Abstract
Study Objectives:
To determine the effect of head posture on upper airway collapsibility and site of collapse of the passive human upper airway.
Design:
Pharyngeal critical closing pressure (Pcrit) and site of airway collapse were assessed during head flexion, extension and rotation in individuals undergoing propofol anesthesia.
Setting:
Operating theatre of major teaching hospital.
Participants:
Fifteen healthy volunteers (8 male), including 7 who were undergoing surgery unrelated to the head or neck.
Measurements and Results:
Applied upper airway pressure was progressively decreased to induce variable degrees of inspiratory flow limitation and to define Pcrit. Upper airway and oesophageal pressure transducers identified the site of collapse. Genioglossus muscle activity (EMGgg) was assessed using intramuscular fine wire electrodes inserted percutaneously. Data from 3 subjects were excluded from analysis due to persistent EMGgg. In the neutral posture Pcrit was –0.4 ± 4.4 cm H2O and collapsed most frequently in the velopharyngeal region. Relative to neutral, Pcrit increased to 3.7 ± 2.9 cm H2O (P < 0.01) and decreased to –9.4 ± 3.8 cm H2O (P < 0.01) when the head was flexed and extended, respectively but was unchanged by rotation (–2.6 ± 3.3 cm H2O; n = 10; P = 0.44). The site of collapse varied, in no consistent pattern, with change in head posture in 5 subjects.
Conclusions:
Head posture has a marked effect on the collapsibility and site of collapse of the passive upper airway (measured by EMGgg) indicating that controlling head posture during sleep or recovery from anesthesia may alter the propensity for airway obstruction. Further, manipulating head posture during propofol sedation may assist with identification of pharyngeal regions vulnerable to collapse during sleep and may be useful for guiding surgical intervention.
Citation:
Walsh JH; Maddison KJ; Platt PR; Hillman DR; Eastwood PR. Influence of head extension, flexion, and rotation on collapsibility of the passive upper airway. SLEEP 2008;31(10):1440–1447.
Keywords: Head extension, upper airway, collapsibility, Pcrit, posture, sedation, anesthesia
QUANTIFYING PHARYNGEAL COLLAPSIBILITY HAS PROVEN USEFUL IN RECENT YEARS IN ELUCIDATING THE PATHOPHYSIOLOGY OF OBSTRUCTIVE SLEEP apnea (OSA) and establishing both its severity and efficacy of treatment(s).1–3 The pharyngeal critical closing pressure (Pcrit) which is defined as the applied pressure below which airflow ceases or the airway occludes4,5 is commonly used to describe pharyngeal collapsibility. A lower Pcrit indicates an airway more resistant to collapse and a higher Pcrit, an airway less resistant to collapse. Measurements of Pcrit during sleep can differentiate snoring, apneic, hypopneic, and healthy adults1 and correlate with improvements in apnea severity after treatment such as weight loss and uvulopalatopharyngoplasty.2,3 Measurements of Pcrit obtained in spontaneously breathing adults during general anesthesia correlate with the apnea hypopnea index (AHI) during sleep, especially REM sleep.6
It has long been recognized that head posture influences upper airway patency. Head extension reduces upper airway obstruction and is a commonly used maneuver to maintain airway patency in resuscitation and in anesthesia.7 In contrast, head flexion reduces airflow8 and pharyngeal space.9 An early study in infant cadavers demonstrated increased and decreased upper airway closing pressures during flexion and extension, respectively, although rotation had no significant effect.10 More recently, in vivo studies have demonstrated decreased pharyngeal collapsibility during head extension in anesthetized and paralyzed OSA subjects11 and in midazolam-sedated healthy subjects.12 Head flexion increased pharyngeal collapsibility in anesthetized and paralyzed OSA subjects.11 The effect of head rotation is not clear with one study in midazolam-sedated healthy subjects reporting no change,12 and another study in anesthetized and paralyzed infants reporting increased collapsibility.13
Comparisons of airway collapsibility between studies are difficult due to differing measurement protocols and populations studied. To date, no in vivo study has examined the effect of all head postures (flexion, extension, rotation, and neutral) on airway collapsibility in the same population. In addition, no study has examined the effect of changing head posture on the region of pharyngeal collapse. Such information has important implications for the conduct of studies of airway collapsibility, as failure to control for its effect could provide misleading results. Furthermore, quantification of the effect of head posture on the propensity for airway obstruction could have significant therapeutic implications for anesthesia and sleep: a substantial effect would provide impetus for development of devices to control head position in vulnerable individuals during sleep or recovery from anesthesia.
