Abstract
Study Objectives:
To relate reproductive hormones (and the preceding 7-year rates of their change) to objectively and subjectively assessed sleep measures, independent of age, vasomotor symptom frequency, depressive symptoms, and body size.
Design:
A cross-sectional sleep substudy nested in the Study of Women's Health Across the Nation (SWAN), a longitudinal study of the menopausal transition.
Setting:
Community-based.
Participants:
365 Caucasian, African American, and Chinese women.
Measurements and Results:
Sleep duration, continuity, and architecture were measured during two nights of in-home polysomnography (PSG) studies. Participants completed the Pittsburgh Sleep Quality Index (PSQI) for sleep quality, sleep diaries for medication, vasomotor symptoms, lifestyle information and questionnaires for depressive symptoms. Blood collected annually in the years prior to sleep study was assayed for follicle stimulating hormone (FSH), estradiol (E2), and total testosterone (T).
More rapid rate of FSH change was significantly associated with higher delta sleep percent, longer total sleep time (TST), but less favorable self-reported sleep quality (PSQI). Baseline E2 was modestly and negatively associated with sleep quality. Women in the lowest total testosterone quartile at baseline had more wake time after sleep onset (WASO) than women in the highest quartile. Lower E2/T ratio, an index reflecting the increasing androgenic environment with the menopause transition, was associated with less WASO.
Conclusions:
More rapid rate of FSH change was associated with longer sleep duration but poor sleep quality. Women with higher T or who were closer to the completion of the transition process (as indexed by a lower E2/T) had less sleep discontinuity (less WASO).
Citation:
Sowers MF; Zheng H; Kravitz HM; Matthews K; Bromberger JT; Gold EB; Owens J; Consens F; Hall M. Sex steroid hormone profiles are related to sleep measures From polysomnography and the pittsburgh sleep quality index. SLEEP 2008;31(10):1339–1349.
Keywords: Menopause, endogenous hormones, FSH, estradiol, testosterone, sleep disturbance, sleep
THE MENOPAUSE IS A 4- TO 10-YEAR MULTIFACETED PROCESS OCCURRING IN WOMEN AT MID-LIFE AND IS ASSOCIATED WITH NUMEROUS FACTORS THAT might trigger or exacerbate sleep disturbances. Attributes of the menopausal transition and its hormone changes that may confer risk for sleep disturbances, beyond the effects of age alone, include: (1) increasing disruption in the chronobiological rhythms synchronized through the actions of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH); (2) changing ovarian hormone levels (especially estradiol) which influence norepinephrine turnover which is associated with REM sleep; (3) increasing disruption of thermoregulatory processes leading to the onset and exacerbation of vasomotor symptoms (hot flashes and night sweats of varying frequency and intensity); and, (4) increases in symptoms of depression, anxiety and stress.1
Primary evidence for a menopause-sleep relationship in women comes from cross-sectional surveys indicating that sleep complaints and insomnia increase during the peri- and postmenopausal periods2–6 as defined by marked changes in menstrual bleeding frequency or no menses for at least 12 months.7 In one of the few longitudinal studies, investigators followed women for 3 years and concluded that trouble falling asleep and earlier waking were associated with the menopause transition and physical health risk factors.8 The use of objective sleep measures to help disaggregate components of sleep that may become disrupted are uncommon in studies of the menopause transition from community-based samples. Using single-night polysomnography, the Wisconsin Sleep Study identified that postmenopausal women had deeper sleep and longer total sleep time than premenopausal women; however, peri-and postmenopausal women were less satisfied with their sleep, particularly sleep initiation.9
Studies relating sleep characteristics to the naturally-occurring changes in the gonadotropins and sex steroid hormones during the menopause transition are relatively sparse.1 In the Study of Women's Health Across the Nation (SWAN) cohort, higher follicular phase estradiol (E2) levels from annual blood sampling were associated with lower odds of self-reported trouble falling and staying asleep.10 Touzet et al.11 identified that urinary FSH levels collected in 3 stages of the menstrual cycle were greater in self-characterized long-time sleepers as compared to short-time sleepers in 106 normally-cycling women aged 19–44 years, after adjusting for age and BMI. Hollander et al.,12 in a study of 536 women aged 35–49 (half were African American and half Caucasian), reported that 17% of women had self-identified sleep disturbance. These investigators reported that low follicular phase plasma E2 (but not FSH), measured at baseline and at 8-month intervals for 2 years, was associated with poor self-reported sleep in women with regular menstrual cycles.12 In contrast, Clark et al.13 found neither self-reported menopausal status nor serum FSH measured in the luteal phase of the menstrual cycle was related to questionnaire-defined sleep disturbances in 23 women aged 40–54 years. Shaver et al.14 measured sleep characteristics polysomnographically is 82 women aged 40–59 years classified as pre-, peri-, or postmenopausal based on menstrual cycle regularity and FSH levels. While identifying no statistically significant polysomnographically defined sleep attributes according to menopause status, they reported that there tended to be lower measures of sleep efficiency in peri- and postmenopausal women with hot flashes compared to those without hot flashes. Thus, studies conducted to date indicate that the menopause transition is likely to be associated with disrupted sleep, but have not simultaneously included specific measures of both menopause and sleep in a large enough sample size to provide the depth of information necessary to more completely delineate the nature of this association.
This report relates selected objectively and subjectively measured sleep characteristics in 365 middle-aged women with endogenous levels of FSH, E2, or testosterone (T), or changes in these levels, over a 5- to 7-year time period while adjusting for important covariates including age, depressive symptoms, body size, and vasomotor symptom frequency. We related hormone values to measures of sleep architecture (delta [stages 3 and 4] sleep percent); sleep duration; and continuity, including total sleep time (TST) and wake time after sleep time (WASO) obtained during in-home polysomnography (PSG); and self-reported sleep quality. We hypothesized that a more rapid rate of change in FSH levels, indicative of accelerated time to the final menstrual period, would be associated with poorer sleep, objectively and subjectively measured. We further hypothesized that women with higher E2 levels in the time period immediately prior to the sleep assessment would have better objectively and subjectively measured sleep. Evaluating the relationship of testosterone and sleep measures was considered exploratory, as most studies of testosterone and sleep have been conducted in men, and animal studies indicate a strong gender differences in the sleep-hormone relationship.1
METHODS
Data for this report are from the Study of Women's Health Across the Nation (SWAN) Sleep Study, a comprehensive study of sleep (single sleep visit for 3 nights) nested within the ongoing longitudinal study and conducted at 4 of the 7 SWAN clinical sites during 2003–2005, across a time frame that overlaps the 5th, 6th, and 7th annual Core SWAN protocol examinations.
