of Ecstasy (MDMA): a review of 15 years of empirical research. Hum Psychopharmacol. 2001;16:557–577. doi: 10.1002/hup.351. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Is ecstasy MDMA? A review of the proportion of ecstasy tablets containing MDMA, their dosage levels, and the changing perceptions of purity. Psychopharmacology (Berl) 2004;173:234–241. doi: 10.1007/s00213-003-1712-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier; Burlington: 2005. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse. Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Ionescu-Pioggia M, Pope KW. Drug use and life style among college undergraduates: a 30-year longitudinal study. Am J Psychiatry. 2001;158:1519–1521. doi: 10.1176/appi.ajp.158.9.1519. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, McBride WJ. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of Wistar rats. J Pharmacol Exp Ther. 2002;303:1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, McQuade LE, Clarke PB. Characterization of dopamine-dependent rewarding and locomotor stimulant effects of intravenously-administered methylphenidate in rats. Neuroscience. 2006a;141:1457–1468. doi: 10.1016/j.neuroscience.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Sellings LH, McQuade LE, Clarke PB. Evidence for multiple sites within rat ventral striatum mediating cocaine conditioned place preference and locomotor activation. J Pharmacol Exp Ther. 2006b;317:1178–1187. doi: 10.1124/jpet.105.100339. [DOI] [PubMed] [Google Scholar]
- Spina L, Fenu S, Longoni R, Rivas E, Di Chiara G. Nicotine-conditioned single-trial place preference: selective role of nucleus accumbens shell dopamine D1 receptors in acquisition. Psychopharmacology (Berl) 2006;184:447–455. doi: 10.1007/s00213-005-0211-4. [DOI] [PubMed] [Google Scholar]
- White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, “Ecstasy”) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Yamamoto BK, Spanos LJ. The acute effects of methylenedioxymethamphetamine on dopamine release in the awake-behaving rat. Eur J Pharmacol. 1988;148:195–203. doi: 10.1016/0014-2999(88)90564-x. [DOI] [PubMed] [Google Scholar]