Abstract
Study Objective:
The inhibitory neuromodulator adenosine has been proposed as a homeostatic sleep factor that acts potently in the basal forebrain (BF) to increase sleepiness. Here 300 μM of adenosine was dialyzed in the BF of rats, and the effect on vigilance was determined in the rat Psychomotor Vigilance Task (rPVT).
Design:
Rats experienced all experimental conditions in a repeated-measures, cross-over design.
Patients or Participants:
Twelve young adult male Fischer-Norway rats.
Interventions:
Sustained attention performance in the rPVT was evaluated following 2 hours of bilateral microdialysis perfusion of vehicle, adenosine (300 μM), or codialysis of 300 μM of adenosine with the A1 receptor antagonist 8-cyclopentyltheophylline.
Measurements and Results:
During rPVT performance, response latencies and performance lapses increased significantly after adenosine dialysis when compared with baseline (no dialysis) or vehicle dialysis sessions. The codialysis of 8-cyclopentyltheophylline with adenosine completely blocked the effects produced by adenosine alone, resulting in performance equivalent to that of the vehicle sessions.
Conclusions:
Pharmacologic elevation of BF adenosine in rats produced vigilance impairments resembling the effect of sleep deprivation on vigilance performance in both man and rats. This effect of exogenous adenosine was completely blocked by codialysis with an adenosine A1 receptor antagonist. The results are consistent with the hypothesis that sleep loss induces elevations of BF adenosine that, acting via A1 receptors, lead to increased sleepiness and impaired vigilance.
Citation:
Christie MA; Bolortuya Y; Chen LC; McKenna JT; McCarley RW; Strecker RE. Microdialysis elevation of adenosine in the basal forebrain produces vigilance impairments in the rat psychomotor vigilance task. SLEEP 2008;31(10):1393–1398.
Keywords: Adenosine, basal forebrain, microdialysis, attention, vigilance, rat, sleep, sleep deprivation, sleepiness, PVT, caffeine
THE ACTIVATION OF CORTICALLY PROJECTING BASAL FOREBRAIN (BF) NEURONS HAS LONG BEEN ASSOCIATED WITH CORTICAL ACTIVATION, AROUSAL, and attention/vigilance.1–3 Although the cholinergic population of these BF neurons has been the most widely studied, at least 2 other populations of BF neurons (ie, GABAergic, and putatively glutamatergic neurons) also play a role in this cortical activation.4,5 An inhibition of these BF neurons leads to reduced arousal and vigilance; hence, recent work has attempted to identify the biologic processes that inhibit the BF neurons during periods of sleepiness and reduced vigilance.6,7 Adenosine, an inhibitory neuromodulator and putative sleep factor, has emerged as a leading neurochemical candidate mediating the inhibition of these wakefulness/vigilance-promoting BF neurons.8,9
Adenosine, a byproduct of energy metabolism (adenosine is the “A” in ATP), has been shown to accumulate in the BF during periods of prolonged wakefulness (ie, when energy demands are high) and to decline during periods of sleep.6,7,10 Caffeine and theophylline are potent adenosine receptor antagonists, explaining the potent stimulant effects of coffee and tea.11 The application of a similar adenosine antagonist directly into the BF increases wakefulness, whereas increasing BF adenosine levels via drug application directly into the BF reduces wakefulness.6,7 These, and additional findings, have led to the hypothesis that an elevation of adenosine in the BF is a neurochemical correlate of the sleepiness associated with prolonged wakefulness, and, as such, adenosine may be a mediator of the homeostatic sleep drive.12 In the present study, we used this foundation of knowledge to test the prediction that microdialysis application of exogenous adenosine in the rat BF will produce a decrease in vigilance performance in the rat Psychomotor Vigilance Task (rPVT).