Anesthesia offers ideal conditions for study of the upper airway as it can be made electromyographically quiescent at clinical anesthesia levels and abolition of the arousal response removes the problem of state changes induced by the measurement procedures. In contrast to other approaches11 our anesthesia model preserves spontaneous ventilation which is important as the associated fluctuations in intraluminal pressure significantly affect upper airway behavior. We have previously demonstrated the parallels in upper airway behavior between anesthesia and sleep.6
The purpose of the present study was to quantify the effect of head extension, flexion, and rotation on collapsibility and site of collapse of a passive upper airway through study of anesthetized volunteers.
MATERIALS AND METHODS
Participants
Subjects were either healthy individuals who had volunteered specifically for this study (n = 8) or patient volunteers who were undergoing general anesthesia for surgery unrelated to the head or neck and were otherwise healthy (n = 7). All subjects provided written informed consent prior to participation in the study which was approved by the hospital's human research ethics committee.
Subject Preparation
No premedication was administered. Standard monitoring was applied including Bispectral Index (BIS) monitoring for electroencephalogram activity. A vein was cannulated and sedation/anesthesia induced with intravenous propofol (Diprivan; Astra Zeneca, Alderley Park, Cheshire, United Kingdom) administered via a Diprifusor (Astra Zeneca) target-controlled infusion system (Alaris PK, Cardinal Health, Switzerland) which calculated effect site concentration on the basis of a 3-compartment pharmacokinetic algorithm.14
Genioglossus electromyogram activity (EMGgg) was measured in 13 of the subjects (including patient volunteers) using 2 pairs of bipolar intramuscular wire electrodes inserted percutaneously in the submental region as previously described.15 The submental skin was pretreated with lidocaine-prilocaine cream (EMLA, AstraZeneca, North Ryde, New South Wales, Australia) ≥30 min under an occlusive dressing. In the 8 non-patient volunteers, these wires were inserted prior to infusion of propofol. In the patient volunteers, wire insertion was performed at a subanesthetic concentration of propofol (calculated effect site concentration of approximately 1.0 μg/mL/kg). The 2 pairs of bipolar EMG electrodes were referenced to a common ground, placed on the mandible. In addition, a bipolar pair was derived from a single wire from each pair, thereby providing a third EMG signal. Each EMGgg signal was amplified, band-pass filtered (10–3,000 Hz, model 7P3; Grass Instruments, West Warwick, RI, USA), full-wave rectified, and processed with leaky integrators with a time constant of 100 ms to yield a moving-time-averaged EMGgg. Immediately following connection of wires and optimization of signals, subjects were asked to perform a series of swallows and maximal tongue protrusions, each effort being separated by a few breaths. Each maneuver was repeated until the peak amplitude of the moving-time-averaged EMGgg was similar for 3 maximal efforts. The propofol target blood concentration was then increased to an effect site concentration of 6.0 μg/mL/kg.
Pharyngeal and esophageal pressure was measured using a 4-sensor pressure transducer catheter (Gaeltec, CTO-4; Dunvegan, Isle of Skye, Scotland) passed via the nares into the esophagus. The oropharyngeal transducer (POP) was visualized through the mouth and positioned just below the soft palate. A transducer 5 cm above the POP transducer measured nasopharyngeal pressure, while transducers 5 and 20 cm below the POP measured hypopharyngeal and esophageal (PES) pressures, respectively. In non-patient volunteers topical lignocaine spray was pre-applied to the nasopharynx and posterior pharynx, and this catheter was inserted while awake, prior to infusion of propofol. In patient volunteers, this procedure was performed following loss of consciousness. The catheter was not inserted in one patient volunteer because of time constraint.
