Abstract
Study Objectives:
To compare a clinical pathway using portable monitoring (PM) for diagnosis and unattended autotitrating positive airway pressure (APAP) for selecting an effective continuous positive airway pressure (CPAP) with another pathway using polysomnography (PSG) for diagnosis and treatment of obstructive sleep apnea (OSA).
Design:
Randomized parallel group
Setting:
Veterans Administration Medical Center
Patients:
106 patients with daytime sleepiness and a high likelihood of having OSA
Measurements and Results:
The AHI in the PM-APAP group was 29.2 ± 2.3/h and in the PSG group was 36.8 ± 4.8/h (P = NS). Patients with an AHI ≥ 5 were offered CPAP treatment. Those accepting treatment (PM-APAP 45, PSG 43) were begun on CPAP using identical devices at similar mean pressures (11.2 ± 0.4 versus 10.9 ± 0.5 cm H2O). At a clinic visit 6 weeks after starting CPAP, 40 patients in the PM-APAP group (78.4% of those with OSA and 88.8% started on CPAP) and 39 in the PSG arm (81.2% of those with OSA and 90.6% of those started on CPAP) were using CPAP treatment (P = NS). The mean nightly adherence (PM-APAP: 5.20 ± 0.28 versus PSG: 5.25 ± 0.38 h/night), decrease in Epworth Sleepiness Scale score (–6.50 ± 0.71 versus –6.97 ± 0.73), improvement in the global Functional Outcome of Sleep Questionnaire score (3.10 ± 0.05 versus 3.31 ± 0.52), and CPAP satisfaction did not differ between the groups.
Conclusions:
A clinical pathway utilizing PM and APAP titration resulted in CPAP adherence and clinical outcomes similar to one using PSG.
Citation:
Berry RB; Hill G; Thompson L; McLaurin V. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. SLEEP 2008;31(10):1423–1431.
Keywords: Sleep apnea, portable monitoring, auto-titration positive airway pressure, CPAP
OBSTRUCTIVE SLEEP APNEA (OSA) IS A COMMON DISORDER, AND ACCESS TO DIAGNOSIS AND TREATMENT IS DELAYED IN SOME AREAS BECAUSE OF limited capacity to perform polysomnography (PSG).1 Attended PSG has been the standard of care for diagnosis2,3 and CPAP titration.3,4 Portable monitoring (PM) using a limited number of bioparameters has been suggested as an alternative to PSG for the diagnosis of OSA.5–8 PM could shorten the time to diagnosis and effective treatment in locations where the access to PSG is limited or delayed and could also offer an alternative to patients who are not willing or unable to undergo an attended PSG. However, in populations with a high proportion of positive studies for moderate to severe OSA, this approach would still require PSG for CPAP titration. In such a circumstance, a clinical pathway consisting of PM for diagnosis followed by PSG for CPAP titration would not have much advantage compared to one using a single partial night (split) PSG consisting of an initial diagnostic portion followed by a CPAP titration. However, unattended autotitration is an alternative to CPAP titration with PSG.9,10 A recent large study11 in a group of patients with severe OSA found that CPAP treatment based on unattended autotitration can result in clinical outcomes similar to CPAP titration with PSG. In this study, patients were carefully selected, evaluated, and followed in a systematic manner. Another study found that diagnosis and treatment of patients with moderate to severe OSA using oximetry and autotitration resulted in equal treatment efficacy and greater CPAP adherence compared to an approach using PSG titration.12 In this study, patients were selected based on clinical evaluation and oximetry before being randomized to PSG or autotitration. A different study found that home monitoring using oximetry and PSG had similar utility in helping clinicians to predict which patients with OSA would benefit from CPAP treatment.13
Obstructive sleep apnea has a high prevalence in the patient population of the Veterans Administration Health Care System (VAHCS).14 In this population, many comorbid conditions in addition to OSA can impair sleep quality. This and a number of other factors such as an appreciable percentage of patients with a low socioeconomic status make this a challenging population to treat. Furthermore, the number of available PSG studies in many VAHCS locales is not sufficient to meet the demand. We hypothesized that a clinical pathway using (1) identification of symptomatic moderate- to high-risk patients, (2) PM for diagnosis, and (3) CPAP treatment based on unattended autotitration would provide equivalent outcomes compared to a clinical pathway using PSG. The goal was to use clinical pathways simulating real world conditions and including symptomatic patients with the entire range of OSA (AHI ≥ 5/h).
METHODS
All patients referred for a sleep study for suspected OSA at the Malcom Randall VAMC attend an education and evaluation class before a sleep study is scheduled. At this class information about sleep apnea, sleep monitoring, and CPAP treatment is presented by a registered nurse using lecture slides, handouts, and videos. Patients fill out a detailed sleep and medical history questionnaire including an Epworth Sleepiness Scale (ESS).15 The patients received a flyer describing the research study and those wishing to participate indicated this intention. Those willing to participate were then screened on the basis of inclusion and exclusion criteria by a physician. All patients included in the study had a primary care physician and had extensive data in the computerized medical record concerning medications, allergies, and medical diagnoses.
Inclusion criteria included daytime sleepiness (ESS ≥ 12) and the presence of ≥ 2 of the following: loud habitual snoring, witnessed apnea/gasping, or treatment for hypertension. A similar approach has been used to identify patients with a moderate to high likelihood ratio of having OSA in previous studies.16,17 Exclusion criteria were residence > 200 miles from the medical center, moderate-to-severe congestive heart failure, use of nocturnal oxygen, moderate-to-severe chronic obstructive pulmonary disease, awake hypercapnia, neuromuscular disease, cataplexy, significant symptoms of the restless legs syndrome, use of daily potent narcotics, uncontrolled psychiatric disorder, night shift or rotating shift work, a prior diagnostic study for a sleep disorder, or prior treatment with positive airway pressure or upper airway surgery. Because of the portable monitoring device being used in the study, patients on α-blockers (terazosin and others) or those not in sinus rhythm were also excluded.