The Psychomotor Vigilance Task (PVT) has been widely used in human studies to detect the sustained attention impairments associated with different types of sleep loss, including chronic sleep restriction,13,14 sleep deprivation,15,16 sleep disordered breathing,17–19 and insomnia.20–22 However, only recently has a rat version of the PVT been described that lends itself to invasive investigations of the role of adenosine and the BF in the control of behavior state and sustained attention.24 Although previous studies have demonstrated that pharmacologic elevation of adenosine, or adenosine agonists, directly in the BF decreases the time animals spend in wakefulness,10,23,28,29,reviewed in 6 & 7 it is unknown how this manipulation alters performance in a sustained attention task such as the rPVT. We here report that increasing BF adenosine levels produce impairments in rPVT sustained-attention performance that resemble those seen in rats24,26 and humans13–22 after various forms of sleep deprivation or disruption.
METHODS
Animals
Twelve adult Fischer-Norway rats (Charles River Breeding Laboratories, Wilmington, MA), weighing between 300 and 350 grams, were housed under constant temperature (23°C ± 1°C) and a 12:12 light-dark cycle (lights on at 07:00.) with food available ad libitum. To encourage responding for water reinforcement in the operant task rats had restricted access to water in their home cage. Rats were introduced to the water-restriction schedule over several days by reducing the amount of time each day that water was available in the home cage. After entering the rPVT training protocol, rats received water as a reward for task performance (20 μL/reinforcement). Additional home-cage access to free water was available for 5 or 10 minutes immediately after each rPVT session. Rats on this water-restriction procedure remain active and very healthy; for example the rats maintained or gained body weight. All animals were treated in accordance with Association for Assessment and Accreditation of Laboratory Animal Care's policy on care and use of laboratory animals. All experiments conformed to U.S. Department of Veterans Affairs, Harvard University, and U.S. National Institutes of Health guidelines on the ethical use of animals.
rPVT Apparatus
The rPVT was performed in a MED-Associates (Burlington, VT) operant chamber, with infrared head-detection beams across the front of the water-delivery port to record nose pokes. The target stimulus was a centrally mounted light directly above the water-delivery port. A nose poke into the water-delivery port during stimulus illumination (0.5 seconds), or during the 2.5-second limited hold period after the stimulus was extinguished, was rewarded with 20 μL of water delivered by automatic dipper.
The rPVT
As previously described,24 rats were trained daily (at 13:00) to nose poke in response to the illumination of the central stimulus light. The performance criterion for rats to enter the experimental arm of the study was a minimum of 120 reinforced responses within a 30-minute session for 3 consecutive sessions with the stimulus duration set at 0.5 seconds. Intertrial intervals varied from 3 to 7 seconds in 1-second increments in a quasi-random fashion (equal density of intervals throughout session) to prevent predictable stimulus onset. Responding during the intertrial interval (a “premature response”) or failing to respond at all (an omission) were treated as errors and resulted in a 10-second “time out” (house light extinguished and absence of trials). At the end of the 10-second time-out, the house light was reilluminated and a new intertrial interval started. The primary measures of performance in the rPVT were response latencies and lapses. Response latencies were calculated from the onset of the light until the rat nose poked the water-delivery port, excluding lapses and omissions (defined next). Parametric analysis of pilot data revealed that the variability of rat response latencies in the rPVT was much greater than that typical of human responses in the PVT. Hence, lapses were individually defined, posthoc, as those trials in which response latencies were greater than 2 times the average basal response latency for each rat. This definition of lapses did not skew the distribution of the remaining response latencies and provided a sensitive measure of performance. In the analysis, lapses also included omissions. Omissions were defined as a failure to respond within the 3-second window of opportunity and were used as an indication that rats did simply stop responding. Sessions in which rats made more than 50 omissions were not included in the final analysis, although large blocks of omissions of this type were very rare. As is typical of human PVT studies, omissions were not analyzed independent of lapses. Premature errors were any response during the intertrial interval. Premature response errors and the overall number of responses were recorded and analyzed as secondary measures of performance. The number of responses per session functioned as both a secondary measure of performance and a rudimentary measure of motivation.
