Abstract
Study Objectives:
The effects of hypnotics on sleep-dependent brain plasticity are unknown. We have shown that sleep enhances a canonical model of in vivo cortical plasticity, known as ocular dominance plasticity (ODP). We investigated the effects of 3 different classes of hypnotics on ODP.
Design:
Polysomnographic recordings were performed during the entire experiment (20 h). After a baseline sleep/wake recording (6 h), cats received 6 h of monocular deprivation (MD) followed by an i.p. injection of triazolam (1–10 mg/kg i.p.), zolpidem (10 mg/kg i.p.), ramelteon (0.1–1 mg/kg i.p.), or vehicle (DMSO i.p.). They were then allowed to sleep ad lib for 8 h, after which they were prepared for optical imaging of intrinsic cortical signals and single-unit electrophysiology.
Setting:
Basic neurophysiology laboratory
Patients or Participants:
Cats (male and female) in the critical period of visual development (postnatal days 28–41)
Interventions:
N/A
Measurements and Results:
Zolpidem reduced cortical plasticity by ∼50% as assessed with optical imaging of intrinsic cortical signals. This was not due to abnormal sleep architecture because triazolam, which perturbed sleep architecture and sleep EEGs more profoundly than zolpidem, had no effect on plasticity. Ramelteon minimally altered sleep and had no effect on ODP.
Conclusions:
Our findings demonstrate that alterations in sleep architecture do not necessarily lead to impairments in sleep function. Conversely, hypnotics that produce more “physiological” sleep based on polysomnography may impair critical brain processes, depending on their pharmacology.
Citation:
Seibt J; Aton SJ; Jha SK; Coleman T; Dumoulin MC; Frank MG. The non-benzodiazepine hypnotic zolpidem impairs sleep-dependent cortical plasticity. SLEEP 2008;31(10):1381–1391.
Keywords: Benzodiazepine, non-benzodiazepine, development, sleep-dependent cortical plasticity, α1-GABAA receptor
SEVERAL STUDIES INDICATE THAT SLEEP PROMOTES MEMORY CONSOLIDATION IN HUMANS1,2 AND IS ASSOCIATED WITH BIOCHEMICAL EVENTS THAT MAY promote synaptic plasticity,3 which is thought to be the cellular substrate of memory formation.4 However, it is not known how drugs commonly prescribed for insomnia influence these processes. For example, while it is known that hypnotics acting at the GABAA-receptor (GABAA-R) produce anterograde amnesia5 and impair daytime cognitive function6 and in vitro synaptic plasticity,7,8 only a handful of studies have examined the impact of hypnotic sleep on sleep-dependent memory formation.9,10 Considering that most hypnotic agents target the GABAA-R, which is critical in certain forms of plasticity and memory formation,6,11 it is possible that different classes of hypnotics have different effects on plastic events that normally occur during sleep. This may also be true for more recently developed hypnotics such as ramelteon, which target MT1 and MT2 melatonin receptors, because high doses of melatonin (10 mg/kg, i.p.) impair the induction of in vitro synaptic plasticity.12 In addition, hypnotics may influence sleep-dependent memory processes by altering the normal expression of sleep related genes13 or the amounts of different sleep states or cortical activity during sleep.14 Benzodiazepines (BDZ), and to a lesser extent, non-benzodiazepines (NBDZ), suppress REM sleep and NREM slow wave activity (SWA) while increasing thalamocortical spindling.14 REM sleep and spindles have in turn been hypothesized to promote different forms of memory consolidation while SWA may promote Hebbian and non-Hebbian synaptic plasticity.3,15
To determine the impact of hypnotic use on memory processes that may normally occur during sleep we examined the effects of 3 different classes of hypnotics on a canonical form of in vivo cortical plasticity. If vision is blocked in one eye (monocular deprivation, MD) during a critical period of development, the responses of most neurons in primary visual cortex (V1) shift in favor of the open eye.16 We have shown that this type of plasticity, known as ocular dominance plasticity (ODP), is consolidated by sleep and inhibited by sleep deprivation or when activity in the visual cortex is reversibly blocked during sleep.17–19 In this study, we investigated the effects of the BDZ triazolam (Tm; Halcion), the NBDZ zolpidem (Zm; Ambien) and the melatonin receptor agonist ramelteon (Rm, Rozerem) on this process.
MATERIALS AND METHODS
Experimental Design and Surgical Procedures
Animals were housed and treated in accordance with University of Pennsylvania IACUC regulations for animal care and use. We used an experimental design similar to that used previously to test the roles of sleep and sleeping cortical activity in ODP (Figure 1a).17,18 Briefly, surgery was performed on cats in the critical period of visual development between P24-P28. They were anesthetized and prepared for chronic implant surgery to obtain polysomnographic recordings as described previously.17 The skull was exposed, 5 EEG electrodes were placed bilaterally in frontal and parietal bone of the skull, and 3 EMG electrodes (braided stainless-steel wire) were placed in the nuchal muscle. The electrodes were connected to an electrical socket that was fixed to the skull with bone screws and dental acrylic. All animals were treated with antibiotics and analgesics as described previously.17 Following at least 4–5 days of postoperative recovery, kittens (at P28 to P41, depending on the age of surgery and the time course of experiment schedule) were brought to the laboratory and placed in an illuminated sleep-recording chamber with a revolving base for sleep/wake recording. After 6 h of baseline recording beginning at approximately 11:00, cats were anesthetized with isoflurane and had their right eyelids treated with topical anesthetic and sutured closed (monocular deprivation, MD) as previously described.17 Following recovery from this procedure the animals were kept awake (through a combination of gentle handling, novel object exploration, vocalization, and floor rotation) under normal room illumination for the next 6 h to provide a common stimulus for remodeling in V1,17,18 after which they were allowed to sleep ad lib for 8 h. Just prior to the post-MD rest period, drugs or vehicle were injected intraperitoneally (i.p.) (see below and Figure 1a). Light-dark schedules and ambient temperature were identical to colony housing with the exception that post-MD periods were always performed in complete darkness so that visual experience was held constant following MD. The number, mean age of animals and drug doses for each group are summarized in Table 1. Preliminary findings indicated that low and high doses in the ramelteon (Rm; 0.1 mg/kg, 1 mg/kg) and triazolam (Tm; 0.1 mg/kg, 1 mg/kg) groups produced similar effects on sleep and plasticity, therefore the results of these experiments were pooled in the Rm and Tm groups, and the lower dosage was used in all subsequent studies.
Figure 1.
Experimental design and representative hypnograms showing effects of hypnotic treatment on post-MD sleep architecture. (a) Experimental protocol. EEGs/EMGs are recorded during the entire 20 h of each experiment. All experiments begin with a 6 h baseline period (approximate start time: 11:00), followed by 6 h of monocular deprivation (MD) in awake animals to induce remodeling of the visual cortex.17 The animals are then provided an ad lib sleep period (8 h) in complete darkness (post-MD period) combined with vehicle (VEH) or drug administration (approximate start time: 23:00). All drugs (Zm, 10 mg/kg; Tm, 1 mg/kg–0.1mg/kg; Rm, 1 mg/kg–0.1 mg/kg) are injected i.p. before the post-MD sleep period, after which animals are prepared for measurements of ocular dominance and microelectrode recordings. (b) Representative hypnograms after injection (and post-monocular deprivation, MD). Wakefulness (W), REM (R), NREM (NR) and “NREM-drowsiness” (ND) were scored in 8-s epochs (starting at approximately 23:00). Note the total suppression of REM sleep during the first 2 h of the post-MD period in the triazolam animal. A similar trend is also found in the zolpidem treated animal. There are no obvious sleep changes in the ramelteon animal compared to the vehicle animal.