Subjects meeting the above criteria signed an informed consent and were randomized to the PSG pathway or the PM–APAP pathway. The project was approved by the Institutional Review Board of the University of Florida and the Human Studies Subcommittee of the Malcom Randall Veterans Administration Medical Center.
PSG Pathway
In the PSG pathway subjects underwent attended polysomnography for diagnosis and CPAP titration. If the apnea + hypopnea index (AHI) was ≥ 10/h during the first 2 hours of monitoring, a split study was performed (diagnostic portion followed by a CPAP titration). If the criterion for a split study was not met, a diagnostic PSG lasting an entire night was performed. If the AHI was ≥ 5/h on the diagnostic PSG, patients underwent a CPAP titration PSG on another night.
The PSG was performed using standard techniques, and data were recorded digitally (Aurora PSG system, Gamma software, Grass Technologies, West Warwick RI) with continuous video and audio monitoring by the technologist. Two central derivations (C4-A1, C3-A2), two occipital derivations (O2-A1, O1-A2), right and left eye derivations (ROC-A1, LOC-A2), a chin EMG derivation, EKG, nasal pressure, nasal-oral airflow (thermal device), snore sensor (piezoelectric), chest and abdominal piezoelectric bands, pulse oximetry, and right and left anterior tibialis EMG were recorded. During the CPAP titration the flow, leak, and pressure signals from a Synchrony Positive Airway Pressure Device (Respironics, Murrysville, PA) were also recorded. Sleep was staged manually in 30-sec epochs according to Rechtschaffen and Kales criteria.18 Respiratory events were defined using published standards for sleep research.19 An apnea was defined as cessation of airflow for ≥ 10 sec. A hypopnea was defined as any reduction in airflow associated with an arousal or 3% reduction in the arterial oxygen saturation. The CPAP titration consisted of slow upward titration of pressure no faster than 1–2 cm H2O every 10 min, with a goal of eliminating apnea, hypopnea, snoring, and respiratory effort related arousals. Subjects watched a CPAP video, had mask fitting, and breathed on CPAP of 4 cm H2O for 10 min prior to the study. The quality of the CPAP titration was graded as follows: optimal (AHI ≤ 5/h, supine REM sleep on the treatment pressure); good (AHI < 10/h, REM sleep on the treatment pressure), adequate (AHI < 10/h, supine NREM on the treatment pressure), or inadequate if none of these criteria were met.
If the AHI criteria for patients requiring an entire diagnostic night was less than 5/h they then had an PM study and were included in that arm if the AHI ≥ 5/h. If an absence of sleep apnea was confirmed by the PSG and the PM study, patients exited the study. Patients with inadequate CPAP titrations were offered a repeat PSG CPAP titration.
PM-APAP Study Arm
The Watch PAT100 (Itamar Medical, Haifa, Israel) was the portable monitoring device used for diagnosis in this study arm.20–25 The Watch PAT100 (WP100) is a 4-channel device based on the peripheral arterial tone (PAT) with 3 additional channels: heart rate (based on the PAT signal), pulse oximetry (SpO2), and actigraphy. The PAT signal measures the arterial pulsatile volume changes that are regulated by the α-adrenergic innervation of the smooth muscle of the vasculature of the finger. Thus, the PAT signal is a measure of sympathetic nervous activity. The WP100 indirectly detects apnea/hypopnea by identifying surges of sympathetic activity (decrease in PAT signal amplitude) associated with the termination of respiratory events. A respiratory event is detected by a combination of a decrease in PAT signal, increase in heart rate, and changes in arterial oxygen saturation (SpO2) using a proprietary algorithm. Total sleep time is estimated using actigraphy adapted for patients with sleep apnea.23,24 The amount of REM sleep (percentage of total sleep time) is estimated using a proprietary algorithm identifying characteristic changes in heart rate and sympathetic tone associated with this sleep stage. The WP100 AHI (pAHI) has been shown to provide a reasonable estimate of the AHI measured by PSG.20–25
Subjects reported to the research sleep laboratory and were shown a video describing operation of the WP100 and placement of the finger probes (PAT and oximetry probes). A technologist reviewed step-by-step device setup instructions and observed a simulated setup. Patients were given a laminated instruction card and took the device home. Prior to bedtime at home, patients applied the device to the wrist of the nondominant hand, the PAT probe to the 3rd finger, and the oximetry probe to the 4th finger of the hand. Prior to lights out, the patients pushed a button on the WP100 to start the study. In the morning the patient returned the device to the hospital. The flash memory card information was transferred to a computer and analyzed using a computer program (ZZPAT). The automated analysis was used to score the results. However, a physician reviewed the tracings to determine if the data were technically adequate and if the events were correctly identified by ZZPAT. The available results included an AHI (PAHI), TST estimate, %REM estimate, arterial oxygen desaturation index, and the lowest SpO2 value.
An acceptable study was one in which all signals were adequate and both the device TST estimate and the patient's reported sleep time were at least 4 h. In the case of device failure, patient error in application, or inadequate sleep, the study was repeated (one time only). The PM criterion for diagnosis of OSA was a pAHI ≥ 5/h. If an adequate PM study was not diagnostic of OSA, subjects were changed to the PSG arm for confirmation of the absence of OSA. If the absence of OSA was confirmed, the patient exited the study. If two PM studies were inadequate because of technical problems or poor sleep, subjects were also changed to the PSG pathway.