SWAN Study Design and Participants
SWAN is a multiethnic, community-based, multisite cohort study of the menopausal transition. A total of 3,302 women were enrolled for longitudinal evaluation from a community based survey conducted at 7 sites: Boston, MA, Chicago, IL, Detroit area, MI, Los Angeles and Oakland, CA, Newark, NJ, and Pittsburgh, PA. Sites used a number of techniques to identify representative samples from the communities targeted for recruitment which have been described.15 These techniques included use of community residential censuses, random digit dialing, and other listings including published telephone directories, voting records, and membership records of a Health Maintenance Organization. Each site recruited a sample of Caucasian and minority women. Women were Caucasian (all sites), African American (Boston, Chicago, Detroit area, Pittsburgh), Chinese (Oakland), Japanese (Los Angeles), and Hispanic (Newark), and aged 42–52 years at their initial screening. Additional baseline (in 1996) SWAN eligibility criteria were: not pregnant, not using exogenous hormones in the 3 months prior to interview, premenopausal or early perimenopausal with an intact uterus and at least one ovary, and at least one menstrual period in the 3 months prior to baseline interview. Each site's institutional review board approved the study, and all women gave written informed consent to participate.
At annual visits beginning in 1996, Core SWAN enrollees provided detailed data on menstrual cycles, vasomotor and other symptoms, and additional factors that might be related to menopause and sleep. At selected annual Core SWAN evaluations, 4 questions were asked about sleep characteristics, and at 2 annual evaluations, a standard measure of sleep quality (Pittsburgh Sleep Quality Index, PSQI) was included, but more detailed measures to characterize sleep architecture, duration, or continuity were not included. Annual examinations included phlebotomy with specimens being assayed for E2, T, and FSH.
SWAN Sleep Study Design and Participants
The SWAN Sleep Study (Sleep I) was a cross-sectional study of sleep patterns being conducted at 4 of 7 SWAN study sites. During 2003–5, investigators at SWAN sites located in Chicago, the Detroit area, Oakland, and Pittsburgh collaborated to develop a more comprehensive evaluation of sleep. There were 370 women between the ages of 48 and 59 years evaluated, including 328 pre- and peri-menopausal and 42 postmenopausal Caucasian, African American, and Chinese women. Data collected at the annual SWAN Core visits from 1996 to the time of women's sleep study were used to relate the amount and rate of change annually in endogenous hormone values from 1996 to subsequent measures of sleep duration, continuity, architecture, and quality from the Sleep Study.
Eligibility for the Sleep Study was determined prior to and during participants' annual Core SWAN assessment. Inclusion criteria were based primarily on the presence of sufficient data to characterize menopausal status. Efforts were focused on recruiting women who were pre- and peri-menopausal as defined by the SWAN Core Study while excluding those women with surgical menopause (< 1%) or using hormone therapy (approximately 23% of the cohort). Exclusion criteria also included factors that could affect sleep, including ongoing treatment for cancer or rotating or night shift employment (exclusion rates for these measures were between 1% and 3%). Among eligible participants at the 4 sites, 30% declined to participate in the Sleep Study; the most commonly cited reasons included “protocol burden,” “too busy,” and “family obligations.” Sleep Study participants did not differ markedly from Core SWAN participants with regard to age, self-assessed sleep quality, ethnicity, self-reported health status, depressive symptoms, hypertension, or diabetes. Sleep Study participants tended to have slightly higher body mass index (BMI) than those who did not participate.
Sleep Study Protocol
The Sleep Study protocol sleep measures included subjective sleep symptoms, sleep diaries, wrist actigraphy, and polysomnography (PSG). In-home PSG studies were conducted on the first 3 nights of the protocol, while sleep diary and actigraphy data were collected in all days (and nights) of the protocol, which was comprised of an entire menstrual cycle or 35 days, whichever was shorter. This extended data collection allowed the simultaneous capture of sleep patterns and vasomotor symptoms from diaries with progression across the same menstrual cycle.
Sleep Measures
The sleep protocol was initiated within 7 days of the beginning of the follicular phase of the menstrual cycle in women who were still menstruating. PSG studies were conducted in a woman's home during the first 3 consecutive days following protocol initiation, using the Vitaport 3 (VP3) PSG monitor (Temec, Netherlands). The PSG montage included 2 channels (C4/A1, C3/A2) of electroencephalographic (EEG) recording, bilateral electro-oculograms (EOG), bipolar submental electromyograms (EMG), and one channel of electrocardiogram (EKG) recording. In addition, sleep disordered breathing and periodic leg movement activity were evaluated on the first night of the protocol. Sleep EEG data for visual scoring of sleep continuity and staging were collected on nights 2 and 3. Sleep disordered breathing was assessed using nasal pressure and oral-nasal thermistors to measure airflow, impedance plethysmography to measure chest and abdominal wall movements, and fingertip oximetry (Nonin X-pod model 3012) to measure oxygen saturation. Criteria for identifying apneas, hypopneas, and flow-limited events were based on current guidelines recommended by the American Academy of Sleep Medicine.16 Bilateral anterior tibialis EMG was used to assess repetitive movements that occurred at fixed intervals of time during sleep (i.e., periodic leg movements, PLM). Wrist motion (data not reported) was assessed with the Actiwatch-64 (AW-64) (MiniMitter, Respironics, Bend, OR).
Electronic data were sent to the University of Pittsburgh study site for processing, evaluation of quality, and scoring. A validation study to assess reproducibility as well as inter- and intra-rater reliability among 8 scorers was completed and only those scores from 6 technicians who demonstrated technical consistency and reproducibility were incorporated into the data base. In the reliability study, intraclass correlations ranged from 0.78 for REM sleep to 0.96 for wakefulness after sleep onset (WASO).
The summary sleep measures reported here were calculated from nights 2 and 3 of study. Sleep was visually scored in 20-sec epochs, supplemented by power spectral analysis of the EEG. Measures of sleep duration included time in bed and TST. Time in bed was calculated as time from reported lights out (with confirmation of PSG signals consistent with reduced activity) to time of reported awakening from sleep (and confirmation of PSG signals consistent with increased activity). TST was calculated as total minutes scored as stages 1 to 4 and REM sleep. Sleep continuity was quantified by measures of sleep latency (SL [time from beginning of the recording period to the first consecutive 10 min of stage 2 or stage 3–4 sleep interrupted by no more than 2 min of stage 1 or wakefulness]); wakefulness after sleep onset (WASO [total minutes of wakefulness between sleep onset and good morning time]), and sleep efficiency (SE [time spent asleep/time in bed × 100]). Measures of sleep architecture included percent of time spent asleep spent in NREM stages 1, 2, and 3 + 4, and REM sleep.