Table 1.
Summary Showing the Total Number of Animals (N), Numbers of Hemispheres for Optical Imaging (OI) and the Numbers of Hemispheres and Neurons Used for Single Unit Electrophysiology (Units) in Each Group
| OI |
Units Neuronal Properties |
|||||
|---|---|---|---|---|---|---|
| N | Mean age | RH | LH | RH (cells) | LH (cells) | |
| VEH | 5 | 34.6 | 5 | 5 | 4 (120) | 4 (120) |
| Zolpidem (10 mg/kg) | 6 | 32.3 | 5 | 5 | 5 (150) | 5 (150) |
| Triazolam (0.1 mg/kg) | 4 | 35.5 | 4 | 4 | 3 (90) | 3 (90) |
| Triazolam (1 mg/kg) | 1 | 28 | 1 | 1 | 1 (30) | 1 (30) |
| Total Triazolam | 5 | 34 | 5 | 5 | 4 (120) | 4 (120) |
| Ramelteon (0.1 mg/kg) | 4 | 36 | 3 | 4 | 4 (60) | 3 (90) |
| Ramelteon (1 mg/kg) | 1 | 31 | 1 | 1 | 1 (30) | 1 (30) |
| Total Ramelteon | 5 | 35 | 4 | 5 | 5 (90) | 4 (120) |
For OI, the number of imaged hemispheres is shown. In the ramelteon group, one hemisphere was not imaged due to vascular artifacts and tissue damage. In the zolpidem group, one animal was included in the sleep analyses, but excluded from plasticity assays because of clouding of the vitreous in one eye. For electrophysiology, the subset of analyzed hemispheres and the corresponding units in brackets are shown. 30 single neurons (sampled using a systematic randomization procedure) per hemisphere were used for neuronal properties analysis (see Materials and Methods). There was no difference in age between any of the groups (1-way ANOVA, ns). All animals shown were used in sleep analyses.
Drugs
We tested widely used short-acting hypnotics which have their plasma concentration peaks ∼2 h after systemic injection5,20,21 and used dose ranges comparable to those previously used in animal studies.14,22–24 Zolpidem (Zm, 10 mg/kg, Sigma-Aldrich; St. Louis, MO), triazolam (Tm; 1–0.1 mg/kg, Sigma-Aldrich; St. Louis, MO), and ramelteon (Rm; 1–0.1 mg/kg, Takeda Pharmaceuticals North America, Inc.) were diluted in vehicle (VEH; DMSO) solution, and injected intraperitoneally in a volume of 0.25 to 0.5 cc immediately before the onset of the post-MD period (Figure 1a).
Sleep/Wake Analysis
Polygraphic signals were routed from the animal via an electrical, counterbalanced tether/commutator to an Astro-Med (West Warwick, RI) amplifier system, processed with a high-pass filter of 0.3 Hz and a low-pass filter of 100 Hz, digitized at 200 Hz, and collected on a personal computer running commercial sleep acquisition/analyses software (SleepSign, Kisei Comtec, Irvine, CA). Anterior parietal (AP) EEG and EMG signals were used to assign polygraphic data into 8-s epochs of NREM sleep, REM sleep, and waking using SleepSign software.18 The proportion of time spent in REM, NREM, and waking (and mean bout duration for each state) was calculated separately for baseline and post-injection periods. A bout was defined as a sustained vigilance state of at least 24 s (3 epochs), not interrupted by the occurrence of any other vigilance state. For measurements of combined (or total) sleep bout durations (Figure 2e, f), “total sleep” bouts were defined as sustained, sequences of REM, NREM, or both of at least 24 s, not interrupted by waking periods ≥ 24 s.
Figure 2.
Effects of Zm, Tm, and Rm on vigilance states during post-MD sleep. Changes in NREM (a), (b), REM (c, d), total sleep (NREM+REM) (e) and wake (g, h) amounts and duration for each group are shown in 2-h bins. The left column shows mean (± SEM) state amounts as a % of total recording time and the right column shows corresponding mean (± SEM) bout duration in minutes during the post-MD period. Mean (± SEM) baseline (Bsl) values for each group are shown in the inset (see Material and Methods). There were no statistical differences between groups for all baseline values (1-way ANOVA, ns). Changes in sleep/wake parameters in the post-MD period were assessed with a repeated-measures 2-way ANOVA followed by Holm-Sidak post hoc tests (see text for details). Statistical differences were assessed for each 2-h bin, and levels of significance are reported for each group individually on the graphic. Different from baseline:*P ≤ 0.05, **P < 0.001, ***P ≤ 0.0001. Different from VEH: aP ≤ 0.05, bP < 0.001, cP ≤ 0.0001. The number of animals in each group is shown in the inset legend (see also Table 1)
Fast-Fourier transforms (FFT; SleepSign) were used to assess AP EEG spectra between 0–40 Hz (collapsed into 0.5-Hz bins) in each sleep state and computed in 2-h segments for the post-MD sleep period. For each cat, EEG power in each frequency band during post-MD sleep was expressed as a percentage of the baseline (6 h mean) recording for quantitative assessment of EEG changes.17,18 The proportion of time spent in a given state, the bout duration of each state, and changes in EEG power spectra were expressed in 2-h bins across the post-MD rest period (Figure 2 and 3). In triazolam (and one zolpidem) treated cats, we occasionally observed a “drowsy” state that appeared intermediate between wakefulness and NREM sleep (medium to high motor tone combined with high amplitude EEGs) that we classified as “NREM-drowsiness” (ND) (Figure 1b and Supplementary Figure S1 available online at www.journalsleep.org). This sleep stage was analyzed separately for EEG power spectra but included in the sleep bout calculations as NREM because of its behavioral and EEG similarities to NREM sleep (Supplementary Figure S1).
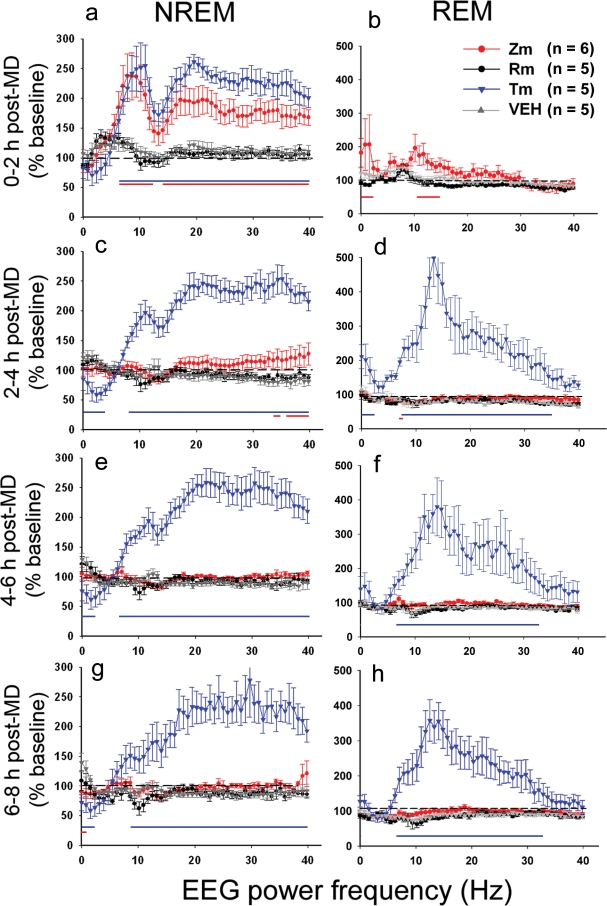
Figure 3.