Subjects with a diagnosis of OSA by PM then came to the sleep laboratory for a CPAP instructional video, mask fitting, and training on the use of the APAP device by a sleep technologist. An Autoset Vantage autotitrating positive airway pressure device (ResMed, Poway, CA) was used for autotitration with a nasal or full face mask as judged appropriate by the technologist. Subjects who reported dryness or nasal congestion were studied with heated humidification. An APAP nap practice session consisted of patients applying the selected mask, turning on the device, and wearing the device for 15–20 minutes. Adjustments in mask type were made on the basis of patient complaints and leak. The pressure range was set to 4–18 cm H2O. Subjects took the APAP device home and wore it for 2–3 nights. The device was returned and information transferred to a computer for analysis. An adequate APAP study was defined as cumulative average device use > 4 h and a cumulative residual AHI ≤ 12/h (and AHI < 10/h on at least one night). The study was repeated only once if the initial study was found to be inadequate. Prior to the second APAP study, the mask was changed, or heated humidification was added if these interventions were deemed necessary by the technologist. If a second APAP failure occurred, patients were offered an attended PSG titration. The APAP analysis and single night tracings of pressure and leak were reviewed by a physician. A CPAP treatment pressure was chosen as the 95th percentile pressure.
CPAP Treatment
Following attended CPAP or unattended APAP titration patients were offered treatment with CPAP. Those choosing to accept CPAP treatment reported to a CPAP setup class. In this class they watched a video about OSA and CPAP treatment and were given a CPAP device set according to the prescribed pressure. At the class they were shown interface options and had mask fitting. The subject's spouse was present if possible. All subjects in the study received the REMstar Pro with C-Flex and heated humidity (Respironics, Murrysville, PA). The device has a memory card to record time at pressure. At one week after CPAP setup, subjects received an unsolicited telephone call to ask how they were doing and what problems were encountered. If necessary, subjects returned to the hospital for a pressure or mask change.
Study Questionnaires
On the first study visit of both study arms, subjects completed the Functional Outcomes of Sleep Questionnaire (FOSQ).26 Subjects also completed a Diagnostic Sleep Study Satisfaction Questionnaire on the morning following the WP100 study, a split PSG, or a diagnostic PSG (Appendix A). The questionnaire asked the patient to comment on the comfort of monitoring equipment and the quality of sleep. The answer options ranged from 1 to 5, with higher being more comfortable and better sleep (total score ranged from 2 to 10). Following the APAP titration nights and the CPAP titration PSG (or second portion of the split study), patients completed a PAP titration satisfaction questionnaire (Appendix B). The scores on the PAP titration satisfaction questionnaire ranged from 2 to 10 with higher being more satisfied.
Clinic Evaluation at 6 Weeks
Subjects came to sleep clinic 6 weeks after CPAP setup. The CPAP memory card information was read and subjects filled out an Epworth Sleepiness Scale, a FOSQ, and a CPAP satisfaction questionnaire (Appendix C). At that time, clinically indicated changes in treatment were made, but the subject's study participation ended. From the memory card adherence information values of the average nightly use in hours (all nights) and the percentage of nights with use > 4 h were recorded. The CPAP satisfaction questionnaire consisted of 3 questions, each with possible answers of 1–5 (total score 3–15), with a higher score indicating better satisfaction. The residual AHI as determined by the CPAP device was also recorded as an estimate of how well the OSA was treated.
Data Analysis
Statistical analysis was performed with Sigma Stat (Systat Software, Inc. Chicago, IL). Reported values are expressed as mean ± SEM unless otherwise stated. Comparisons between treatment arms used the unpaired t test unless data failed a test of normality. In that case, the Mann-Whitney rank sum test was used. A chi-squared test was use for proportions.
To identify possible factors associated with higher adherence, correlation coefficients were calculated for CPAP adherence (nightly hours of use) and a number of variables including some associated with higher CPAP adherence in prior studies.27,28 The variables analyzed included the diagnostic AHI, BMI, prestudy ESS, lowest SpO2 during the diagnostic study, treatment pressure, baseline FOSQ, and satisfaction with PAP titration (PSG titration or APAP titration). We also divided the patients in each clinical pathway into those adherent (≥ 70% of nights with use > 4 h) and those non-adherent. A 2-way analysis of variance of the variables used in the correlation analysis was performed to identify significant differences between adherent and non-adherent patients using the 2 factors (PSG vs PM-APAP and adherent vs non-adherent). Such an analysis could also determine if any significant difference found between adherent and non-adherent patients depended on the clinical pathway (interaction term).
A non-inferiority analysis29,30 was planned in case the major endpoint (mean hourly CPAP usage) values were not statistically different. The goal of this type of analysis is to determine if a method being studied (PM-APAP) is not inferior to a standard method (PSG) by more than a chosen amount (equivalence margin). The non-inferiority analysis was performed with PASS software (NCSS, Kaysville UT). The analysis used an alpha of 0.05, the number of subjects in each group, the standard deviation in adherence in each group, and the real difference in adherence between the groups. A one-sided test was used, as the goal of the study was to demonstrate that the adherence with the PM-APAP method was not inferior to PSG. We computed the smallest equivalence margin that would result in a power of 0.80. In the non-inferiority analysis the null hypothesis (H0) is that the actual difference is larger than a specified value. If H0 is true this means that the tested method is inferior to the standard by more than the specified difference. The alternative hypothesis (H1) is that the difference between the methods is equal to or smaller than the specified value. For example, one might choose a difference in adherence of 1 hour. Computation might show that the null hypothesis was rejected at a power of.68. The difference would then be sequentially increased until computation showed a power of 0.80.
RESULTS
The demographic characteristics of patients randomized to each arm of the study and those using CPAP at 6 weeks are shown in Table 1. The patients were predominantly male, obese, and sleepy (mean ESS > 16). The demographic characteristics did not differ between the 2 arms of the study at randomization or for the patients using CPAP at the 6 week clinic visit. Flow diagrams for the 2 study arms are illustrated in Figures 1 and 2.
Table 1.