The Pittsburgh Sleep Quality Index (PSQI) was based on a 19-item, self-report questionnaire used to evaluate habitual sleep quality.17 The global score ranges from of 0 to 21 with high scores indicating poor sleep quality; scores > 5 suggest clinically significant sleep complaints.17 The PSQI has good internal consistency (Cronbach α = 0.83 for component scores); good test-retest reliability (r = 0.85 for the global score); and, sensitivity of 88.5% for distinguishing clinically referred patients from healthy control participants.
Hormone Assays
Serum hormone levels from the SWAN Core data were used to characterize the endogenous levels closest to, but preceding the Sleep Study evaluation. Additional blood was not drawn as a part of the Sleep Study protocol. The annual endogenous hormone measures values from the SWAN Core visits (baseline to follow-up visit 07) were used to develop trajectories or patterns of change up to the time of the Sleep Study evaluation.
At baseline and annual Core SWAN visits, fasting blood specimens were drawn from participants timed to days 2 to 5 of the follicular phase of their menstrual cycles and a variable representing this timing was included in statistical modeling as a 3-level categorical covariate: day 2–5, not day 2–5, unknown. If a timed specimen could not be obtained after 2 attempts to achieve the day 2–5 sampling, a random fasting specimen was taken within 90 days of the annual assessment. Blood was refrigerated about 1 hour after phlebotomy, centrifuged, and the serum aliquoted, frozen at −80°C, and shipped on dry ice to the University of Michigan CLASS Endocrine Laboratory. Specimens were assayed continuously upon arrival using an ACS-180 automated analyzer (Bayer Diagnostics Corp., Norwood, Massachusetts).
Serum FSH concentrations were singular measures from a 2-site chemiluminescence immunoassay to the β subunit. Inter- and intra-assay coefficients of variation were 12.0% and 6.0%, respectively, and the lower limit of detection was 1.1 IU/L. The absolute concentrations of FSH are higher in this assay than values from many clinical laboratories, based on differences in the standards selected.
Estradiol (E2) was measured using a modified, off-line assay in duplicate on an ACS-180 automated analyzer. Inter- and intra-assay coefficients of variation averaged 10.6% and 6.4%, respectively, over the assay range; the lower limit of detection was 1 pg/mL.
Serum total testosterone (T) concentrations were determined by competitive binding of a 2-dimethylacridinuimester (DMAE)-labeled T derivative to a rabbit polyclonal anti-testosterone antibody premixed with monoclonal anti-rabbit IgG antibody immobilized on solid phase paramagnetic particles. Inter- and intra-assay coefficients of variation were 10.5% and 8.5%, respectively, with a lower limit of detection of 2 ng/dL.
A ratio of E2 to total T values was used as a measure of the disequilibrium in the sex steroid status with the menopausal transition.
Menopausal Status and Vasomotor Symptoms
Using bleeding criteria to determine menopause transition status in annual Core SWAN visits, participants were classified into one of the following 4 categories: premenopausal (no change in menstrual bleeding regularity), early perimenopausal (menses in the preceding 3 months with an increase in bleeding irregularity), late perimenopausal (menses in the previous 12 months, but not the previous 3 months, and postmenopausal (≥ 12 months of amenorrhea).18–20
The frequency of vasomotor symptoms per 24-h period in the 14 days commencing with the polysomnographic studies were identified from the daily sleep diaries.
Sociodemographic Information
Sociodemographic data were collected at the annual Core SWAN assessments. Race/ethnicity was determined by self-identification as African American, Caucasian, or Chinese. Other sociodemographic variables included age (continuous variable); marital status (single/never married, married or living as married, separated/widowed/divorced) and educational level (high school graduate or less, some college, college graduate, graduate studies). A 3-level response to a question about difficulty in paying for basics (very, somewhat, or not very difficult) including food, shelter, and health care was used as an indicator of economic strain. Study site designation was included in statistical models.
Physical and Mental Health Variables
Self-perceived overall health was coded as excellent, very good/good, fair/poor. Body mass index (BMI) was computed as weight in kilograms/height in meters squared. Depressive symptoms were assessed at annual Core SWAN visits with the Center for Epidemiologic Studies Depression (CES-D) Scale.21 Data were used as a continuous measure (20-item scale, each item scored 0–3, reflecting the frequency of experiencing the item in the previous week; range 0–60). Depressive symptoms were also evaluated concurrently with the Sleep Study with the 16-item Inventory of Depressive Symptoms (IDS).22 Data analysis with the IDS was based on scores in which the contribution from the 4 sleep-related items had been removed.
Daily medication use (prescription and over-the-counter), recorded at Sleep Study protocol inception and on daily diaries, was coded according to the World Health Organization Anatomical Therapeutic Chemical (ATC) classification.23 For study purposes, medications that could potentially affect sleep parameters were those products associated with the following ATC classification codes: N02A, N03A, N05B, N05C, N06A, and R06A.
Health Behavior Variables
Responses about smoking frequency, alcohol consumption, and caffeine consumption were determined from the first 14 days of the daily sleep diaries. Current smokers were those who reported smoking ≥ 7 cigarettes in the 2-week period initiated by the sleep protocol. Physical activity was measured at the Core annual SWAN visit by a composite score based on the Kaiser Permanente Activity Score,24 a modification of the Baecke scale,25 assessing 3 domains (sports, leisure, and household activities); it was treated as a continuous variable.
Data Analysis
The analytic sample included 365 women with PSG data and Core SWAN data points from annual follow-up assessments (a total of 2757 annual hormone observations). Univariate statistics were computed for continuous variables and frequencies were determined for categorical variables. Variables with highly skewed distributions were transformed or categorized. Attribution of statistical significance was based on P-values from 2-sided tests at a value of P < 0.05; P-values between 0.06 and 0.09 were identified as a trend toward statistical significance.