Drug effects on EEG power spectra in the post-MD period. Mean ± SEM EEG power densities for NREM and REM sleep represented in 2-h bins and expressed as a % of corresponding baseline values (see Material and Methods). Both Zm and Tm decreased spectral energy in the delta frequency range and increased energies in sigma, beta, and gamma frequencies in NREM sleep during the first 2 h of sleep post-MD (a) This effect was more persistent in Tm treated animals (c, e, g). Tm also significantly altered REM EEG power spectra across the 8 h post-MD period (b, d, f, h). Significant changes in EEG power compared to VEH are represented by color coded bars on the bottom of the graphs (Holm-Sidak test vs. VEH, P < 0.05, and see text for detailed statistics). The number of animals in each group is shown in the inset legend (see also Table 1).
Intrinsic Signal Imaging and Ocular Dominance (OD) Analysis
Optical imaging was performed using previously described methods.18,25 Briefly, all animals were fully anesthetized by quick induction with oxygen + isoflurane (0.5%–5%) by mask. The animals underwent neuromuscular paralysis through intravenous injection of pancuronium bromide (0.04–0.01 mg/kg/0.5h, IV). Before paralysis, a tracheal cannula was inserted (lidocaine gel 1%–2% was applied topically to incision margins) and connected to a respirator delivering oxygen and anesthesia gas. The animal was then placed in a stereotaxic frame. The skull and dura over right and left primary visual cortex were removed, and intrinsic signals were recorded using a CCD camera (Dalstar 1M30; Dalsa; Waterloo, ON) focused 600 μm below the pial surface. Contact lenses were placed in the eyes (for optimum focus to a monitor positioned at a distance of 40 cm). Responses were measured for slowly rotating full-field grating stimuli, presented to either the re-opened right/deprived eye (DE) or left/non-deprived eye (NDE). Images of the cortical surface were continuously acquired at a rate of 30 frames/s and saved after temporal (4 frames) and spatial (2 × 2 pixels) binning. Optical maps were constructed by determining the preferred stimulus and response magnitude at the preferred stimulus for each pixel. OD at each pixel was determined by comparison of delay-corrected response at the preferred orientation for stimuli presented to the two eyes, and measured using a 7-point scoring system (with 4 corresponding to equal response for both eyes, and 1 and 7 corresponding to pixels exclusively driven by the contralateral and ipsilateral eye, respectively18,26). Overall measures of OD in each hemisphere were quantified using the Contralateral Bias Index (CBI) as previously described.17,18 The optical CBI (or comparable scalar measures) is a weighted average of the OD distribution and is routinely used to asses OD17,18,26,27 and shown to closely correspond with microelectrode recordings.28 It is calculated according the following formula:
CBI = [(n1−n7) + ((2/3) * (n2−n6)) + ((1/3) * (n3−n5)) + nT] / (2 * nT), in which nT is the total number of visual responsive pixels and nx is the number of pixels with ocular dominance rating x.
CBI values of 1 indicates complete contralateral-eye dominance in the hemisphere under study, 0 complete ipsilateral eye dominance, and 0.5 indicating equal representation of both eyes. For comparisons of combined hemisphere data, CBIs were adjusted so that scores of 0 indicate complete right (deprived) eye dominance, and scores of 1 indicate complete left (non-deprived) eye dominance. Cortical regions that were obscured by vasculature or other artifacts were excluded from OD analysis.
Single-Unit Electrophysiology
Immediately after completion of the optical imaging session (which lasted typically between 3 to 4 h), the anesthesia was switched to nembutal (boluses of 2–4 mg/kg IV) and single unit visual response properties were assessed in a subset of hemispheres from each group using previously described methods.18 Briefly, single neuronal responses to grating stimuli presented in either eye were recorded using a 1 × 1 mm array of 16 electrodes (Frederick Haer; Bowdoinham, ME). For each set of neurons recorded, 8 full-field, slowly-drifting, reversing gratings (0.2 cycles/degree, 5-s presentation) were presented randomly to each eye 4 times (4 × 8 different orientations at 22.5° intervals + blank screen per eye). Single-unit data were discriminated offline (Offline Sorter; Plexon, Inc.; Dallas, TX) and mean response properties for all stimuli were calculated for each sorted neuron by a custom computer program. Because the number of sampled hemispheres was too small for CBI assessments, we restricted our analyses to the following measurements.
A systematic, random sampling procedure was employed to ensure an equal contribution of neurons from each hemisphere. We selected every kth element from our sampling frame (one hemisphere), where k, the sampling interval, is calculated as:
k = population size (N) / sample size (n)
This makes systematic sampling functionally similar to simple random sampling but allows for even sampling across our population of recordings. Using this approach 30 representative units were selected from each recorded hemisphere. Once this sampling was completed, we then calculated the following visual response properties for all neurons in all hemispheres:
Evoked Response Index (ERI): ERI is calculated by the equation: ERI= 1-(Rspon/Rpf), where Rspon = the mean number of spikes during blank screen presentations and Rpf= the mean number of spikes at the preferred orientation (maximal response). The ERI ranges from 1 (a very responsive neuron, i.e., no blank screen responses) to 0 (a nonresponsive neuron, i.e., neuron fires equally or more to blank screen as to visual stimuli).19
Orientation Selectivity Index (OSI): The selectivity for a given orientated stimulus was assessed for both eyes using a previously used metric (OSI).29 Mean firing rates were computed at each neuron's preferred stimulus orientation and the orthogonal (90° from preferred; OSI90) or the 45° (45° from preferred; OSI45°) orientation. Ratios of unit firing rates were calculated (response at 90°/preferred, or 45°/preferred) and subtracted from 1. Indices of 1 indicate a high degree of selectivity, and indices of 0 indicate lack of selectivity.29
Maximum firing rate (Max) at the preferred stimulus orientation for both eyes was assessed for each neuron. We also calculated a Normalized Maximum firing rate which is the Max normalized to the mean firing rate for all neurons recorded within that hemisphere. This normalization corrects for background differences in spike rates between recordings due to inter-animal variability and normal periodic fluctuations in anesthetic depth.