Study Group Demographics
| Randomized PM-APAP | Randomized PSG | Completed PM-APAP | Completed PSG | |
|---|---|---|---|---|
| Subjects | 53 | 53 | 40 | 39 |
| Age (years) | 51.9 ± 1.7 | 55.1 ± 1.5 | 50.9 ±1.8 | 54.8 ± 1.9 |
| BMI (kg/m2) | 34.0 ± 0.08 | 34.4 ± 0.9 | 35.2 ± 0.9 | 35.5 ± 1.8 |
| Male/Female | 46/7 | 47/6 | 34/6 | 35/4 |
| Epworth Sleepiness Scale | 16.6 ± 0.47 | 16.2 ± 0.54 | 16.4 ± 0.7 | 16.6 ± 0.6 |
| No OSA by testing in randomized arm | 4 | 6 | ||
| No OSA confirmed by crossover | 3 | 4 | ||
| Crossover to other arm after diagnosis of OSA | 1 | 2 | ||
| Crossover to other arm after CPAP titration | 1 | 0 | ||
| OSA after crossover | 51 | 48 | ||
| CPAP setups | 45 | 43 |
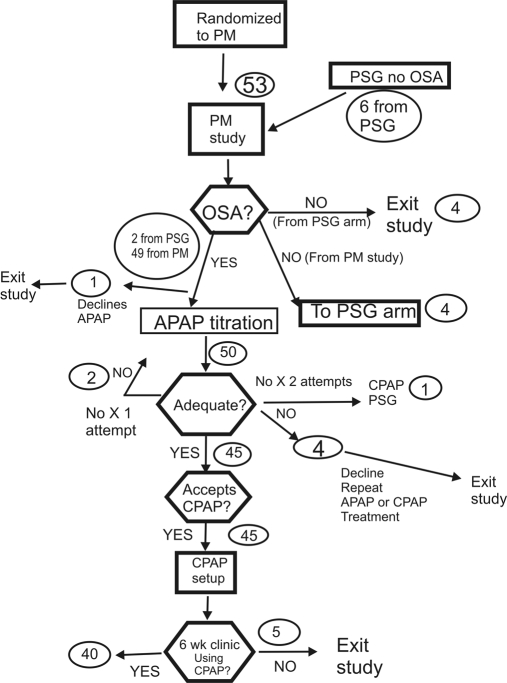
Figure 1.
The patient flow through the PM-APAP arm of the study is illustrated. The round circles show the number of patients in that part of the study.
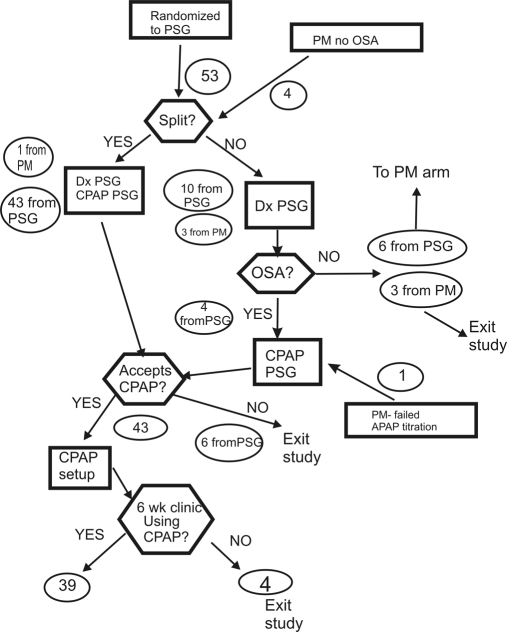
Figure 2.
The patient flow through the PSG arm of the study is illustrated. The round circles show the number of patients in that part of the study.
Diagnostic Testing
The results of diagnostic testing are shown in Table 2. The mean AHI values in both study arms were in the severe range. The AHI was slightly but not significantly higher in the PSG group but not when based on total recording time instead of total sleep time. The lowest arterial oxygen desaturation values were also similar in the two study arms. The total sleep time value in the PM-APAP arm is an estimate to total sleep time supplied by the WP100. Of the 53 patients randomized to the PSG arm, there were 44 split studies (Figure 2). Thus, total sleep time reflects the diagnostic portion of a split study in most patients in the PSG arm. Two of 53 PM studies (3.7%) were repeated because of technical failure and 2 because of insufficient sleep. Of the 53 patients randomized to the PM-APAP arm, 49 had OSA. The 4 patients without OSA on PM testing underwent a PSG; one was found to have OSA. This patient had a split PSG and was started on CPAP treatment. Of the 53 patients randomized to the PSG arm, 6 did not have OSA by PSG testing. These 6 underwent PM testing, and 4 were confirmed to not have OSA. The other 2 had an AHI ≥ 5/h and underwent APAP titration and CPAP treatment. Thus, a very high percentage of patients (99 of 106 = 93%) had OSA in this study. The patient satisfaction with the diagnostic PM study was slightly but not significantly higher than the satisfaction with the diagnostic PSG or diagnostic portion of the split studies.
Table 2.
Diagnostic Results
| Randomized PM-APAP | Randomized PSG | Completed PM-APAP | Completed PSG | |
|---|---|---|---|---|
| Subjects | 53 | 53 | 40 | 39 |
| AHI | 29.2 ± 2.3 | 36.8 ± 4.8 | 33.3 ± 3.8 | 39.8 ± 4.6 |
| 27.8 ± 3.3 (based on TRT) | 33.5 ± 3.7 (based on TRT) | |||
| Low SpO2 (%) | 82.3 ± 0.9 | 84.5 ± 1.4 | 81.3 ± 4.0 | 83.6 ± 1.8 |
| TST (min) | 363.5 ± 12.4 | 167.4 ± 12.5 | ||
| Diagnostic satisfaction (2–10), higher better | 6.5 ± 0.33 | 5.6 ± 0.7 |
Notes: TRT = total recording time, TST in PM-APAP pathway estimated by actigraphy, TST in the PSG group is the amount of sleep during the diagnostic portion, if a split study was performed. Mean TST values in the PM-APAP and PSG groups were not compared statistically as the value in PSG group was smaller by design (most were split studies). No other differences between the PM-APAP and PSG groups were statistically significant.