Once the catheters were in position, the subject was fitted with a chin strap, the mouth taped, and a tight-fitting nasal mask applied via which oxygen was delivered with a Bain circuit (fresh gas flow rate ≥14 L/min). A calibrated Fleisch pneumotachograph (Hewlett Packard, Waltham, MA, USA) was attached to the nasal mask to monitor airflow. In series, an expiratory port and bilevel positive pressure pump (BiPAP; Respironics, Murrysville, PA, USA) enabled continuous positive airway pressure (CPAP) to be maintained using the device's inspiratory positive airway pressure mode. A preset lower pressure, using the device's expiratory positive airway pressure mode, or subatmospheric pressure, generated from a regulated vacuum source (model VFC204P; Fuji Electric Co., Tokyo, Japan), could be abruptly attained when required.16 A sample port in the mask was connected to a pressure transducer (model 143PC, micro Switch; Honeywell, Morristown, NJ, USA) for continuous measurement of mask pressure (Pmask). All pressure transducers were calibrated simultaneously prior to subject set-up with 5 known pressures. The head was carefully placed in a neutral position (Frankfort plane [line between tragus of the ear and infraorbital rim] perpendicular to the bed surface) on a Shea headrest, and a fluid goniometer was fitted to the mask to measure the degree of head flexion and extension produced during subsequent maneuvers.
All signals were digitally recorded continuously at 1,000 Hz on a PowerLab data acquisition and analysis system (model 16s; ADInstruments, Sydney, New South Wales, Australia).
Protocol
A sequential pressure drop technique was used to measure Pcrit. Once a propofol effect site concentration of 6.0 μg/mL/kg was obtained and stable ventilation was established, Pmask was rapidly changed (during early expiration) from the maintenance level to a lower pressure for 5 successive breaths (Figure 1). Immediately following the fifth breath, Pmask was further lowered for the next 5 breaths. Pmask continued to be reduced through a range of positive and, where necessary, negative pressures to produce increasing degrees of inspiratory flow () limitation. The sequence was terminated at a Pmask sufficient to abolish (i.e., Pcrit). Each sequence of successive pressure drops lasted a total of approximately 60–120 seconds and included ≥3 pressure levels in which flow limitation was observed. Immediately following each sequence, airway pressure was immediately returned to the maintenance level.
Figure 1.
Polygraph example from one subject (#12) showing the effects of sequential decreases in mask pressure (Pmask) on respiratory flow, esophageal pressure (Pes) and the moving-time-averaged (MTA) genioglossus EMG (EMGgg) when the head was neutral, flexed (10°), extended (18°), and rotated (47°). Selected non-flow limited and flow limited breaths are highlighted in the expanded view (bottom panel).
This pressure drop sequence was performed initially with the head in the neutral posture (the Frankfort plane perpendicular to the bed surface), then repeated with the head flexed, extended, and in all but 2 subjects, rotated. The order of application of flexion, extension, and rotation was randomized. The head/neck was maintained in each posture by one of the experimental team using digital pressure applied to the mentum and vertex. The teeth were held in centric occlusion and care taken not to exert any pressure on the submental region. Flexion and extension were largely restricted to the atlanto-occipital joint, such that the occiput continued to rest on the Shea head rest and measured (goniometer) as the deviation of the Frankfort plane from neutral. Rotation, always to the left side, was measured as deviation of the mid-sagittal plane (line from centre of head to nasion) from vertical, with care taken to avoid flexion or extension. In all but 2 subjects, a final sequence was performed with the head returned to the neutral position. At least 30 seconds of stable breathing at the maintenance pressure was required at each posture before initiating a pressure drop sequence.
Immediately after measurements were completed, EMGgg wires and catheters were removed, and in the patient volunteers, the nasal mask removed and a laryngeal mask (LMA-Classic; Pacific Medical, Victoria, Australia) inserted in preparation for surgery. In the non-patient volunteers, propofol infusion was ceased and the nasal mask was left in place until return of consciousness, at which time it was removed.