At the end of neurophysiological studies (optical imaging followed by single-unit recordings), the animals were euthanized by intravenous injection of 150 mg/kg pentobarbital. Euthanasia procedures were consistent with recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
Statistics
All values are expressed as means ± SEM or median and quartile values as indicated. Statistical analyses were performed using SigmaStat software (Systat Software Inc.; San Jose, CA). Each data set was tested for normality using the Kolmogorov-Smirnov and equal variance tests. Mean percentage and mean bout duration of sleep/wake data were analyzed using 2-way repeated measures ANOVA to test for effects of drugs over time. Post hoc comparisons were made using the Holm-Sidak test. EEG power spectra were compared separately in each 2-h bin in the post-MD period using 2-way ANOVAs (group and frequency as main factors) followed by Holm-Sidak post hoc tests. Unit neuronal properties data were subject to nonparametric Kruskal-Wallis 1-way ANOVA on Ranks followed by post-hoc Dunn test for multiple comparisons.
RESULTS
Effect of Hypnotics on Sleep Architecture
Polysomnography showed that the 3 hypnotics had profoundly different effects on sleep architecture. Two-way repeated measures ANOVAs with group (factor A), time (factor B), and group × time (interaction) were used to test the effects relative to baseline and between groups. When ANOVAs indicated statistical significance, Holm-Sidak post hoc tests were performed to determine which groups differed from control values (matched baseline or VEH, Figure 2).
Figure 2 shows the effects of each drug on sleep architecture in the 8 h post-MD period, in terms of amounts (as % of total recording time: TRT, left column) and duration in minutes (right column). We found a main effect of time and an interaction (X) between time and group for NREM amounts (time, F4,67 = 24.8; X, F12,67 = 4, P < 0.001). There were also main effects and an interaction between time and group for bout duration (group, F3,67 = 8.8; time, F4,67 = 16.9; X, F12,67 = 7.9, P < 0.001 for all). Post hoc tests showed that both Tm and Zm significantly increased NREM sleep time by approximately 49% and 27%, respectively within the first 2 h post-injection when compared with VEH and respective baseline values (Figure 2a). This was accompanied by an increase in NREM bout duration in the Tm and Zm groups in the first 2 h of approximately 76 min and 20 min, respectively (Figure 2b). These effects were most pronounced in the Tm animals as they persisted for almost the entire post-MD period, whereas the effects of Zm dissipated after approximately 2 h (Figure 2a, b). In a subset of animals (Tm, n = 3; Zm, n = 1), we also occasionally observed a state that appeared intermediate between waking and NREM sleep which we scored as “NREM-drowsy” (ND) (Figure 1b and Supplementary Figure 1). The amount of ND in the Tm and Zm groups was very low across the 8 h of post-MD sleep (3% ± 1.4% and 0.16% of total recording time, respectively).
We found significant main effects of group, time and an interaction between these factors for REM amounts (group, F3,67 = 10.3; time, F4,67 = 13.8; X, F12,67 = 5.5, P < 0.001 for all) and bout duration (group, F3,67 = 5.1, P < 0.05; time, F4,67 = 5.5, P < 0.001; X, F12,67 = 2.9, P < 0.005). This reflected the fact that Zm and Tm both reduced REM amounts and bout duration to varying degrees. For example, as shown in a representative cat (Figure 1b), REM sleep was completely suppressed in the first 2 h following Tm injection (see also Figure 3b). Relative to baseline, Tm reduced both REM sleep time (∼20%) and bout duration (∼4 min) over the entire 8-h post-MD sleep period (Figure 2c, d). Zm also reduced REM sleep time relative to baseline and VEH-injected cats (Figure 2c), but this effect was no longer present after the second hour post-injection. We also observed a slight rebound in REM sleep amounts in the latter portion of the post-MD sleep period in the Zm group (Figure 2c).
There were also significant changes in total sleep amounts as % TRT across groups that varied with time (group, F3,67 = 3.4, P < 0.05; time, F4,67 = 46.4, P < 0.001; X, F12,67 = 3.5, P < 0.001). Total sleep time was elevated in all cats after MD relative to baseline for most of the post-MD period (Figure 2e). However, this increase was no longer significant after 2 h in the Tm group, and in both the Tm and Rm group, total sleep time fell below baseline values by the end of the post-MD period. In contrast, total sleep consolidation was only enhanced in the drug treated cats (time, F4,67 = 16.4, P < 0.001, Figure 2f) and was more uniform across the groups as total sleep bout duration was elevated in the Tm, Zm, and Rm cats only in the first 2 h of the post-MD period. These changes in sleep were accompanied by overall decreases in wake amounts as % TRT relative to baseline (time, F4,67 = 19.5, P < 0.001) and to a more variable degree, wake bout duration (time, F4,67 = 19.5, P < 0.001).
Effects of Hypnotics on Sleep EEGs
Both Tm and Zm produced abnormal NREM (Figure 3, left column) and REM (Figure 3, right column) EEGs during the post-MD sleep period. The ANOVA showed a main effect of group and an interaction between group and EEG frequency band (see below). As described in previous studies,30,31 Tm (and, to a lesser extent, Zm) decreased NREM SWA (0.5–4 Hz) and increased sigma and beta-gamma activity (10–15 Hz and 20–40 Hz, respectively). These effects were more pronounced in the Tm treated cats as they persisted for the entire post-MD period (0–2h; group, F3,884 = 370.4, X, F153,884 = 2.6; 2–4h; F3,884 = 897.7, X, F153,884 = 8; 4–6h; group, F3,884 = 1065.7, X, F153,884 = 8.3; 6–8h; group, F3,884 = 527.9, X, F153,884 = 4.3; P < 0.001 for all). In Zm cats, these effects were restricted to the first 2 h (Figure 3a). Tm and Zm also increased sigma and SWA in REM sleep and again these effects were more persistent in Tm treated cats (Tm: 2–4h; group F3,884 = 591, X, F153,884 = 4.1; 4–6h; group, F3,884 = 298, X, F153,884 = 2.3; 6–8h; group, F3,884 = 402.3, X, F153,884 = 3.8, P < 0.001 for all; Zm: 0–2h; group, F2,624 = 79.8, p < 0.001, X, F102,624 = 1.3, P < 0.05). In contrast, Rm did not produce any significant alterations in NREM or REM sleep EEGs in agreement with findings from other investigators.32 We also analyzed potential changes in SWA as a consequence of sleep deprivation (6h of MD). NREM SWA in the post-MD period was first expressed as a % of respective baseline values and a 1-way repeated measures ANOVA was performed on the data in 2-h bins. We did not find any significant differences in SWA amounts in the VEH, Zm or Rm groups across the post-MD period (Supplementary Figure S2). We did find a significant decrease in SWA in the Tm treated animals (Supplementary Figure S2), but this likely reflects nonspecific effects of Tm on EEG expression rather than homeostatic mechanisms. For example, as shown in Figure 3, Tm produced a profound overall suppression of SWA in the post-MD period.