PAP Titration
In the PM-APAP arm, 50 patients had at least one APAP titration (including 2 patients crossed over from the PSG arm). One of the patients diagnosed with OSA by PM declined an APAP titration. A total of 7 patients (14% of 50) had an inadequate APAP titration on the first attempt. Three of the patients had an additional APAP titration. Two of these 3 had an adequate repeat APAP titration and were eventually started on CPAP. The other patient had an unsuccessful repeat APAP titration and was crossed over to PSG titration and the PSG arm. Four patients had an inadequate APAP trial but declined further APAP titration or CPAP treatment. Thus, 45 patients had a satisfactory APAP titration and were offered CPAP treatment. For these 45 patients, the mean nightly APAP usage was 6.3 ± 0.3 h, and the mean residual AHI was 7.3 ± 0.5/h. All 45 patients accepted CPAP and were started on treatment.
In the PSG arm, 44 patients underwent CPAP titration during a split study (including 1 crossover from the other arm), and 5 underwent a separate night of PSG CPAP titration (including one crossover from the other arm). Of these 49 CPAP titrations, 26 were considered optimal, 18 good, 2 adequate, and 3 inadequate. The 3 patients with inadequate studies declined repeat study and CPAP treatment. Of the 46 with adequate or better CPAP titrations, 3 patients declined CPAP treatment, and 43 started on CPAP. The APAP and CPAP titration satisfaction scores (N = 50 and 49 respectively) were 6.48 ± 0.5 for PM-APAP and 6.85 ± 0.3 for PSG (P = NS).
CPAP Treatment and Adherence
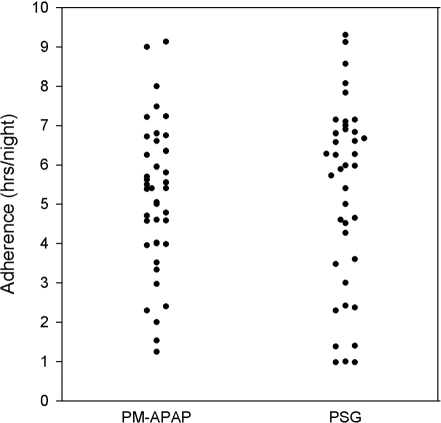
The CPAP treatment statistics and outcomes are shown in Table 3. The mean levels of CPAP chosen for treatment in both groups were very similar. In the PM-APAP arm, 5 of the 45 patients stopped using CPAP before being seen at the 6-week follow-up clinic. In the PSG arm, 4 of 43 patients stopped using CPAP before being seen at the 6-week clinic. The percentage of patients using CPAP did not differ between the 2 treatment arms. The average nightly CPAP use (all nights) was slightly over 5 h in both study groups and did not differ significantly (Figure 3). A non-inferiority analysis showed that our data had an 80% power to determine that the PM-APAP group adherence was not inferior to the PSG adherence by more than 1.2 h. The percentage of nights with greater than 4 h of use was around 70% in both groups and did not differ statistically. The number of patients with > 4 h on 70% or more nights was similar in number or as percentage of patients eligible for treatment (intention to treat) or those accepting CPAP treatment.
Table 3.
CPAP Treatment and Adherence
| PM-APAP | PSG | |
|---|---|---|
| For those randomized: | ||
| Number randomized | 53 | 53 |
| Number with OSA | 51 | 48 |
| Number CPAP setups | 45 | 43 |
| CPAP pressure (cm H2O) | 11.2 ± 0.4 | 10.9 ± 0.5 |
| Using CPAP at 6 weeks | 40 | 39 |
| % CPAP use of those with OSA | 78.4% | 81.2% |
| % CPAP use of those with CPAP setup | 88.8% | 90.6% |
| For those using CPAP at 6 weeks: | ||
| Number using CPAP | N = 40 | N = 39 |
| Average night CPAP use (h/night) | 5.20 ± 0.28 | 5.25 ± 0.38 |
| % of nights > 4 h | 71.7 ± 4.6 | 67.4 ± 6.4 |
| Patients with > 4 h on 70% or more of nights | ||
| Number: | 24 | 22 |
| % with OSA | (47% of 51) | (45.8% of 48) |
| % of CPAP setups | (53.3% of 45) | (51.1% of 43) |
No differences between the PM-APAP and PSG groups were statistically significant.
Figure 3.
Individual CPAP adherence values (all nights) are shown for the PM-APAP and PSG study arms.
We found no significant correlation between nightly CPAP adherence and any of the variables we analyzed (AHI, BMI, baseline ESS, baseline FOSQ, lowest SpO2, treatment pressure, and PAP titration satisfaction) in either clinical pathway or for the group as a whole. A 2-way analysis of variance to identify variables differing between adherent and non-adherent patients found no difference in BMI, baseline ESS, baseline FOSQ, treatment pressure, or lowest SpO2. The pretreatment AHI in adherent patients was higher (40.6 ± 3.8 vs 31.1 ± 4.5/h), but the difference was not statistically significant (P = 0.11). Adherent patients had a higher PAP titration satisfaction (8.2 ± 0.32 vs 6.9 ± 0.4 with a maximum value of 10, P < 0.05). There was no effect of clinical pathway on this difference.
Treatment Outcomes
The effects of CPAP treatment on the patients using CPAP at 6 weeks are shown in Table 4. In both groups there was a sizable and equivalent decrease in subjective sleepiness as assessed by the Epworth Sleepiness Scale. The improvements in the quality of life assessed by the Functional Outcomes of Sleep Questionnaire were very similar. The patient assessment of satisfaction with CPAP treatment was also essentially the same. The mean residual AHI values determined from machine download were also similar and quite low.