Surgery
Guide cannula surgery was carried out under general anesthesia (1% isoflurane). The coordinates for guide cannula placement were; A/P −0.04, M/L 2.2, D/V −6.6 (microdialysis probe tip extended to DV −8.6). Rats were given 1 week on free water for postsurgical recovery before being reintroduced to water restriction over a 3-day period before resuming daily rPVT testing. Each rat entered the experimental phase only after achieving 3 consecutive days of rPVT performance at the presurgery criterion.
BF Dialysis and rPVT
After postsurgical recovery, rats were habituated to being connected to the dialysis cable and dialysis lines for at least 3 days prior to dialysis. Microdialysis probes (CMA Stockholm, Sweden, model CMA 11, 2-mm membrane length) were inserted between 18 and 24 hours prior to dialysis. On adenosine-only dialysis days, vehicle (artificial cerebrospinal fluid; aCSF) dialysis started at 09:00 and continued until 11:00 when the 2 hours of adenosine dialysis was started (without handling the rats), running from 11:00 to 13:00. On control dialysis days, aCSF was continuously dialyzed from 09:00 to 13:00. During all dialysis (and baseline) sessions, rats were disconnected from the dialysis equipment at 13:00 and immediately placed in the operant chambers for a 30-minute rPVT session.
Cyclopentyltheophyline + Adenosine Codialysis
Five of the rats underwent a single dialysis session with adenosine and 8-cyclopentyltheophyline (CPT), an A1 receptor antagonist. rPVT performance was assessed immediately after the completion of dialysis. During adenosine and CPT codialysis sessions, aCSF dialysis was discontinued at 10:30, at which point 1-μM CPT-alone dialysis began and lasted for 30 minutes until 11:00, at which point codialysis of CPT with 300 μM of adenosine began and lasted until 13:00. The choice of 300 μM of adenosine concentration was based on previous work showing that 300 μM effectively decreased the amount of time spent in wakefulness.6,23 Previous work indicates that the concentration of pharmacologic agents outside the membrane is about 1/10th of the inside concentration.23
Histology
After completion of experiments, rats were euthanized with sodium pentobarbital. Rats were perfused transcardially, first with 0.1 M of phosphate buffer and then with 4% buffered paraformaldehyde. Brains were placed in 4% paraformaldehyde overnight, followed by transfer to 30% sucrose-0.1 M phosphate buffer until the tissue block sank. Fifty-micrometer coronal sections were cut on a freezing microtome, and a series of 1-in-6 sections was processed with 0.5% cresyl violet to verify the dialysis site. For the purposes of this experiment, the BF was defined as consisting of the horizontal diagonal band, substantial innominata, magnocellular preoptic nucleus, and the immediately adjacent ventral region with large cholinergic neurons.
Statistical Analysis
rPVT behavior measures were averaged within a session to generate 4 measures from each session (response-latency, number of responses, number of premature errors, and number of lapses) for each rat. To account for interindividual differences behavior measures were analyzed via a mixed-model analysis of variance (SPSS, SPSS Inc., Chicago, IL). Any significant analyses were further analyzed with posthoc t-tests, and all reported p-values (original and posthoc) have been adjusted with a modified (ranked ordered) Bonferroni correction.
RESULTS
Histology
One rat was removed from the study due to an inappropriately placed dialysis probe. Two other rats were euthanized during the study due to postsurgical complications, leaving a total of 9 rats with histologically confirmed cannulae placement in the BF from which data were collected.
rPVT and 300-μM Adenosine BF Dialysis
Observation of the rats during the 30-minute rPVT sessions postdialysis (aCSF and adenosine) revealed that the rats did not sleep during task performance and consistently made a large number of responses (> 100) before making any short pauses in their task performance. Performance was not different between the 2 control conditions (baseline and aCSF) for any measure.