Effect of Hypnotics on Sleep-Dependent Cortical Plasticity
We found that only Zm significantly inhibited cortical plasticity based on optical imaging of intrinsic cortical signals. Using a 2-factor ANOVA, we found a significant effect of group (F3,31 = 5.27, P = 0.005) and hemisphere (F1,31 = 13.22, P < 0.001) on optical CBI values. Post hoc Holm-Sidak tests in combined hemisphere data sets showed that CBI (± SEM) values in the Zm group (0.609 ± 0.011) were significantly lower than VEH (0.684 ± 0.022, P < 0.0025), Tm (0.686 ± 0.022, P < 0.0021), or Rm (0.68 ± 0.017, P < 0.0052, Figure 4b). Similar results were obtained from the hemisphere ipsilateral (right hemisphere, RH) to the deprived eye (Zm [0.627 ± 0.0142] vs. VEH [0.721 ± 0.0237], P < 0.007; Zm [0.627 ± 0.011] vs. Tm [0.741 ± 0.0193], P < 0.001, Holm-Sidak; Figure 4c). The impairment of ODP in the ipsilateral hemisphere for the Zm group was also evident when pixel responses to stimulation of either eye were ranked on a scale from 1 to 7 and represented as a standard OD histogram as previously described18,33 (and see Material and Methods). As shown in Figure 4e, the shift in response toward the non-deprived eye in the VEH, Tm, and Rm groups is more pronounced in the right (ispislateral, IPSI) hemisphere compared to the Zm treated cats. These latter results were consistent with a previous study that also found that ODP is more pronounced in the ipsilateral hemisphere.18 The effect of Zm on ODP was further quantified by expressing the % change in CBI relative to cats with normal vision (CBI: 0.53).27 As shown in Figure 4d, the normal shift in ocular dominance observed after sleep (in VEH-treated cats) was reduced by ∼50% in cats treated with Zm (ANOVA, F3,31 = 13.72, P < 0.001; shift in VEH cats, 32.88% ± 4.06%, in Tm cats 33.18% ± 4.72%, in Zm cats 16.82% ± 2.4%, Zm < Rm < VEH < Tm, P < 0.005, Holm-Sidak).
Figure 4.
Zolpidem impairs sleep-dependent ocular dominance plasticity. (a) Representative optical maps from each of the treatment groups. Maps are from hemispheres ipsilateral to the right (deprived) eye (DE). The first column shows maps of surface vasculature above primary visual cortex (V1). The highlighted area in all maps corresponds to the cortical region that was optimally focused and free of vascular artifacts. The second and third columns show angle maps generated by stimulating the left (non-deprived) eye (NDE) and the right eye (DE), respectively. The fourth and fifth columns are corresponding polar maps. For both angle and polar maps, the color of each pixel represents the orientation of the stimulus that most strongly activated the corresponding neurons (key to color assignment is given at the bottom of the third column). For polar maps, pixel brightness represents the magnitude of the response driven by either eye. The last column represents ocular dominance ratio (OD) maps where pixels driven by the DE are color-coded as magenta and pixels driven by the NDE blue. Contour lines demarcate regions preferentially activated by the DE, and values to the right are corresponding optical Contralateral Bias Index (CBI) values (for details, see Materials and Methods). Note that DE optical polar maps are darker than NDE maps (indicative of a ‘shift’ in ocular dominance) in all cases except Zm. In addition, the shrinkage of DE territories observed in the OD ratio maps is less pronounced in the Zm cat. (b-c) Comparison of optical CBIs for combined hemispheres (b) and in hemispheres ipsilateral (right, RH) and contralateral (left, LH) to the DE (c). The shift in CBI values towards the NDE is decreased only in the Zm group (*P ≤ 0.01, **P ≤ 0.005, see text for details). The grey horizontal bar represents the range of values for normal sighted animals (∼0.51–0.55). (d) Percentage of shift in OD normalized to values from cats with normal binocular vision (mean CBI for normal sighted animals = 0.53). There is approximately a ∼50% decrease in the amount of plasticity in the Zm group. The number of hemispheres is shown in the inset legend. (e) Ocular dominance pixel histograms in hemispheres ispilateral (IPSI) and contralateral (CONTRA) to the deprived eye for each group. Histograms for each hemisphere are ranked such that “1” represents pixels exclusively responsive to the ipsilateral eye, “7” represents pixels exclusively responsive to the contralateral eye, and “4” represents pixels that are driven equally by the two eyes. Note the decreased number of cells ranked as “1” and “2” and the relative increase in binocular cells (“4”) in the IPSI hemispheres in the Zm treated animals (red arrows). White scale bar = 1 mm.
Effects of Hypnotics on Visual Response Properties
Microelectrode single-unit recordings in a subset of hemispheres from each group were used to verify that none of the hypnotics under investigation grossly impaired visual response properties at the time of OD assays (Table 2). For example, we found a slight decrease in raw deprived-eye (DE) firing rates (Max.) in the Zm group compared to VEH (Kruskal-Wallis 1-way ANOVA, H = 12.1, P < 0.05, Dunn test), but this was no longer significant when data were corrected for background variability (Kruskal-Wallis 1-way ANOVA, H = 0.428, P = 0.934). We also found that the DE responses in the Zm group were significantly more selective for orientation based on the OSI45 index compared to VEH (Kruskal-Wallis 1-way ANOVA, H = 11.14, P < 0.05, Dunn test), consistent with diminished plasticity following MD. There was also a significant decrease in DE ERI in the Tm group, consistent with heightened plasticity (i.e., a loss of response to DE). There were no other major differences between groups for visual responsiveness (ERI, a measure of signal-to-noise), orientation selectivity (OSI90), or firing rates at the preferred orientation (Table 2).
Table 2.
Neuronal Response Properties for the Main Groups
| VEH (240) | Rm (210) | Zm (300) | Tm (210) | |
|---|---|---|---|---|
| ERI | ||||
| DE | ||||
| H = 21.92 | 0.975 | 0.88 | 0.93 | 0.77 |
| P < 0.001 | (0. 68/1) | (0.57/1) | (0.50/1) | (0.43/1) |
| NDE | ||||
| H = 6.51 | 1 | 0.96 | 0.98 | 0.97 |
| P = 0.095 | (0.85/1) | (0.67/1) | (0.76/1) | (0.78/1) |
| OSI90 | ||||
| DE | ||||
| H = 4.04 | 0.76 | 0.77 | 0.8 | 0.88 |
| P = 0.258 | (0.5/1) | (0.54/1) | (0.56/1) | (0.60/1) |
| NDE | ||||
| H = 0.95 | 0.79 | 0.82 | 0.78 | 0.84 |
| P = 0.815 | (0.5/1) | (0.56/0.96) | (0.56/1) | (0.6/0.97) |
| OSI45 | ||||
| DE | ||||
| H = 11.14 | 0.67 | 0.67 | 0.75 | 0.66 |
| P = 0.011 | (0.5/0.87) | (0.5/0.83) | (0.0.56/0.88) | (0.5/0.83) |
| NDE | ||||
| H = 2.92 | 0.68 | 0.67 | 0.70 | 0.70 |
| P = 0.404 | (0.5/0.83) | (0.5/0.83) | (0.5/0.86) | (0.53/0.83) |
| Norm. Max | ||||
| DE | ||||
| H = 0.43 | 0.94 | 0.87 | 0.86 | 0.97 |
| P = 0.934 | (0.46/2.15) | (0.46/1.99) | (0.44/2.21) | (0.42/2.18) |
| NDE | ||||
| H = 6.31 | 1.53 | 1.52 | 1.63 | 1.8 |
| P = 0.098 | (0.69/3.1) | (0.78/2.7) | (0.7/3.59) | (0.96/3.99) |
| Max. | ||||
| DE | ||||
| H = 12.1 | 2 | 1.75 | 1.25 | 1.5 |
| P = 0.007 | (0.75/3.75) | (0.75/3.5) | (0.5/3.25) | (0.75/3.75) |
| NDE | ||||
| H = 10.78 | 3 | 3 | 2.25 | 2.875 |
| P = 0.013 | (1.25/6.5) | (1.5/6) | (1/4.75) | (1.5/6.75) |
The number of units used for the analysis of neuronal properties is shown in parentheses. Median values and the 25th/75th percentiles (in parentheses) for the Evoked Response Index (ERI), Orientation Selectivity Indices (OSI90 and OSI45), normalized maximal response (Norm. Max), and maximal response (Max) for the right (deprived, DE) and left (non-deprived, NDE) eyes are shown. Multiple comparisons (Kruskal-Wallis 1-way ANOVA) were performed to determine overall significance between groups. H and probability values are shown for corresponding Kruskal-Wallis for multiple comparisons (between-group differences for a given eye). Statistically significant differences compared to VEH for the same eye are shown in bold (P < 0.05, Dunn test).