Table 4.
Treatment Outcomes
| PM-APAP | PSG | P | |
|---|---|---|---|
| Change in ESS | −6.50 ± 0.71 | −6.97 ± 0.73 | NS |
| Change in FOSQ | 3.10 ± 0.05 | 3.31 ± 0.52 | NS |
| CPAP satisfaction Questionnaire (3–15), 15 most satisfied | 12.8 ± 0.4 | 12.2 ± 0.2 | NS |
| Machine estimate of residual AHI (/hour) | 3.5 ± 0.3 | 5.3 ± 0.7 | NS |
DISCUSSION
This study compared two clinical pathways for the diagnosis and CPAP treatment of OSA in a patient population with daytime sleepiness and a high likelihood of having OSA. A clinical pathway utilizing unattended portable monitoring for diagnosis and APAP titration to establish an effective level of CPAP was compared with one using polysomnography. Patients were required to be sleepy, but a low AHI (≥ 5/h) was acceptable for the diagnosis of OSA and continued study participation. The major findings of the study are that in those patients using CPAP at 6 weeks, the CPAP adherence and improvement in subjective sleepiness and a quality of life measures were similar in the two study arms. Patients in both clinical pathways reported equivalent satisfaction with CPAP treatment.
Portable monitoring has been advocated to improve timely access to diagnosis and treatment for patients with suspected OSA. Validating the use of portable monitoring has been challenging for a number of reasons, including use of PSG as a gold standard (despite its own limitations), night-to-night variability, an AHI computed on the basis of total recording time versus total sleep time, and the large number of different portable monitoring devices, each utilizing different numbers and types of physiological measurements.5–8 In the unattended setting there is always the problem of data loss. In populations with a significant number of negative PM studies, the need for PSG to confirm that a patient does not have OSA could increase the cost and decrease the utility of PM. Conversely, in patient populations with a high likelihood of OSA, a large majority of patients studied with PM devices will need CPAP titration. In this case, one could argue that PM followed by a CPAP PSG has little advantage over a split-night PSG. However, several recent studies11,12 have suggested that use of unattended autotitration can result in equivalent outcomes to attended CPAP titration with PSG in carefully selected patient populations. In these studies, careful protocols for patient education and in one study11 a period of “CPAP practice” was used to identify patients who might not do well with unattended titration. Our study also used a period of APAP practice to identify problems before patients took the device home.
The goal of this study was to compare a clinical pathway designed around portable monitoring and autotitration with one using polysomnography. Such an approach was also taken by Mulgrew and coworkers.12 They selected study patients by symptoms of sleepiness and the Sleep Apnea Clinical Score (using neck size, snoring/gasping during sleep, and hypertension) coupled with an oxygen desaturation index > 15/h. They randomized 86 patients to polysomnography or unattended autotitration. We randomized 106 patients before any diagnostic study and included some milder patients. However, our patients had mean ages, BMIs, and ESS values similar to those of their patients. In their study CPAP adherence was significantly higher in the ambulatory group with a difference in the median values of 1.12 hours. However, both clinical pathways showed similar improvements in sleepiness and quality of life. Thus, our findings are similar and suggest that a pathway utilizing PM and unattended autotitration can result in similar CPAP adherence and outcomes to a pathway using polysomnography.
Study Limitations
This study was performed with a group of patients with a very high likelihood of having obstructive sleep apnea. The results may not apply to populations with a lower prevalence of sleep apnea or in patients without subjective sleepiness. A high proportion of patients in our study population were male; further studies are needed in populations with more females. We obtained our endpoints 6 weeks after CPAP treatment was initiated. It is possible that the results could have differed with longer follow-up. However, previous studies have suggested that the pattern of CPAP use is often set relatively early.31
The AHI in the PM-APAP group was lower than the PSG group, although the difference was not statistically significant. Of note, in the PSG group the AHI was based on the diagnostic portion of a split sleep study in the majority of cases versus an entire night with the PM device. The AHI based on a PM device is usually lower than one based on PSG as the number of events detected by the PM device is divided by the monitoring time instead of the total sleep time to determine the AHI. For example, Whitelaw and coworkers13 randomized a large number of patients with suspected OSA to PSG or home monitoring. The patients had almost identical BMI values, but the AHI was 26/h in the PSG group and 16.6/h in the home monitoring group. In our study the WP100 provides a total sleep time estimate based on actigraphy. This helped eliminate long periods of wakefulness but likely still overestimated total sleep time. When we computed an AHI in the PSG group based on total monitoring time in the diagnostic study (or portion) the AHI was very similar to that in the PM-APAP group. In addition, the CPAP treatment pressures and BMI were quite similar in our two groups. Even if the AHI in the PSG group were significantly higher, we contend that this would favor an increase in CPAP adherence in the PSG group. Some, but not all, studies of CPAP adherence have suggested that a higher AHI predicts improved adherence.27,28 In our study, adherent patients had a higher AHI, but the difference was not statistically significant.
To assess treatment efficacy in the PSG and PM-APAP groups, we compared the residual AHI values obtained from data stored in the CPAP devices. The ability of CPAP devices to accurately determine residual AHI has not been well addressed in peer-review publications. The accuracy could vary between different CPAP manufactures and even between different models from the same manufacturer. Woodson and coworkers32 found the Autoset APAP device (ResMed, Poway, CA) to overestimate the AHI by a mean of only 1.4/h compared to PSG. However, AHI values by the two methods could vary as much as 10/h in individual patients. A recent investigation33 published to date only in abstract form, compared machine and PSG determination of AHI on subtherapeutic CPAP (4 cm H2O) and found AHI mean values (PSG versus CPAP) of 49.7 versus 53.5/h. A similar study34 also published in abstract form found mean AHI values of 20.2 (CPAP) versus 17.1/h (PSG). In this study the correlation between values was 0.925. Both these studies used devices from the same manufacturer of the CPAP devices used in our study. One could argue that even if the CPAP algorithm for AHI determination was not perfect, the same method was used for both patient groups and was not subject to the variability in scoring of respiratory events associated with human judgment. Thus, it seems likely that both groups in our study had equivalent and effective CPAP treatment. This contention is supported by similar treatment outcomes and patient satisfaction with CPAP.