Response Latencies
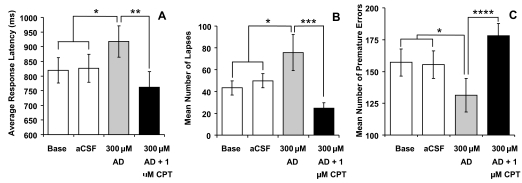
The mixed-model analysis of variance for the response-latency measure was significant (F3,20 = 5.6, p < 0.01). Posthoc analysis revealed that response latencies slowed significantly in the rPVT sessions performed immediately after 2 hours of adenosine dialysis, when compared with both nondialysis baseline and aCSF dialysis (t8 = 2.81, p < 0.05, t8 = 2.53, p < 0.05, respectively, see Figure 1A).
Figure 1.
Rat Psychomotor Vigilance Task. Response latencies are slowed (Panel A), and the mean number of lapses, including omissions, increased significantly (Panel B) after a 300-μM adenosine (AD) infusion into the basal forebrain (BF), compared with baseline and artificial cerebrospinal fluid (aCSF) controls. These behavior effects are reversed by codialysis of 300 μM of adenosine with 1 μM of 8-cyclopentyltheophyline (CPT, an adenosine A1 receptor antagonist) in comparison with the 300-μM adenosine-only condition (Panels A & B). The number of premature errors per session decreased after the 300-μM adenosine infusion into the CBF, compared with baseline and aCSF control, but this reduction was reversed after codialysis of 300 μM of adenosine with 1 μM of CPT (Panel C). *P < 0.05, **P < 0.025, ***P < 0.01, ****P < 0.005. Base refers to nondialysis baseline. Error bars ± SEM.
Lapses
The number of lapses per session increased significantly after adenosine dialysis (F3,20 = 4.98, p < 0.01). In the rPVT session immediately after adenosine dialysis, rats made more lapses than in either the nondialysis baseline or aCSF conditions (t8 = 2.23, p < 0.05, t8 = 2.21, p < 0.05, respectively), see Figure 1B.
Premature Errors
The mixed-model analysis of variance for the premature errors measures was also significant (F3,20 = 3.85, p < 0.05). However, in this case, premature errors actually decreased in the session immediately after adenosine dialysis when compared with aCSF (t8 = 2.44, p < 0.05) and approached significance when compared with nondialysis baseline (t8 = 1.85, p = 0.053), see Figure 1C.
BF Dialysis and rPVT: CPT + Adenosine Codialysis
As predicted, codialysis of the adenosine A1 receptor antagonist CPT (1 μM) with adenosine blocked the effects of 300 μM of adenosine alone on the 3 rPVT measures (see Figure 1A, B, C). Thus, both response latencies and lapses decreased significantly immediately after codialysis with CPT compared with dialysis with adenosine alone (response latencies, t4 = 2.89, p < 0.025, see Figure 1A; Lapses, (t4 = 4.13, p < 0.01, see Figure 1B). The reduction of premature errors produced by dialysis with adenosine alone was reversed by codialysis of adenosine with CPT (t4 = 5.56, p < 0.005, see Figure 1C).
Mean Number of Responses
The mean number of responses per 30-minute session averaged 140. The total number of reinforced responses per operant session did not differ among any experimental condition, indicating motivation was not affected by dialysis of adenosine alone or codialysis of adenosine + CPT.
DISCUSSION
Rats that received bilateral dialysis perfusion of 300 μM of adenosine in the BF immediately prior to performing the rPVT demonstrated a behavior impairment analogous to that of sleep-deprived humans13–16,25 and rats24,26: response latencies slowed and lapses increased significantly. This effect was blocked by the codialysis of an A1-receptor antagonist, demonstrating that the performance impairments were due to elevated adenosine in the BF rather than nonspecific factors. Importantly, however, the rats did not fall asleep during the rPVT sessions, indicating that the carefully selected adenosine dose produced sleepiness and vigilance impairments but did not produce profound sleep that could grossly interfere with operant task performance.