Three aspects of serum hormone concentrations were examined in relation to the sleep measures. These included: (1) concurrent hormone concentrations measured at the Core SWAN visit that temporally preceded the sleep visit; (2) baseline hormone levels; (3) slopes or rates of change per unit of time. A 2-stage modeling approach was used to evaluate hormone change over time in relation to the sleep measures. In the first stage, each woman's annual measures of hormone concentrations or their indices were decomposed into a subject-specific intercept and slope (with linear and quadratic terms). In the second stage, these explanatory variables, along with covariates and interaction terms specified a priori, were incorporated as independent variables into mixed models to be related to sleep characteristics.26 The appropriateness of model fitting was assessed both graphically and using residual analyses. The first-order autoregressive (AR(1)) correlation specification was used to model covariance structures. A spatial covariance structure (sp(pow)(night)) was used to capture repeated effects of 2 nights' sleep measures with correlation decreasing over time between 2 study nights (12 women had non-consecutive nights).
We evaluated models that included both a hormone value and a variable representing menopause status (based on variability in menstrual bleeding). Covariates retained in all models included chronological age, clinical site, difficulty in paying for basics, blood draw time, and BMI. Covariates considered included CES-D and the modified IDS (though not simultaneously in the same model) to consider the impact of depressive symptoms as well as measures of vasomotor symptoms (hot flashes, night sweats, and cold sweats). The following covariates were retained in the final models only if they were statistically significant at the P < 0.05 level: education, race/ethnicity, menopausal status, marital status, overall health status, daily hormone usage, smoking, alcohol and caffeine consumption, and physical activity score.
For purposes of more intuitive presentation, when statistically significant associations were identified, the hormone associations were expressed as categorical variables, usually tertiles or quartiles, and the relationship shown using bar graphs. However, the statistically significant models, assumptions and regression β coefficients are shown in Appendix 1. SAS 9.1 and Macro facilities (SAS Institute, Cary, NC) were used to perform the statistical analyses and plot the findings.
RESULTS
The racial/ethnic composition of the sample was 47% Caucasian, 37% African American, and 16% Chinese (Table 1). Two-thirds of participants were married or living with a partner (62%), and 82% had at least some college education. Only 4% of women classified themselves as having substantial difficulty in paying for basics, while 22% of women identified that they had some difficulty in paying for basics. On average and by Sleep Study I design, 67% of women were classified as premenopausal/early perimenopausal, 21% as late perimenopausal, and 11% as postmenopausal. The median BMI was 28 kg/m2.
Table 1.
SWAN Sleep Study: Number and Frequency (%) of Demographic, Menopause, Health, and Behavioral Characteristics
| N (Percent) | |
|---|---|
| Demographic Data | |
| Site | |
| Michigan | 69 (19%) |
| Chicago | 89 (24%) |
| Oakland | 110 (30%) |
| Pittsburgh | 97 (27%) |
| Race/Ethnicity | |
| Caucasian | 170 (47%) |
| African American | 136 (37%) |
| Chinese | 59 (16%) |
| Marital Status | |
| Married | 225 (62%) |
| Not married | 133 (36%) |
| Missing | 7 (2%) |
| Educational Attainment | |
| ≤ High school | 61 (17%) |
| Some college | 115 (32%) |
| ≥ Baccalaureate degree | 184 (50%) |
| Missing | 5 (1%) |
| Difficulty in Paying for Basics | |
| Very hard | 15 (4%) |
| Somewhat hard | 79 (22%) |
| Not hard at all | 261 (72%) |
| Missing | 10 (2%) |
| Menopause and Health Data | |
| Menopausal Status | |
| Pre/Early Perimenopausal | 246 (67%) |
| Late Perimenopausal | 78 (21%) |
| Postmenopausal | 41 (11%) |
| Hot flashes during days 1–4 of sleep diary | |
| Any | 225 (70%) |
| None | 110 (30%) |
| Depressive symptoms (CES-D) ≥16 | |
| Yes | 50 (14%) |
| No | 310 (85%) |
| Missing | 5 (1%) |
| Behavorial Data | |
| Caffeinated drinks, days 1–14 of sleep diary | |
| Yes | 255 (70%) |
| No | 110 (30%) |
| Alcoholic drinks, days 1–14 of sleep diary | |
| Yes | 38 (10%) |
| No | 327 (90%) |
| Smoked cigarettes, days 1–14 of sleep diary | |
| Yes | 39 (11%) |
| No | 326 (89%) |
In these women with a median age of 52 years, the median time asleep was 387.3 minutes (almost 6.5 h) with a median of 40 min of wakefulness following sleep onset (Table 2). The median sleep efficiency was 86.6%. The median PSQI score was 6 and the median apnea-hypopnea index (AHI) was 5/h of sleep.
Table 2.
Descriptive Statistics of Sleep Measures and Continuous Covariates
| Median (IQR) | |
|---|---|
| Age (years) | 52 (3) |
| BMI (body mass index) kg/m2 | 28.1 (10.9) |
| Total Physical Activity Score (without work activity) | 7.85 (2.45) |