There is always the possibility of type II statistical error when comparison of values between two groups is not statistically significant. Although the means of our major outcomes were virtually identical, our study did not have sufficient power to eliminate the possibility that a difference does exist. One method of dealing with this problem is non-inferiority testing.29,30 In this procedure one can determine a confidence limit for how much lower than a standard (PSG) the comparator (PM-APAP) could be with a selected power. Our study had a power of 0.80 to show that the mean hourly adherence in the PM-APAP group was not inferior to PSG by more than 1.2 hours. It is likely that a similar magnitude of difference in CPAP adherence would be the smallest likely to produce an effect on outcomes. For example, in the study by Mulgrew12 and coworkers the difference in CPAP adherence between groups treated with and without PSG (difference in medians) was 1.12 hours. However, there was no difference in improvement in the ESS or the Sleep Apnea Quality of Life Index at 3 months between the groups.
It is possible that some characteristics of patients might predict less success with the PM-APAP pathway. The only factor we found associated with higher CPAP adherence was higher patient satisfaction with the preceding titration study. However, higher titration satisfaction predicted better CPAP adherence in both clinical pathways. We were not able to identify factors predictive of CPAP adherence that were unique to the PM-APAP pathway. This might be explained by choosing study inclusion and exclusion criteria designed to select patients likely to have a good outcome in either clinical pathway.
In summary, clinical pathways utilizing PSG and portable monitoring and autotitration resulted in similar CPAP treatment acceptance, adherence, and clinical outcomes. Our study group, typical of VAHCS patients, was predominantly male and a very high percentage of the patients had positive studies. Patients in both groups had comprehensive education about sleep apnea and possible treatments before entering the study. Patients in both groups also had careful evaluation via detailed sleep history questionnaires and review of medical records. Recent guidelines for portable monitoring emphasize the importance of clinical evaluation before portable monitoring.8 A substantial amount of time was spent training the patients how to use both diagnostic PM and autotitrating equipment. The results of this study may not be generalizable unless sufficient time is invested in patient education and training on device use. However, this study suggests that a systematic pathway using PM and unattended autotitration can be effective in patients with a high likelihood of having OSA.
DISCLOSURE STATEMENT
This study was sponsored by Itamar Medical with a grant to the North Florida Foundation for Research and Education. The authors did not receive any salary or other compensation form Itamar. Mr. Hill is the owner of a sleep testing company, TimeShare Sleep Services. This company did not perform any testing for this study.
Appendix A
| PM Study Satisfaction | |||||
| Very Uncomfortable | Undecided | Very Comfortable | |||
| How comfortable was the monitoring equipment? | 1 | 2 | 3 | 4 | 5 |
| Poor Sleep | Undecided | Good Sleep | |||
| How well did you sleep last night? |
1 |
2 |
3 |
4 |
5 |
| Split study – diagnostic portion: | |||||
| Very Uncomfortable | Undecided | Very Comfortable | |||
| How comfortable was the monitoring equipment during the first part of the sleep study (no mask) ? | 1 | 2 | 3 | 4 | 5 |
| Poor Sleep | Undecided | Good Sleep | |||
| How well did you sleep during the first part of the study? (no mask) |
1 |
2 |
3 |
4 |
5 |
| Diagnostic PSG | |||||
| Very Uncomfortable | Undecided | Very Comfortable | |||
| How comfortable was the monitoring equipment ? | 1 | 2 | 3 | 4 | 5 |
| Poor Sleep | Undecided | Good Sleep | |||
| How well did you sleep during the study? | 1 | 2 | 3 | 4 | 5 |
Appendix B
| On APAP return | |||||
| Very Uncomfortable | Undecided | Very Comfortable | |||
| How comfortable was using the APAP at home? | 1 | 2 | 3 | 4 | 5 |
| Poor Sleep | Undecided | Good Sleep | |||
| How well did you sleep with the mask on? |
1 |
2 |
3 |
4 |
5 |
| Split study-CPAP titration portion: | |||||
| Very Uncomfortable | Undecided | Very Comfortable | |||
| How comfortable was the CPAP? | 1 | 2 | 3 | 4 | 5 |
| Poor Sleep | Undecided | Good Sleep | |||
| How well did you sleep with the mask on? (second part of the study) |
1 |
2 |
3 |
4 |
5 |
| PSG CPAP titration | |||||
| Very Uncomfortable | Undecided | Very Comfortable | |||
| How comfortable was the CPAP? | 1 | 2 | 3 | 4 | 5 |
| Poor Sleep | Undecided | Good Sleep | |||
| How well did you sleep with the mask on? | 1 | 2 | 3 | 4 | 5 |
Appendix C
CPAP Satisfaction questionnaire
| Dissatisfied | Undecided | Very pleased | |||
| How do you feel about CPAP treatment in general? | 1 | 2 | 3 | 4 | 5 |
| How do you feel about improvement in your symptoms? | 1 | 2 | 3 | 4 | 5 |
| CPAP Less effective | Undecided | CPAP more effective | |||
| How do you feel regarding control of your daytime sleepiness compared to the way you felt before starting CPAP? | 1 | 2 | 3 | 4 | 5 |
REFERENCES
- 1.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–72. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 2.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 3.Kushida CA, Littner MR, Hirschkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 4.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 5.Flemons WW, Littner MR, Rowley JA, Gay P, et al. Home diagnosis of sleep apnea: a systematic review of the literature: an evidence based review. Chest. 2003;124:1543–79. doi: 10.1378/chest.124.4.1543. [DOI] [PubMed] [Google Scholar]