Abundant evidence now supports the hypothesis that adenosine is an endogenous sleep factor that acts potently in the BF to reduce cortical activation and wakefulness. In this hypothesis, elevations of the homeostatic sleep drive produced by sleep disruption lead to an accumulation of adenosine in the BF, which increases sleepiness by inhibiting the cortically projecting/wakefulness-promoting neurons of the BF.8–10,12,27 For example, pharmacologic elevations of adenosine, or adenosine agonist, in the BF lead to a decrease in wakefulness and an increase in sleep in animal studies,10,23,28,29, reviewed in 6 &7 whereas BF dialysis of adenosine antagonists produces opposite effects on sleep and wakefulness.6 Recent work indicates that sleep loss also leads to an upregulation of adenosine A1 receptors in the BF: quantitative positron emission tomographic imaging has demonstrated that cerebral adenosine receptors of the A1 subtype are upregulated in humans after 24 hours of sleep30; a comparable autoradiographic study in rats also found an increase in BF A1 receptors.31
In addition to homeostatic sleep regulation, the BF region has been implicated in the control of sustained attention (for review see2,3). Thus, the fact that deficits in sustained attention are one of the first signs of sleepiness is consistent with studies indicating that the same BF region implicated in the control of the homeostatic sleep drive is involved in the regulation of attention.32,33 Furthermore, neurons of this BF region project to components of the cortical sustained attention network, the activation of which is associated with optimal human performance in the PVT.34 Finally, several studies have examined the effect of caffeine, an adenosine antagonist, on human PVT performance. Caffeine has been shown to reverse PVT impairments in sleepy humans.35–39 As predicted, the effect of adenosine dialysis in the BF of rats on rPVT performance in rats was the opposite of the effect of caffeine ingestion in humans performing the PVT. 35–39
The results of the current study are consistent with those of our previous studies examining the effect of 4-, 7-, and 10-hour total sleep deprivation on attention in the 5-Choice Serial Reaction Time (5CSRT) task26 and the effect of 24 hours of total sleep deprivation on rPVT performance.24 In both tasks, response latencies and lapses (‘omissions’ in the 5CSRT) increased significantly as a result of manipulations predicted to increase sleepiness. Relative to the 5CSRT, the main advantage of the rPVT is that it is directly analogous to the human PVT. Hence, neurobiologic findings, such as those of the present study, can be more convincingly extended to explain vigilance impairments observed in sleepy human subjects. In addition, the rPVT has the practical advantage that rats learn the rPVT task much more quickly than the 5CSRT.
Although the rPVT was developed as a direct analog of the human PVT, the rat and human PVT differ in 2 ways that warrant mention. For one, rats require water restriction to motivate them to perform the operant task, whereas humans do not. As discussed in Christie et al,24 24 hours of total sleep deprivation do not alter water consumption in water-restricted rats; hence, it is unlikely that changes in thirst motivation influenced rPVT performance in the present study. Secondly, the definition used for lapses in the rPVT is slightly different than that often used in human studies. Many human PVT studies focus on response latencies as the primary measure of performance (while also reporting error rates), whereas other studies focus solely on lapses.13,18,20,40 In human PVT studies, lapses are typically defined as a failure to respond within 500 milliseconds, although the cutoff point is at the discretion of the experimenter. The criterion for lapses in the present rPVT study was individually defined for each rat as 2 times their postsurgical, nondialysis, baseline, response-latency performance. Although this differs from the human PVT, setting the lapse criterion at 2 times the baseline was necessary because of the high variability of response latencies between rats in the rPVT, which was largely due to different starting positions when each rat initiated a nose poke. Nonetheless, lapses increased significantly after adenosine dialysis, when compared with both nondialysis baseline and aCSF dialysis, in the same way that lapses increase in sleepy human subjects.13,18,20,40
In the present study, rats made fewer premature errors in the rPVT after 300-μM adenosine dialysis in the BF. This was unexpected because sleep disruption has been shown to increase premature errors in humans15 while having no effect on premature errors in rat studies of sustained attention.24,26 It is generally thought that sleepiness interferes with inhibitory control and, as such, is predicted to produce more, not fewer, anticipatory (premature) errors. A dose-response study employing adenosine dialysis in the BF is one approach to address this apparent contradiction.