DISCUSSION
Using a combination of polysomnography, optical imaging of intrinsic cortical signals and microelectrode recording in vivo, we investigated the impact of commonly prescribed hypnotics on a classic form of cortical plasticity. We found that different classes of hypnotics have profoundly different effects on sleep architecture and EEG activity, but only a hypnotic that preferentially targets the α1-GABA-R subunit interferes with sleep-dependent brain plasticity. These findings have important implications because they demonstrate that major alterations in sleep architecture do not necessarily impair important brain functions mediated by sleep. Conversely, agents that produce more ‘physiological’ sleep based on polysomnography may grossly impair these very same functions and these effects may be particularly acute in pediatric populations. This is an especially important area of future investigation because hypnotic use in children is increasingly common despite a paucity of basic research on this topic.34,35
Hypnotics and Plasticity in the Developing and Adult Brain
Our principal findings can not be ascribed to nonspecific effects of Zm on visual processing at the time of OD assays. First, as shown in Figures 2 and 3, the behavioral and electrophysiological effects of Zm dissipate after 2 h and therefore are unlikely to be at effective brain concentrations when optical imaging and microelectrode measurements are performed. This is further supported by measurements of Zm metabolism, which show that Zm reaches peak plasma concentrations within 1–2 h21 with a half-life of 1.5–2.4 h.36 Second, in agreement with intracortical infusion studies performed in mice37,38 drugs acting at the GABAA-R do not produce gross alterations in visual response properties based on microelectrode recordings of single neurons (Table 2). This is also demonstrated by optical imaging as shown in Figure 4. Representative optical angle maps and polar maps in the Zm cat did not show major impairments in orientation selectivity or overall response strength compared to the other groups (Figure 4a). Therefore, although the precise neural site of action (e.g., thalamic vs. cortical) cannot be determined using systemic drug administration, our findings are best explained by an alteration of GABAergic neurotransmission via the α1-GABAA-R during post-MD sleep.
This idea is consistent with the role of the α1-GABAA-R in both memory and cortical plasticity. Among the different GABAA-R subtypes, both α1-GABAA-R and α5-GABAA-R have been shown to modulate memory,39 but the α5-GABAA-R is unlikely to play a role in visual cortical plasticity because it is mainly located within the hippocampus and is bound by Zm with very low affinity.39 On the other hand, the α1-containing GABAA-R is the major subtype in the cerebral cortex and several studies have demonstrated a selective increase in its expression40 and function41 during postnatal development suggesting that it influences brain development. For example, BDZs normally inhibit gene expression important for synaptic plasticity by binding to the α1-GABAA-R42 and this particular subunit is also critically involved in the timing of the critical period in visual cortex.11 In addition, mice that lack functional α1-subunit ([α1(H101R)]) do not display BDZ-induced memory impairment.43
Since both Tm and Zm bind to the α1-subunit in vitro44, one might have predicted similar effects of both compounds on sleep-dependent cortical plasticity. One likely explanation for the specific effect of Zm on ODP is that at the physiological doses used in this study, only Zm activates the α1-GABAA-R enough to impair plasticity. BDZs, like Tm, are nonselective for subunit composition and bind with equal affinity for various GABAA-R subtypes to the so called benzodiazepine site (α/γ2 interface),5 explaining most of the anxiolytic effects and EEG changes45 seen after BDZ administration.46 In contrast, Zm binds preferentially to α1-containing GABAA-R with an affinity almost 30 times higher than for other α-GABAA-R subunits.47,48 For these reasons, within physiological ranges, Zm is more likely to activate the α1 subunit of the GABAA-R compared to Tm. Therefore while we can not exclude other potential mechanisms, our data are best explained by the selective binding of Zm to the α1 subunit.
Although we focused on a specific form of developmental brain plasticity, our findings may be applicable to adults as well. A number of the cellular mechanisms governing ODP are known to influence adult forms of cortical and hippocampal based memory and plasticity, and ODP itself has recently been shown to persist beyond the classically defined critical period.49 For example, monocular deprivation in adult monkeys decreases GABAA-R expression in deprived cortical territories50 and adult recovery from amblyopia is accompanied by a specific reduction in GABAergic neurotransmission.51
It is not yet clear if GABAergic neurotransmission during sleep is also critical for adult memory and brain plasticity. In agreement with the findings of the present study, the benzodiazepines brotizolam and Tm do not impair declarative memory formation observed after sleep in adult humans.9,10 On the other hand, the NBDZ zopiclone (but not Zm) impaired memory formation for this particular task (word list memorization). These studies must be cautiously interpreted because not all forms of memory were assessed, but they suggest that GABAergic neurotransmission during sleep may indeed influence memory consolidation in adults.
Hypnotic Effects on Sleep Expression in the Developing Cat
To our knowledge, this is the first study to measure the effects of three different classes of hypnotics on sleep architecture and EEG activity in developing animals. In general, Rm, Tm, and Zm produced changes in sleep architecture and sleep EEGs comparable to what has been reported in adults. As is true for adult monkeys,52 Rm increased sleep consolidation (Figure 2f) but had no significant effects on either REM or NREM sleep EEGs (Figure 3). In contrast, Tm and to a lesser extent, Zm reduced REM sleep, increased NREM sleep and increased energies in sigma, beta and gamma frequencies of the EEGs; effects which are also reported in adults.14 The similar effects of these agents in kittens and adult animals suggest that the underlying sleep mechanisms have reached adult-levels of maturation by the 4th postnatal week. Melatonin receptors develop prenatally in mammals and appear to be fully functional at birth53,54 and GABAA-receptors and their associated BDZ sites are present in the fetal brain and attain adult-like properties by at least the second postnatal week in the rat.55–57 Studies in kittens also indicate that several markers of GABAergic neurotransmission are at or near adult values by the 4th–5th postnatal week.58,59 There are, however, important design differences between the present study and previous studies in adult animals which should also be considered. For example, kittens are essentially aperiodic with respect to sleep/wake organization,60 thus time-of-day differences in hypnotic effect reported in adults are unlikely to be present in kittens. In addition, we administered hypnotics after a period of enforced waking, thus a preexisting sleep pressure may have masked certain hypnotic effects while exacerbating others. This may explain some persistent effects of Tm on sleep expression and sleep EEGs (Figures 2 and 3, Supplementary Figure S2), and the appearance of a state intermediate between NREM and wake (“NREM-drowsy,” see Supplementary Figure 1) in some animals. Nevertheless, while these caveats should be kept in mind, our results suggest that Tm, Zm, and Rm act on neonatal sleep mechanisms in a manner comparable to the adult brain.