- 6.Chesson AL, Jr, Berry RB, Pack A. Practice parameters for use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep. 2003;26:907–13. doi: 10.1093/sleep/26.7.907. [DOI] [PubMed] [Google Scholar]
- 7.Executive summary on the systematic review and practice parameters for portable monitoring in investigation of suspected sleep apnea in adults. Am J Resp Crit Care Med. 2004;169:1160–3. doi: 10.1164/rccm.169.1160. [DOI] [PubMed] [Google Scholar]
- 8.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitoring in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med. 2007;3:737–48. [PMC free article] [PubMed] [Google Scholar]
- 9.Berry RB, Parish JM, Hartse KM. The use of auto-titrating positive airway pressure for treatment of adult obstructive sleep apnea. Sleep. 2002;25:148–73. [PubMed] [Google Scholar]
- 10.Littner M, Hirshkowitz M, Davila D, et al. Practice parameters for the use of auto-titrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome. An American Academy of Sleep Medicine Report. Sleep. 2002;25:143–7. doi: 10.1093/sleep/25.2.143. [DOI] [PubMed] [Google Scholar]
- 11.Masa JF, Jimenez A, Duran D, Capote F, et al. Alternative methods of titrating continuous positive airway pressure. Am J Respir Crit Care Med. 2004:1218–24. doi: 10.1164/rccm.200312-1787OC. [DOI] [PubMed] [Google Scholar]
- 12.Mulgrew AT, Fox N, Ayas NT, Ryan F. Diagnosis and Initial management of Obstructive Sleep Apnea without polysomnography. Ann Intern Med. 2007;146:157–66. doi: 10.7326/0003-4819-146-3-200702060-00004. [DOI] [PubMed] [Google Scholar]
- 13.Whitelaw WA, Brant RF, Flemons WW. Clinical usefulness of home oximetry compared with polysomnography for assessment of sleep apnea. Am J Respir Crit Care Med. 2005;171:188–93. doi: 10.1164/rccm.200310-1360OC. [DOI] [PubMed] [Google Scholar]
- 14.Mustafa M, Erokwu N, Ebose I, Strohl K. Sleep problems and the risk for sleep disorders in an outpatient veteran population. Sleep Breath. 2005;9:57–63. doi: 10.1007/s11325-005-0016-z. [DOI] [PubMed] [Google Scholar]
- 15.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 16.Flemons WW. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 17.Flemons WW, Whitelaw WA, Brant R, Remmers JE. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279–85. doi: 10.1164/ajrccm.150.5.7952553. [DOI] [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A. A manual of standardized terminology techniques and scoring system for human stages of sleep. Los Angeles: Brain Information Service/Brain Institute, UCLA; 1968. [Google Scholar]
- 19.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of the American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 20.Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP. Using a wrist-worn device based on peripheral tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004;27:923–33. doi: 10.1093/sleep/27.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003;123:695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 22.Ayas NT, Pittman S, MacDonald M, White DP. Assessment of a wrist-worn device in the detection of obstructive sleep apnea. Sleep Med. 2003;4:435–42. doi: 10.1016/s1389-9457(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 23.Hedner J, Pillar G, Pittman SD, Zou D, Grote L, White DP. A novel adaptive wrist actigraphy algorithm for sleep-wake assessment in sleep apnea patients. Sleep. 2004;27:1560–6. doi: 10.1093/sleep/27.8.1560. [DOI] [PubMed] [Google Scholar]
- 24.Penzel T, Kesper K, Pinnow I, Becker HF, Vogelmeier C. Peripheral tonometry and actigraphy for ambulatory recording of sleep apnea. Physiol Meas. 2004;25:1025–36. doi: 10.1088/0967-3334/25/4/019. [DOI] [PubMed] [Google Scholar]
- 25.Pittman SD, Pillar G, Berry R, Malhotra A, MacDonald MM, White DP. Follow-up assessment of cpap efficacy in patients with obstructive sleep apnea using an ambulatory device based on peripheral arterial tonometry. Sleep Breath. 2006;10:123–31. doi: 10.1007/s11325-006-0058-x. [DOI] [PubMed] [Google Scholar]
- 26.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 27.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnea/hypopnea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 28.Meurice JC, Dore P, Paquereau J, et al. Predictive factors of long-term compliance with nasal continuous positive airway pressure treatment in the sleep apnea syndrome. Chest. 1994;105:429–33. doi: 10.1378/chest.105.2.429. [DOI] [PubMed] [Google Scholar]
- 29.Piaggio G, Elbourne DR, Altman DG, Pocok SJ, Evans SJW. Reporting of noninferiority and equivalence randomized trials. JAMA. 2006;295:1152–60. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- 30.Christensen E. Methodology of superiority versus equivalence trials and non-inferiority trials. J Hepatol. 2007;46:947–54. doi: 10.1016/j.jhep.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Weaver TE, Kribbs NB, Pack AI, et al. Night to night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 32.Woodson BT, Saurejan A, Brusky LT, Han JK. Nonattended home automated continuous positive airway pressure titration: comparison with polysomnography. Otolaryngol Head Neck Surg. 2003;128:353–7. doi: 10.1067/mhn.2003.35. [DOI] [PubMed] [Google Scholar]
- 33.Prasad B, Herdegen JJ. REM Star Pro M Series detects breathing events comparable to attended polysomnography. Am J Resp Crit Care Med. 2008:A 940. [Google Scholar]
- 34.Ikeda Y, Kasai T, Takaya H, Maeno K, et al. Validation of breathing event detection of the RemStarAuto-m series compared to clinical polysomnography. Am J Resp Crit Care Med. 2008;177:A177. [Google Scholar]