In conclusion, this study demonstrates the usefulness of the rPVT for investigating the neurobiologic processes underlying the attention impairments associated with sleep loss and provides additional evidence for the role of adenosine in the BF (and specifically the A1 receptor) in regulating the sleepiness and impaired vigilance associated with the homeostatic sleep drive.
DISCLOSURE STATEMENT
This was not an industry supported study. Drs. Christie, Bolortuya, and Strecker have received research support from Takeda. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Meghan Frisbie, Lance Morin, Nina Connolly, and Glenn Russo for technical assistance, and John Franco for care of the animals. This research was supported by: Department of Veterans Affairs Medical Research Service Award to RES, NHLBI - P50 HL060292 (RES & RWM), NIMH - R37 MH039683 (RWM), NHLBI - T32 HL07901 (MAC & JTM), NIMH - F32 MH070156 (JTM).
Author contributions: MAC designed and conducted the rPVT & microdialysis experiments, analyzed data and wrote the paper; YB conducted the rPVT and microdialysis experiments; JTM and LCC designed and conducted the microdialysis component; RES and RWM designed research, analyzed data, and wrote the paper.
REFERENCES
- 1.McGaughy J, Sarter M. Sustained attention performance in rats with intracortical infusions of 192 IgG-saporin-induced cortical cholinergic deafferentation: effects of physostigmine and FG 7142. Behav Neurosci. 1998;112:1519–25. doi: 10.1037//0735-7044.112.6.1519. [DOI] [PubMed] [Google Scholar]
- 2.Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–83. doi: 10.1016/s0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- 3.Detrain L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Prog Neurobiol. 1999;58:249–77. doi: 10.1016/s0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 4.Lin SC, Gervasoni D, Nicolelis MA. Fast modulation of prefrontal cortex activity by basal forebrain noncholinergic neuronal ensembles. J Neurophysiol. 2006;96:3209–19. doi: 10.1152/jn.00524.2006. [DOI] [PubMed] [Google Scholar]
- 5.Manns ID, Alonso A, Jones BE. Rhythmically discharging basal forebrain units comprise cholinergic, GABAergic, and putative glutamatergic cells. J Neurophysiol. 2003;89:1057–66. doi: 10.1152/jn.00938.2002. [DOI] [PubMed] [Google Scholar]
- 6.Strecker RE, Morairty S, Thakkar MM, et al. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res. 2000;115:183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- 7.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–96. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Thakkar MM, Delgiacco RA, Strecker RE, McCarley RW. Adenosinergic inhibition of basal forebrain wakefulness-active neurons: a simultaneous unit recording and microdialysis study in freely behaving cats. Neuroscience. 2003;122:1107–13. doi: 10.1016/j.neuroscience.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol. 1999;521:679–90. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–23. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 12.McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–30. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 14.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 15.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 16.Urrila AS, Stenuit P, Huhdankoski O, Kerkhofs M, Porkka-Heiskanen T. Psychomotor vigilance task performance during total sleep deprivation in young and postmenopausal women. Behav Brain Res. 2007;180:42–7. doi: 10.1016/j.bbr.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 18.Dinges DF, Weaver TE. Effects of modafinil on sustained attention performance and quality of life in OSA patients with residual sleepiness while being treated with nCPAP. Sleep Med. 2003;4:393–402. doi: 10.1016/s1389-9457(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 19.Sforza E, Haba-Rubio J, de BF, Rochat T, Ibanez V. Performance vigilance task and sleepiness in patients with sleep-disordered breathing. Eur Respir J. 2004;24:279–85. doi: 10.1183/09031936.04.00091903. [DOI] [PubMed] [Google Scholar]