| IDS-Inventory of Depressive Symptomatology defined depression score on Day 04 (without sleep items) from the Sleep Study | 7 (3) |
| Sleep measures * | |
| TST - Total time asleep (min) † | 387.3 (87. 3) |
| WASO - Wakefulness after sleep onset (min)‡ | 40.3 (36.3) |
| SE - Sleep efficiency (%)§ | 86.6 (9.4) |
| Delta (stage 3 + 4) sleep (%)‖ | 1.71 (4.74) |
| Pittsburgh Sleep Quality Index(PSQI) from interview | 6 (3) |
| Sleep related events | |
| Apnea/hypopnea Index (episodes/hour of sleep) | 5.0 (10.5) |
| PLM - Periodic leg movement index | 2.4 (4.5) |
| Hormone measures from the Core SWAN Study | |
| Baseline hormone levels | |
| FSH – Follicle stimulating hormone (mIU/mL) | 13.4 (8.1) |
| E2 - Estradiol (pg/mL) | 56.1 (42.2) |
| T - Testosterone (ng/mL) | 40.9 (24.5) |
| Core SWAN hormone values closest to and preceding the sleep study | |
| FSH – Follicle stimulating hormone (mIU/mL) | 34.9 (62.1) |
| E2 - Estradiol (pg/mL) | 31.1 (55.5) |
| T - Testosterone (ng/mL) | 36.8 (25.4) |
Sleep measures are from nights 2 and 3 of the Sleep Study Protocol;
Times spent asleep = total recording period minus (sleep latency + wakefulness after sleep onset);
Wakefulness after sleep onset = total number of minutes scored as awake, following sleep onset;
Sleep efficiency = (time spent asleep/total recording period)*100;
Stage 3+4 (delta) NREM sleep = (total number of minutes scored as stage 3 or 4 NREM sleep/time spent asleep)*100
Associations with FSH
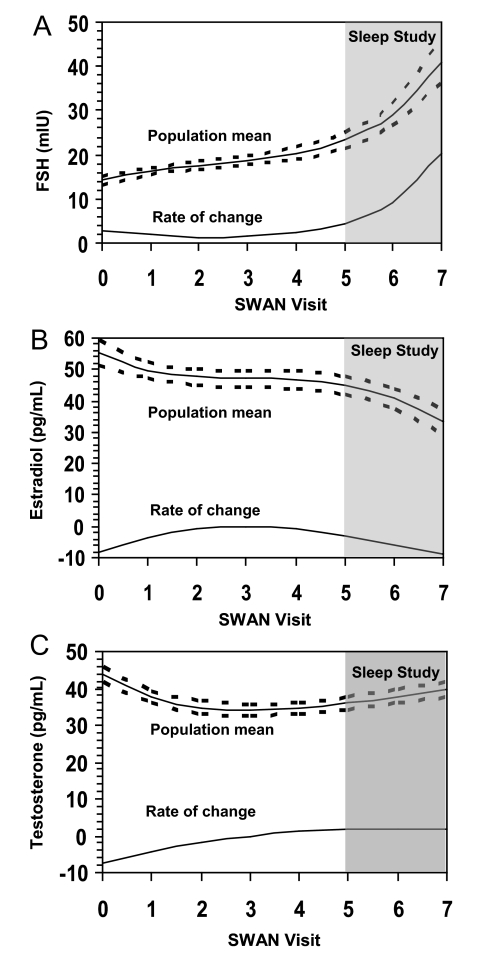
The median (IQR) FSH level was 13.4 mIU/mL, which is consistent with FSH values seen in women who are premenopausal and early peri-menopausal in the overall SWAN study.27 The changing population mean FSH over the time period preceding the Sleep Study, as well as the rate of change, in reference to the time in which the Sleep Study was implemented (shaded area) are displayed in Figure 1A. The mean value of FSH increased over the 7-year period, and the rate of change more than doubled by the 7th year of study.
Figure 1.
Mean hormone profiles of the Sleep Study population over time with corresponding 95% confidence intervals (dashes) for (A) follicle stimulating hormone (FSH), (B) estradiol (E2), and (C) testosterone (T), and their corresponding instantaneous rates of change.
The higher FSH levels measured at the Core visit preceding the Sleep Study were associated with greater WASO and higher AHI. However, these associations between FSH and the sleep measures were no longer statistically significant following adjustment for covariates including age, race/ethnicity and body size. FSH values measured at baseline were negatively related to PSQI-defined sleep quality and to sleep efficiency (P < 0.05) before and after adjusting for covariates.
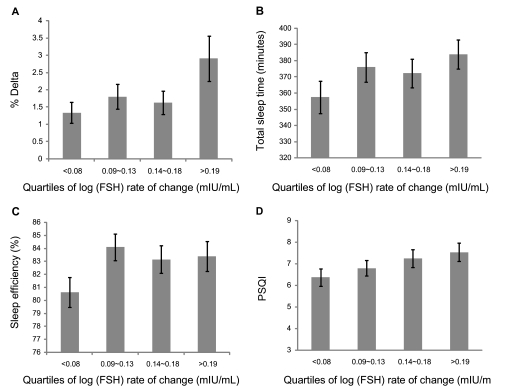
A greater rate of FSH change from the baseline value to the time of the Sleep Study was significantly associated with higher delta sleep percent, longer TST, and more subjective sleep complaints as measured by PSQI (Figure 2A-D). Sleep efficiency was significantly lower in those who had a slower FSH change. These results remained statistically significant following adjustment for covariates (data not shown).
Figure 2.
Association of follicle stimulating hormone (FSH) (mIU/mL) rates of change with (A) % delta sleep; (B) total sleep time; (C) sleep efficiency %; and, (D) Pittsburgh Sleep Quality Index (PSQI) score. Error bars, ± SEM.
The use of an FSH cutpoint of 40 mIU/mL, a frequently espoused value to delineate the transition to postmenopause, was not associated with sleep measures being evaluated.
Associations with Estradiol
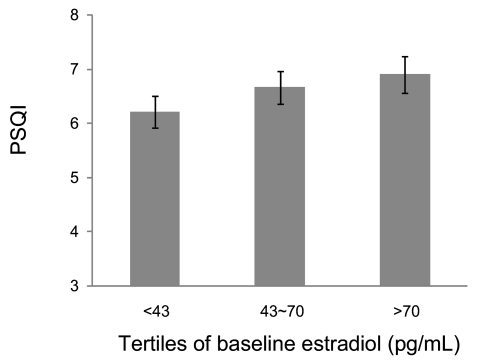
The median estradiol (E2) concentration from blood collected at the visit preceeding the Sleep Study, 56 pg/mL, was consistent with E2 levels observed in days 2–5 of the early follicular phase in pre- and early perimenopausal women of the Core SWAN Study.27 Although estradiol levels declined over the 7-year period, the rate of change was much slower than the observed FSH changes, as shown in Figure 1B. Higher baseline E2 levels were related to higher PSQI score from the Sleep Study, but not to the measures of percent delta sleep or measures of sleep pathology. These findings indicate that women at baseline with a higher E2 reported a slightly poorer sleep quality 7 years later (see Figure 3). There were no other statistically significant associations of E2 values from the Core SWAN evaluation preceding the Sleep Study or with E2 change with any of the sleep measures.
Figure 3.
Association of baseline estradiol (E2) (pg/mL) levels with Pittsburgh Sleep Quality Index (PSQI) score. Error bars, ± SEM.
Associations with Testosterone or the E2/T Ratio
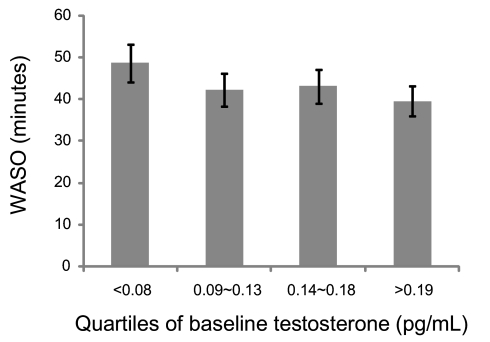
Relatively limited change in T occurred across the visits preceding the Sleep Study (see Figure 1C). Lower baseline levels of T were modestly, but significantly, associated with greater WASO before and after adjusting for covariates. On average, women in the lower baseline quartile of total T values had about 10 more min of WASO than women in the highest quartile (Figure 4).
Figure 4.