CONCLUSIONS
Hypnotics that target the GABAA-receptor are known to inhibit memory formation and cognitive functioning in alert subjects, but their effects on mnemonic processes that occur during sleep are poorly understood.5,6 We now show that certain hypnotics may indeed impair these processes and do so without grossly altering sleep expression. Therefore functional assays of sleep, in addition to polysomnographic screening, must be considered when evaluating hypnotic medications. Future studies are needed to address several questions raised by this investigation. First, how does GABAergic neurotransmission during sleep influence brain plasticity? The α1- GABAA-R is located both intracortically and in the thalamus and changes in inhibition in either brain region during sleep may influence cortical remodeling. Second, does GABAergic neurotransmission in sleep play similar roles in adult forms of brain plasticity? The recent demonstration that ODP extends beyond the classic critical period provides an experimental approach to addressing this question.49 Lastly, what are the acute and long-term consequences of hypnotic use in children? Our findings suggest that critical developmental events may be altered by certain classes of hypnotics. Therefore, more research is needed into the effects of these substances on the developing brain.
ABBREVIATIONS
- AP
anterior parietal
- BDZ
benzodiazepine
- Bsl
baseline
- CBI
Contralateral Bias Index
- DE
deprived eye
- ERI
Evoked Response Index
- FFT
Fast-Fourier transforms
- GABAA-R
GABAA-Receptor
- LH
left hemisphere
- MD
monocular deprivation
- MT1
melatonin receptor subtype1
- MT2
melatonin receptor subtype2
- NBDZ
non-benzodiazepine
- ND
NREM-drowsiness
- NDE
non-deprived eye
- NREM
non-rapid eye movement
- OD
ocular dominance
- ODP
ocular dominance plasticity
- OSI45
Orientation Selectivity Index (firing rates at 45°/firing rate at preferred orientation)
- OSI90
Orientation Selectivity Index (firing rates at 90°/firing rate at preferred orientation)
- Rm
Ramelteon
- REM
rapid eye movement
- RH
right hemisphere
- SEM
Standard error of the mean
- SWA
slow wave activity
- Tm
Triazolam
- V1
primary visual cortex
- VEH
vehicle
- Zm
Zolpidem
DISCLOSURE STATEMENT
This study was supported in part by Takeda Pharmaceuticals North America, marketers of ramelteon. Dr. Frank has participated in speaking engagements for Takeda. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the University of Pennsylvania and by Takeda Pharmaceuticals North America, a Pickwick postdoctoral fellowship from the National Sleep Foundation (to SJA), and a National Institutes of Health Systems and Integrative Biology Training Grant T32GM07517 (to MCD). The authors are grateful to Clement Richard for providing data-analysis software and to Nina Hsu for technical assistance.
Supplementary data is provided in Supplementary Figures. These are available online at www.journalsleep.org
REFERENCES
- 1.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 2.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–66. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 3.Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Progress Neurobiol. 2003;69:77–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 4.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 5.Wagner J, Wagner ML. Non-benzodiazepines for the treatment of insomnia. Sleep Med Revi. 2000;4:551–81. doi: 10.1053/smrv.2000.0126. [DOI] [PubMed] [Google Scholar]
- 6.Mohler H. Molecular regulation of cognitive functions and developmental plasticity: impact of GABAA receptors. J Neurochem. 2007;102:1–12. doi: 10.1111/j.1471-4159.2007.04454.x. [DOI] [PubMed] [Google Scholar]
- 7.Wan H, Warburton EC, Zhu XO, et al. Benzodiazepine impairment of perirhinal cortical plasticity and recognition memory. Eur J Neurosci. 2004;20:2214–24. doi: 10.1111/j.1460-9568.2004.03688.x. [DOI] [PubMed] [Google Scholar]
- 8.Higashima M, Kinoshita H, Koshino Y. Differences in the effects of zolpidem and diazepam on recurrent inhibition and long-term potentiation in rat hippocampal slices. Neurosci Lett. 1998;245:77–80. doi: 10.1016/s0304-3940(98)00178-5. [DOI] [PubMed] [Google Scholar]
- 9.Bozon B, Kelly A, Josselyn SA, Silva AJ, Davis S, Laroche S. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:805–14. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melendez J, Galli I, Boric K, et al. Zolpidem and Triazolam do not affect the nocturnal sleep-induced memory improvement. Psychopharmacology. 2005;181:21–6. doi: 10.1007/s00213-005-2228-0. [DOI] [PubMed] [Google Scholar]
- 11.Hensch T. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 12.Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci. 2005;22:2231–7. doi: 10.1111/j.1460-9568.2005.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wisor JP, Morairty SR, Huynh NT, Steininger TL, Kilduff TS. Gene expression in the rat cerebral cortex: comparison of recovery sleep and hypnotic-induced sleep. Neuroscience. 2006;141:371–8. doi: 10.1016/j.neuroscience.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22:33–42. doi: 10.1093/sleep/22.1.33. [DOI] [PubMed] [Google Scholar]
- 15.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Wiesel TN, Hubel DH. Single cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;28:1029–40. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 17.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–87. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 18.Jha SK, Jones BE, Coleman T, et al. Sleep-dependent plasticity requires cortical activity. J Neurosci. 2005;25:9266–74. doi: 10.1523/JNEUROSCI.2722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank MG, Jha SK, Coleman T. Blockade of postsynaptic activity in sleep inhibits developmental plasticity in visual cortex. Neuroreport. 2006;17:1459–63. doi: 10.1097/01.wnr.0000233100.05408.e4. [DOI] [PubMed] [Google Scholar]
- 20.Pandi-Perumal SR, Srinivasan V, Poeggeler B, Hardeland R, Cardinali DP. Drug Insight: the use of melatonergic agonists for the treatment of insomnia-focus on ramelteon. Nat Clin Pract Neurol. 2007;3:221–8. doi: 10.1038/ncpneuro0467. [DOI] [PubMed] [Google Scholar]
- 21.Blumer JL, Reed MD, Steinberg F, et al. Potential Pharmacokinetic Basis for Zolpidem Dosing in Children With Sleep Difficulties. Clin Pharmacol Ther. 2007 doi: 10.1038/sj.clpt.6100380. [DOI] [PubMed] [Google Scholar]
- 22.Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. Br J Pharmacol. 2000;131:1251–4. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto M, Nishikawa H, Doken Y, Hirai K, Uchikawa O, Ohkawa S. The sleep-promoting action of ramelteon (TAK-375) in freely moving cats. Sleep. 2004;27:1319–25. doi: 10.1093/sleep/27.7.1319. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto M, Higuchi H, Kamata M, Yoshida K, Shimizu T, Hishikawa Y. The effects of benzodiazepine (Triazolam), cyclopyrrolone (zopiclone) and imidazopyridine (zolpidem) hypnotics on the frequency of hippocampal theta activity and sleep structure in rats. Eur Neuropsychopharmacol. 1999;9:29–35. doi: 10.1016/s0924-977x(97)00102-8. [DOI] [PubMed] [Google Scholar]