- 20.Czeisler CA, Walsh JK, Roth T, et al. U.S. Modafinil in Shift Work Sleep Disorder Study Group. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005;353:476–86. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- 21.Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J Sleep Res. 2005;14:49–59. doi: 10.1111/j.1365-2869.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- 22.Raymann RJ, Van Someren EJ. Time-on-task impairment of psychomotor vigilance is affected by mild skin warming and changes with aging and insomnia. Sleep. 2007;30:96–103. doi: 10.1093/sleep/30.1.96. [DOI] [PubMed] [Google Scholar]
- 23.Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW. Role of adenosine in behavioral state modulation: a microdialysis study in the freely moving cat. Neuroscience. 1997;79:225–35. doi: 10.1016/s0306-4522(96)00640-9. [DOI] [PubMed] [Google Scholar]
- 24.Christie MA, McKenna JT, Connelly NP, McCarley RW, Strecker RE. 24-hours of sleep disruption in the rat increases sleepiness and decreases vigilance: introduction of the rat-psychomotor vigilance task. J Sleep Res. 2008 doi: 10.1111/j.1365-2869.2008.00698.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam M, Retey JV, Khatami R, Landolt HP. Age-related changes in the time course of vigilant attention during 40 hours without sleep in man. Sleep. 2006;29:55–7. doi: 10.1093/sleep/29.1.55. [DOI] [PubMed] [Google Scholar]
- 26.Cordova CA, Said BO, McCarley RW, Baxter MG, Chiba AA, Strecker RE. Sleep deprivation in rats produces attentional impairments on a 5-choice serial reaction time task. Sleep. 2006;29:69–76. [PMC free article] [PubMed] [Google Scholar]
- 27.McKenna JT, Tartar JL, Ward CP, et al. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146:1462–73. doi: 10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basheer R, Porkka-Heiskanen T, Stenberg D, McCarley RW. Adenosine and behavioral state control: adenosine increases c-Fos protein and AP1 binding in basal forebrain of rats. Brain Res Mol Brain Res. 1999;73:1–10. doi: 10.1016/s0169-328x(99)00219-3. [DOI] [PubMed] [Google Scholar]
- 29.Satoh S, Matsumura H, Koike N, Tokunaga Y, Maeda T, Hayaishi O. Region-dependent difference in the sleep-promoting potency of an adenosine A2A receptor agonist. Eur J Neurosci. 1999;11:1587–97. doi: 10.1046/j.1460-9568.1999.00569.x. [DOI] [PubMed] [Google Scholar]
- 30.Elmenhorst D, Meyer PT, Winz OH, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27:2410–5. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basheer R, Bauer A, Elmenhorst D, Ramesh V, McCarley RW. Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. Neuroreport. 2007;18:1895–9. doi: 10.1097/WNR.0b013e3282f262f6. [DOI] [PubMed] [Google Scholar]
- 32.Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–78. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 33.Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 34.Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–68. [PubMed] [Google Scholar]
- 35.Van Dongen HP, Price NJ, Mullington JM, Szuba MP, Kapoor SC, Dinges DF. Caffeine eliminates psychomotor vigilance deficits from sleep inertia. Sleep. 2001;24:813–9. doi: 10.1093/sleep/24.7.813. [DOI] [PubMed] [Google Scholar]
- 36.Kamimori GH, Johnson D, Thorne D, Belenky G. Multiple caffeine doses maintain vigilance during early morning operations. Aviat Space Environ Med. 2005;76:1046–50. [PubMed] [Google Scholar]
- 37.McLellan TM, Kamimori GH, Bell DG, Smith IF, Johnson D, Belenky G. Caffeine maintains vigilance and marksmanship in simulated urban operations with sleep deprivation. Aviat Space Environ Med. 2005;76:39–45. [PubMed] [Google Scholar]
- 38.Balkin TJ, Bliese PD, Belenky G, et al. Comparative utility of instruments for monitoring sleepiness-related performance decrements in the operational environment. J. Sleep Res. 2004;13:219–27. doi: 10.1111/j.1365-2869.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 39.Retey JV, Adam M, Gottselig, et al. Adenosinergic mechanisms contribute to individual differences in sleep deprivation-induced changes in neurobehavioral function and brain rhythmic activity. J Neurosci. 2006;26:10472–9. doi: 10.1523/JNEUROSCI.1538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]