Data Analysis
Upper Airway Collapsibility
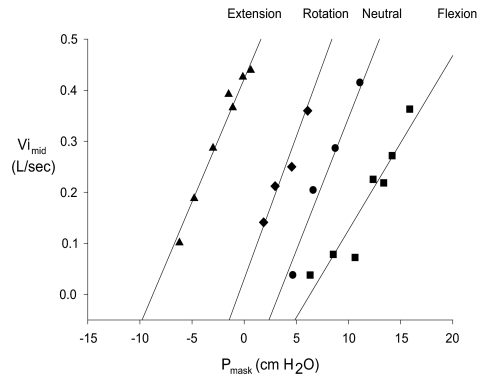
In each head posture, upper airway pressure-flow relationships were evaluated as previously described for sleeping and anesthetized subjects.5,16 Briefly, at each level of Pmask, V̇i and corresponding PES signals were examined. A breath was considered to be flow limited if there was ≥1 cm H2O decrease in PES without any corresponding increase in V̇i.17 Where PES was not measured (one subject), flow-limited breaths were identified as those in which a plateau in the shape of the V̇i profile was observed.5 For each flow-limited breath, the relationship between mid-inspiratory flow (V̇imid) and Pmask were examined.4,5 The mean V̇imid and Pmask from the final 3 breaths at each level of Pmask was calculated and in each head posture a least squares regression equation was computed for the V̇imid and Pmask relationship and solved for Pcrit, the Pmask at which flow became zero (Figure 2). Airway resistance upstream (Rua) to the site of pharyngeal collapse was calculated as the reciprocal of the slope of the regression equation.5
Figure 2.
Pressure-flow relationships for one subject (#12) during head extension (triangles), rotation (diamonds), neutral (circles) and flexion (squares). Pcrit for this subject was −8.1, −0.4, 3.8, and 6.1 cm H2O in the extended, rotated, neutral, and flexed head posture, respectively. Pmask = mask pressure; V̇imid = mid inspiratory flow.
Site of Collapse
The site of pharyngeal collapse was determined from inspection of esophageal and pharyngeal pressures during sequences in which Pmask was lowered to a level sufficient to abolish V̇i. Nasopharyngeal (retropalatal) collapse was considered to be present when esophageal pressure fluctuations were transmitted to the hypopharyngeal and oropharyngeal transducers, but not the nasopharyngeal transducer. Retroglossal pharyngeal collapse was considered to be present when esophageal pressure fluctuations were transmitted to the hypopharyngeal but not oropharyngeal or nasopharyngeal transducers.
Genioglossus Muscle Activity
The moving-time averaged EMGgg was analyzed during breaths at the maintenance pressure and when Pmask was reduced. For each breath, tonic activity was defined as the difference between electrical zero and end-expiratory activity, while phasic activity was defined as the difference between end-expiratory and peak activity during inspiration. Measurements were expressed as a percent of the maximal value obtained during voluntary tongue protrusions and swallows.
Statistical Analyses
The Pcrit measured in each head posture was compared using repeated measures ANOVA. A Holm-Sidak post hoc test was used to determined significance when differences were detected. A Student paired t-test was used to compare measurements of Pcrit from the first and second neutral posture sequences. The Pearson correlation coefficient was also calculated for measurements obtained from these 2 sequences. Unless otherwise stated, all values are reported as mean ± SD. A value of P < 0.05 was considered significant.
RESULTS
Eight male and 7 female subjects participated in the study. Data from 3 male subjects (all non-patient volunteers) were not included in the analyses due to persistent phasic EMGgg (>5% wakeful maximum) at a propofol effect site concentration of 6.0 μg/mL/kg. Of the remaining 12 subjects, mean age was 37.4 ± 11.2 years (range 19–60) and BMI 23.8 ± 2.4 kg/m2 (20.4–29.3).
The depth of anesthesia, as assessed by BIS, remained constant throughout the study, being 30 ± 7 (17–42), 27 ± 6 (22–42), 26 ± 4 (22–36), and 24 ± 3 (22–30) units in the neutral, flexed, extended, and rotated head postures, respectively (P = 0.55).
Effect of Head Posture on Upper Airway Collapsibility
Measurements of airway collapsibility were obtained with the head neutral and in 10 ± 2 (7–13), 20 ± 3 (15–28) and 41 ± 8 (32–55) degrees of flexion, extension, and rotation, respectively. A linear relationship was obtained between Pmask and V̇imid of flow limited breaths at each head posture (Figure 2).