Association of baseline total testosterone (pg/mL) levels with wake time after sleep onset (WASO). Error bars, ± SEM.
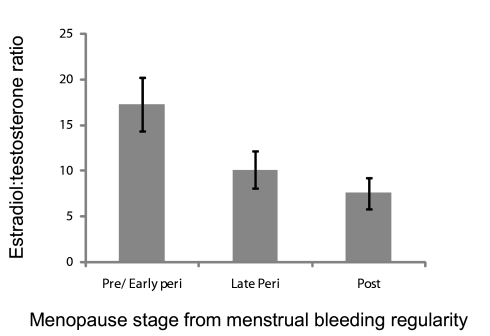
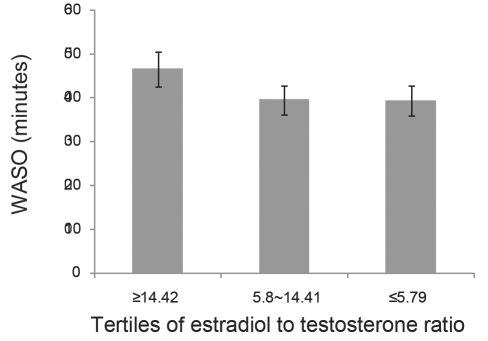
The E2/T ratio was reduced progressively across the menopause transition stages, as shown in Figure 5. This is interpreted as marking the ensuing increasing androgenic environment with the menopause transition. A lower E2/T preceding the Sleep Study was significantly associated with less WASO (Figure 6).
Figure 5.
Estradiol-to-testosterone (E2:T) ratio showing progression of the transition and increasing androgenicity according to menopause status, defined by regularity of menstrual bleeding patterns. Error bars, ± SEM.
Figure 6.
Association of estradiol-to-testosterone (E2:T) ratio (from Core SWAN study preceding the Sleep study) in relation to wake time after sleep onset (WASO) measured in the Sleep Study. Error bars, ± SEM.
No associations of the sleep measures with menopause status were observed in models that included FSH, E2, or T before or after adjusting for other covariates.
DISCUSSION
Studies relating the longitudinal changes in the sex steroid hormones and gonadotropins to measures of sleep patterns, particularly measures of sleep duration, continuity, and architecture are rare. There have been studies of gonadotropin concentrations (FSH and luteinizing hormone) and sleep in men, precocious puberty, and in disease states, but such studies of the normal menopausal transition are particularly infrequent despite the frequency of sleep complaints during the menopause transition.1,6,11 Changes in gonadotropins, such as FSH, typically precede changes in those indices of menopause based on defining menstrual bleeding regularity; therefore, evaluating these changes in greater detail could contribute to a greater understanding of specific sleep patterns among women during midlife.
Stronger associations of sleep measures were identified with FSH than E2. Further, sleep measures were associated with rate of FSH change over the 7 years prior to the Sleep Study rather than with baseline FSH levels or those FSH levels measured 3–6 months prior to the Sleep Study data collection. Specifically, it was the faster rate of change in FSH that was associated with PSG-assessed slow wave sleep and sleep duration, even though many women were still classified by menstrual bleeding criteria as being pre- and early perimenopausal. This indicates that as women transitioned more rapidly from an endocrine perspective, they slept longer. Simultaneously, however, we found greater FSH change was associated with poorer self-reported sleep quality. This apparent contradiction has parallels with 2 other studies. A cross-sectional study of premenopausal women indicated that higher levels of urinary FSH collected in 1 to 4 menstrual cycles were associated with self-reported longer sleep duration.11 Polysomnographic studies from the Wisconsin Sleep Study, which did not include hormone measures, identified that postmenopausal women had deeper sleep and longer total sleep time than premenopausal women; however, like SWAN Sleep Study enrollees, the peri-and postmenopausal women were less satisfied with their sleep.9
The reporting of these associations of sleep with greater FSH change should not be interpreted as intimating a direct causal effect, in spite of their robustness following adjustment for covariates. Rather FSH change can be considered an indicator for the dynamic re-equilibration of hormones during the transition that can influence chronobiological rhythms, which are synchronized through the actions of the gonadotropins, LH and FSH.1 It is recognized that FSH changes are associated with fluctuating estradiol levels that may influence neurotransmission and that FSH changes are associated with the thermoregulatory events that affect the onset and exacerbation of vasomotor symptoms.1 Further, the associations with the sleep measures are with the FSH rate of change, not the absolute FSH levels, suggesting the potential for re-equilibration to occur much more rapidly in some women than others. Though we have multiple annual measures of FSH, these do not include measures of pulsatility magnitude, or frequency from these days which have been associated with sleep variations in the follicular and luteal phases of the menstrual cycle.28
In addition to FSH rate of change, we also identified that lower E2/T ratios (seen in later stages of the transition) immediately preceding the Sleep Study assessment was associated with less WASO. The E2/T ratio is a reasonable marker of the degree of progression through the perimenopausal hormone environment and reflects the increasingly greater androgenic nature of this period.
Our data suggest that selected aspects of sleep in women may also be sensitive to variations in testosterone levels, and understanding these relationships may be important in understanding sleep patterns. We observed that testosterone in the highest quartile was associated with less WASO, compared to women in the lowest total T quartile, in spite of adjustment for multiple covariates. This is consistent with our findings relative to the E2/T ratio. Whereas there is an extensive literature that describes relatively specific and discrete effects of testosterone on sleep in men, the literature that describes effects of T in women is quite sparse and inconsistent in those sleep characteristics that have been addressed. Investigators from the Seattle Midlife Women's Health Study reported no association of urinary T excretion with interview-based studies of day and nighttime awakening from 3 days of diaries in the early follicular phase (completed by 41 women).29
We identified that a higher baseline E2 level, but not its change over time, were associated with slightly more impaired sleep quality. Objective measures of sleep duration, continuity, and architecture were unrelated to E2 levels or rate of change. These findings are inconsistent with those of Hollander et al.,12 who examined a self-reported measure of sleep quality in women of late reproductive age (37–49 years at follow-up). Several attributes of estradiol levels across the transition period may influence associations with E2, explaining these inconsistent findings. The E2 decline is not necessarily monotonic and a sporadic rise in E2 levels prior to the final menstrual period has been reported.30 The major estradiol change frequently occurs within a relatively short time of the final menstrual period,31 a time period more likely to be captured in women of the SWAN cohort. Importantly, the diminished estradiol levels frequently associated with the menopause transition occur approximately 2 years prior to the final menstrual period (FMP),32 while changes in FSH levels frequently occur more than 5 years prior to the FMP.33
Excluding hormone therapy (HT) users from this study was advantageous in addressing questions about the influence of endogenous hormones on sleep characteristics because HT use would obviously confound the hormone results. However, it is not clear what the effect of including HT users in the might have had on the results. It is possible that women who were experiencing more severe symptoms began hormone use, and thus, were excluded from the study. But hormone use represents a mix of characteristics thought to influence sleep. In the Michigan Bone Health and Metabolism Study, women who developed more bothersome hot flashes were more likely to use HT use, but less likely to be obese.34 Further, in the full sample of SWAN women, women who ever used HT during the 6 years of follow-up more often reported at baseline having hot flashes, night sweats, and vaginal dryness.35 However, women who reported worse role emotional and physical functioning at a given visit during the follow-up period were less likely to initiate HT use at the next visit than women who reported better functioning. Furthermore, reports of bodily pain did not lead women subsequently to initiate HT use.