- 25.Kalatsky VA, Stryker MP. New paradigm for optical imaging: temporally encoded maps of intrinsic signal. Neuron. 2003;38:529–45. doi: 10.1016/s0896-6273(03)00286-1. [DOI] [PubMed] [Google Scholar]
- 26.Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the ferret's visual cortex. J Neurosci. 1999;19:6955–78. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiter HO, Waitzman DM, Stryker MP. Cortical activity blockade prevents ocular dominance plasticity in the kitten visual cortex. Exp Brain Res. 1986;65:182–8. doi: 10.1007/BF00243841. [DOI] [PubMed] [Google Scholar]
- 28.Dadvand L, Stryker MP, Frank MG. Sleep does not enhance the recovery of deprived eye responses in developing visual cortex. Neuroscience. 2006;143:815–26. doi: 10.1016/j.neuroscience.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao DS, Krahe TE, Prusky GT, Medina AE, Ramoa AS. Recovery of cortical binocularity and orientation selectivity after the critical period for ocular dominance plasticity. J Neurophysiol. 2004;92:2113–21. doi: 10.1152/jn.00266.2004. [DOI] [PubMed] [Google Scholar]
- 30.Achermann P, Borbely AA. Dynamics of EEG slow wave activity during physiological sleep and after administration of benzodiazepine hypnotics. Hum Neurobiol. 1987;6:203–10. [PubMed] [Google Scholar]
- 31.Brunner DP, Dijk DJ, Munch M, Borbely AA. Effect of zolpidem on sleep and sleep EEG spectra in healthy young men. Psychopharmacology (Berl) 1991;104:1–5. doi: 10.1007/BF02244546. [DOI] [PubMed] [Google Scholar]
- 32.Yukuhiro N, Kimura H, Nishikawa H, Ohkawa S, Yoshikubo S-i, Miyamoto M. Effects of ramelteon (TAK-375) on nocturnal sleep in freely moving monkeys. Brain Research. 2004;1027:59–66. doi: 10.1016/j.brainres.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 33.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–36. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owens JA, Rosen CL, Mindell JA. Medication use in the treatment of pediatric insomnia: results of a survey of community-based pediatricians. Pediatrics. 2003;111:e628–35. doi: 10.1542/peds.111.5.e628. [DOI] [PubMed] [Google Scholar]
- 35.Glaze DG. Childhood insomnia: why Chris can't sleep. Pediatr Clin North Am. 2004;51:33–50. doi: 10.1016/s0031-3955(03)00176-7. [DOI] [PubMed] [Google Scholar]
- 36.Mendelson W. Hypnotic medications: mechanisms of action and pharmacologic effects. In: Kryger M, Roth TC, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier; 2005. pp. 444–51. [Google Scholar]
- 37.Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–8. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–3. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- 39.Mohler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–16. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- 40.Heinen K, Bosman LW, Spijker S, et al. GABAA receptor maturation in relation to eye opening in the rat visual cortex. Neuroscience. 2004;124:161–71. doi: 10.1016/j.neuroscience.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Bosman LW, Rosahl TW, Brussaard AB. Neonatal development of the rat visual cortex: synaptic function of GABAA receptor alpha subunits. J Physiol. 2002;545:169–81. doi: 10.1113/jphysiol.2002.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huopaniemi L, Keist R, Randolph A, Certa U, Rudolph U. Diazepam-induced adaptive plasticity revealed by alpha1 GABAA receptor-specific expression profiling. J Neurochem. 2004;88:1059–67. doi: 10.1046/j.1471-4159.2003.02216.x. [DOI] [PubMed] [Google Scholar]
- 43.Rudolph U, Crestani F, Benke D, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 44.Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol Pharmacol. 1993;43:970–5. [PubMed] [Google Scholar]
- 45.Tobler I, Kopp C, Deboer T, Rudolph U. Diazepam-induced changes in sleep: role of the alpha 1 GABA(A) receptor subtype. Proc Natl Acad Sci U S A. 2001;98:6464–9. doi: 10.1073/pnas.111055398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Langer SZ, Arbilla S, Benavides J, Scatton B. Zolpidem and alpidem: two imidazopyridines with selectivity for omega 1- and omega 3-receptor subtypes. Adv Biochem Psychopharmacol. 1990;46:61–72. [PubMed] [Google Scholar]
- 48.Pritchett DB, Seeburg PH. Gamma-aminobutyric acidA receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990;54:1802–4. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 49.Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–26. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Hendry SH, Huntsman MM, Vinuela A, Mohler H, de Blas AL, Jones EG. GABAA receptor subunit immunoreactivity in primate visual cortex: distribution in macaques and humans and regulation by visual input in adulthood. J Neurosci. 1994;14:2383–401. doi: 10.1523/JNEUROSCI.14-04-02383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sale A, Maya Vetencourt JF, Medini P, et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–81. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- 52.Yukuhiro N, Kimura H, Nishikawa H, Ohkawa S, Yoshikubo S, Miyamoto M. Effects of ramelteon (TAK-375) on nocturnal sleep in freely moving monkeys. Brain Res. 2004;1027:59–66. doi: 10.1016/j.brainres.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 53.Fujieda H, Scher J, Lukita-atmadja W, Brown GM. Gene regulation of melatonin and dopamine receptors during eye development. Neuroscience. 2003;120:301–7. doi: 10.1016/s0306-4522(03)00298-7. [DOI] [PubMed] [Google Scholar]
- 54.Vanecek J. The melatonin receptors in rat ontogenesis. Neuroendocrinology. 1988;48:201–3. doi: 10.1159/000125008. [DOI] [PubMed] [Google Scholar]
- 55.Leinekugel X, Khalilov I, McLean H, et al. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv Neurol. 1999;79:189–201. [PubMed] [Google Scholar]
- 56.Hebebrand J, Hofmann D, Reichelt R, et al. Early ontogeny of the central benzodiazepine receptor in human embryos and fetuses. Life Sci. 1988;43:2127–36. doi: 10.1016/0024-3205(88)90363-3. [DOI] [PubMed] [Google Scholar]
- 57.Richards JG, Schlumpf M, Lichtensteiger W, Mohler H. Ontogeny of benzodiazepine binding sites in fetal rat brain: an in vitro autoradiographic study. Monogr Neural Sci. 1983;9:111–8. doi: 10.1159/000406883. [DOI] [PubMed] [Google Scholar]
- 58.Guo Y, Kaplan IV, Cooper NG, Mower GD. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of the cat visual cortex. Brain Res Dev Brain Res. 1997;103:127–41. doi: 10.1016/s0165-3806(97)81789-0. [DOI] [PubMed] [Google Scholar]
- 59.Hogan D, Berman NE. The development of somatostatin immunoreactive neurons in cat visual cortical areas. Brain Res Dev Brain Res. 1993;71:221–38. doi: 10.1016/0165-3806(93)90174-9. [DOI] [PubMed] [Google Scholar]
- 60.Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–39. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]