Some studies provide evidence that HT can improve some, but not all, aspects of sleep quality in postmenopausal women, and HT has been proposed for therapeutic use in the menopause for sleep related breathing disorders.36–38 Care must be taken in extrapolating from these therapeutic studies to the endogenous hormone environment and sleep performance. For example, in studies of the menstrual cycle, it has been reported that women taking oral contraceptives had more stage 2 NREM sleep in the active phase compared to their own placebo phase and to naturally cycling women. The naturally cycling women, however, had slow wave sleep in the luteal phase comparable to women using contraceptives. This suggests exogenous reproductive steroids influence sleep differently than endogenous progesterone and estrogen.39 By design, women using exogenous hormones were excluded from enrollment in the Sleep Study.
We chose to adjust for depressive symptoms and the presence of hot flashes. In these analyses, the associations of the hormones were independent of associations with measures of depressive symptoms. Further, no consistent associations were observed with the report of hot flashes or night sweats.
This study has a number of strengths. The study design allowed the long-term characterization of sex steroid hormone profiles over a 5 to 7-year period preceding a sleep study profile to better describe the physiological changes occurring in the menopause transition that may affect sleep. The Sleep Study protocol included sleep measures estimated from polysomnographic studies conducted in a large sample of women from 3 racial/ethnic groups. Further, these studies were conducted in the homes of participants to try to minimize the modification of sleep patterns arising from trying to sleep in an atypical or institutional environment. We focused on data from the second and third night of the polysomnographic studies to minimize the effect of the “first night effect.”
Nonetheless, there are also limitations. While hormone profiles were longitudinal, the sleep measures represented a single window in the menopause transition. Currently, efforts are underway to complete longitudinal measures of sleep in this cohort in Sleep Study II. In timing the acquisition of the detailed PSG sleep measures to the early follicular phase of the menstrual cycle, we may have excluded hormone-sleep profiles that are more frequently expressed in the ovulatory or luteal phases of the menstrual cycle and which may differ than follicular phase profiles. We will subsequently address measures of sleep from follicular phase sleep measures versus measures from the luteal phase using information acquired through sleep diaries and actigraphy. Further, the design of the current study did not allow us to address the short-time GnRH or gonadotropin pulsatility that had been the primary focus in understanding sleep patterns in relation to circadian rhythms.
In summary, we observed that the 2 most prominent measures of sex steroid hormones that predicted subsequent sleep characteristics in women at mid-life were the rate of change in FSH and T. More rapid FSH change was associated with higher delta sleep percent, longer TST, but more subjective sleep quality dissatisfaction. Higher total T and the decreasing E2/T ratio, a hormone-based measure of the increasing androgenism of the menopause transition, was associated with less WASO. We conclude that during the menopause transition period, more rapid rate of FSH change was associated with measures of sleep architecture, continuity, and duration, but also with more frequent sleep complaints. Women with higher T and who are moving toward completion of the transition process, as reflected by the decreasing E2/T ratio, had more sleep consolidation. Our results suggest the value of having measures of the rapidity of the menopause transition in addition to objective measures of sleep in women who report sleep dissatisfaction. Clinicians whose patients include women with rapid transitions may want to consider interventions (therapeutic and/or lifestyle) that address menopausal symptomatology, if any, rather than being limited to interventions that target only sleep characteristics.
DISCLOSURE STATEMENTS
This was not an industry supported study. Dr. Consens is on the advisory board for GlaxoSmithKline. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Institutions where work was performed:
Rush University Medical Center, Chicago, IL
University of Michigan, Ann Arbor, MI
University of California-Davis, Davis, CA
University of Pittsburgh, Pittsburgh, PA
Funding for the SWAN Sleep Study is from the National Institute on Aging (Grants AG019360, AG019361, AG019362, AG019363). The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, DHHS, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women's Health (Grants NR004061, AG17104, AG017719, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495, AG17104). Sleep data were processed with the support of RR024153.
Appendix 1
Hormone Profiles from Baseline to Sleep Initiation
| Let t = 0, 1,2,3,…,7 be the number of years since the baseline. By fitting the mixed model using AR(1), the mean population measures of logFSH at time t was given by: | |
| logFSH (at time t) = 2.6467*** + 0.0948***t − 0.0084(t − 3)t + 0.0057***(t − 3)2t | (B1) |
| The mean population rate of change of logFSH at time t was given by: | |
| Rate of change of logFSH (at time t) = (2.6467 + 0.0948t − 0.0084[t − 3]t + 0.0057[t − 3]2t) = 0.0171t2 − 0.0852t + 0.1713 | (B2) |
| The mean population measures of logE2 at time t was given by: | |
| logE2 (at time t) = 4.0166*** − 0.0546**t + 0.0173*(t − 3)t − 0.0055**(t − 3)2t | (B3) |
| The mean population rate of change of logE2 at time t was given by: | |
| Rate of change of logE2 (at time t) = (4.0166 − 0.0546t + 0.0173[t − 3]t − 0.0055[t − 3]2t) = −0.0164t2 + 0.0999t − 0.1554 | (B4) |
| The mean population measures of sqrt(T) at time t was given by: | |
| sqrt(T) (at time t) = 6.6089*** − 0.2612***t + 0.0842***(t − 3)t − 0.0075**(t − 3)2t | (B5) |
| The mean population rate of change of sqrt(T) at time t was given by: | |
| Rate of change of sqrt(T) (at time t) = (6.6089 − 0.2612t + 0.0842[t − 3]t − 0.0075[t − 3]2t = −0.0225t2 + 0.2584t − 0.5814 | (B6) |
Note: ***P-value < 0.0001; **P-value < 0.001; *P-value < 0.03
REFERENCES
- 1.Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22:540–55. [PubMed] [Google Scholar]
- 2.Ballinger CB. Subjective sleep disturbance at the menopause. J Psychosom Res. 1976;20:509–13. doi: 10.1016/0022-3999(76)90015-5. [DOI] [PubMed] [Google Scholar]
- 3.Lee KA, Taylor DL. Is there a generic midlife woman? The health and symptom experience of employed midlife women. Menopause. 1996;3:154–64. Editorial. [Google Scholar]
- 4.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Shaver JL, Zenk SN. Sleep disturbance in menopause. J Womens Health Gend Based Med. 2000;9:109–18. doi: 10.1089/152460900318605. [DOI] [PubMed] [Google Scholar]
- 6.Parry BL, Fernando Martínez L, Maurer EL, López AM, Sorenson D, Meliska CJ. Sleep, rhythms and women's mood. Part II. Menopause. Sleep Med Rev. 2006;10:197–208. doi: 10.1016/j.smrv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.McKinlay SM, Jefferys M. The menopausal syndrome. Br J Prev Soc Med. 1974;28:108–15. doi: 10.1136/jech.28.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas. 1998;30:41–50. doi: 10.1016/s0378-5122(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 9.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–72. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 10.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- 11.Touzet S, Rabilloud M, Boehringer H, Barranco E, Ecochard R. Relationship between sleep and secretion of gonadotropin and ovarian hormones in women with normal cycles. Fertil Steril. 2002;77:738–44. doi: 10.1016/s0015-0282(01)03254-x. [DOI] [PubMed] [Google Scholar]
- 12.Hollander LE, Freeman EW, Sammel MD, Berlin JA, Grisso JA, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001;98:391–7. doi: 10.1016/s0029-7844(01)01485-5. [DOI] [PubMed] [Google Scholar]
- 13.Clark AJ, Flowers J, Boots L, Shettar S. Sleep disturbance in mid-life women. J Adv Nurs. 1995;22:562–8. doi: 10.1046/j.1365-2648.1995.22030562.x. [DOI] [PubMed] [Google Scholar]
- 14.Shaver J, Giblin E, Lentz M, Lee K. Sleep patterns and stability in perimenopausal women. Sleep. 1988;11:556–61. doi: 10.1093/sleep/11.6.556. [DOI] [PubMed] [Google Scholar]
- 15.Sowers MF, Crawford S, Sternfeld B, et al. Design, survey sampling and recruitment methods of SWAN: A multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Wren J, Lobo RA, Kelsey J, Marcus R, editors. Menopause: biology and pathobiology. Academic Press; 2000. pp. 175–88. [Google Scholar]
- 16.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 18.Brambilla DJ, McKinlay SM, Johannes CB. Defining the perimenopause for application in epidemiologic investigations. Am J Epidemiol. 1994;140:1091–5. doi: 10.1093/oxfordjournals.aje.a117209. [DOI] [PubMed] [Google Scholar]
- 19.Dudley EC, Hopper JL, Taffe J, Guthrie JR, Burger HG, Dennerstein L. Using longitudinal data to define the perimenopause by menstrual cycle characteristics. Climacteric. 1998;1:18–25. doi: 10.3109/13697139809080677. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Scientific Group. Research on the menopause in the 1990s. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1996;866:1–107. [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 22.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation of patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. [Accessed December 10, 2007];Guidelines for ATC Classification. Available at: www.whocc.no/atcddd.
- 24.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–23. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 25.Baecke JA, Burema J, Fritjers JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Amer J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 26.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 27.Randolph JF, Jr, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89:1555–61. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 28.Filicori M, Santoro N, Merriam GR, Crowley WF., Jr Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab. 1986;62:1136–44. doi: 10.1210/jcem-62-6-1136. [DOI] [PubMed] [Google Scholar]
- 29.Woods NF, Smith-Dijulio K, Percival DB, Tao EY, Taylor HJ, Mitchell ES. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: observations from the Seattle Midlife Women's Health Study. J Womens Health (Larchmt) 2007;16:667–77. doi: 10.1089/jwh.2006.0138. [DOI] [PubMed] [Google Scholar]
- 30.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81(4):1495–501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 31.Burger HG, Dudley EC, Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84:4025–30. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 32.Sowers MFR, Zheng H, McConnell D, Nan B, Harlow S, Randolph JF. Estradiol rates of change in relation to the final menstrual period from a population-based cohort of women. J Clin Endocrinol Metab. 2008 Jul 22; doi: 10.1210/jc.2008-1056. [Epub ahead of print] MPID: 18647803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sowers MFR, Zheng H, McConnell D, Nan B, Harlow S, Randolph JF. Follicle stimulating hormone and its rate of change to define menopause transition stages. J Clin Endocrinol Metab. 2008 Jul 22; doi: 10.1210/jc.2008-0482. [Epub ahead of print] MPID: 18647816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford K, Sowers M, Crutchfield M, Wilson A, Jannausch M. A longitudinal study of the predictors of prevalence and severity of symptoms commonly associated with menopause. Menopause. 2005;12:308–17. doi: 10.1097/01.gme.0000163869.89878.d9. [DOI] [PubMed] [Google Scholar]
- 35.Hess R, Colvin A, Avis NE, et al. The impact of hormone therapy on health-related quality of life: longitudinal results from the Study of Women's Health Across the Nation. Menopause. 2008;15:422–28. doi: 10.1097/gme.0b013e31814faf2b. [DOI] [PubMed] [Google Scholar]
- 36.Saletu B. Sleep, vigilance and cognition in postmenopausal women: placebo-controlled studies with 2 mg estradiol valerate, with and without 3 mg dienogest. Climacteric. 2003;6(Suppl 2):37–45. [PubMed] [Google Scholar]
- 37.Polo-Kantola P, Rauhala E, Erkkola R, Irjala K, Polo O. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548–54. doi: 10.1016/s0029-7844(00)01191-1. [DOI] [PubMed] [Google Scholar]
- 38.Keefe DL, Watson R, Naftolin F. Hormone replacement therapy may alleviate sleep apnea in menopausal women: a pilot study. Menopause. 1999;6:196–200. doi: 10.1097/00042192-199906030-00004. [DOI] [PubMed] [Google Scholar]
- 39.Baker FC, Mitchell D, Driver HS. Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Arch. 2001;442:729–37. doi: 10.1007/s004240100582. [DOI] [PubMed] [Google Scholar]