The effect of head posture on upper airway collapsibility is shown for each individual and for the group in Figs. 3A and 3B, respectively. Pcrit was just below atmospheric pressure when the head was in the neutral posture (−0.4 ± 4.4 cm H2O). Relative to neutral, Pcrit increased during flexion (by 4.9 ± 3.1 cm H2O, P < 0.01) and decreased during extension (by 7.4 ± 3.7 cm H2O, P < 0.01) (Figure 3B). A similar pattern was also observed in the 3 subjects who were excluded from analysis on the basis of persistent EMGgg activity. In these 3 subjects, Pcrit when neutral was –2.1 ± 8.3 cm H2O and increased (relative to neutral) by 5.7 ± 3.8 cm H2O during flexion and decreased by 5.9 ± 4.2 cm H2O during extension.
Figure 3.
Changes in upper airway collapsibility (Pcrit) in each individual (A) and the group (B) (Error bars, Mean ± SD) when the head was in flexion, neutral, rotation and extension. Note that Pcrit was not measured during head rotation in 2 subjects (dashed lines). *P < 0.01
The effect of head rotation on collapsibility was less consistent than the effects of flexion or extension. Relative to the neutral posture, head rotation increased Pcrit in 5 subjects and decreased Pcrit in 5 subjects (Figure 3A). For this group, Pcrit when neutral and during rotation were not significantly different (−1.8 ± 3.4 vs −2.6 ± 3.3 cm H2O; n = 10; P = 0.44).
Pcrit was higher in males than females when the head was extended (−3.7 ± 5.6 vs −10.7 ± 3.3 cm H2O, respectively, P < 0.01) and rotated (1.8 ± 2.2 vs −4.5 ± 2.3 cm H2O, respectively, P = 0.03), but was similar when the head was neutral or flexed (P = 0.18 and P = 0.08, respectively).
Resistance upstream to the site of collapse was unaffected by head posture, being 11.8 ± 3.9, 16.8 ± 6.6, 18.1 ± 10.7 and 16.6 ± 8.8 cm H2O/L/sec in the neutral, flexed, extended, and rotated head postures, respectively (P = 0.55)
Reproducibility of Pcrit
In the 10 subjects in which Pcrit was measured twice in the neutral posture, mean Pcrit for the first and second measurements were similar, being −1.9 ± 3.4 and −1.8 ± 2.8 cm H2O, respectively (P = 0.85). The intraclass correlation coefficient for these measurements was 0.912 (P < 0.01).
Muscle Activity
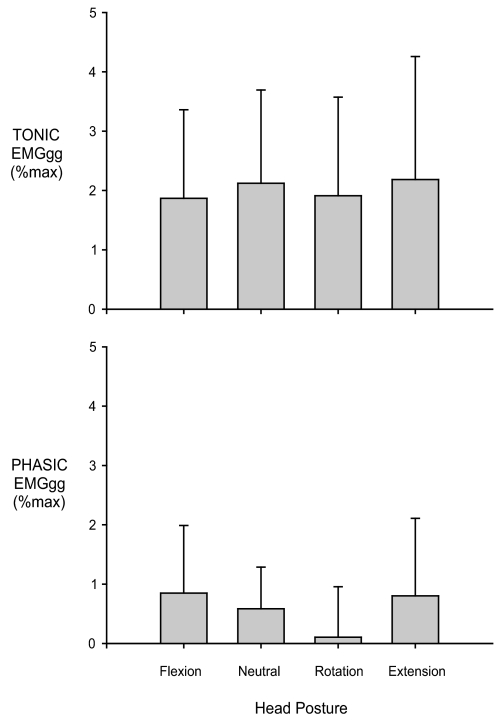
Measurements of EMGgg were obtained in all but 2 subjects. The amplitude of the moving-time-averaged EMGgg tended to increase over the course of a sequence of pressure drops (Figure 1). However, even during the most severe flow limitation (i.e., at a Pmask very close to Pcrit) both tonic and phasic EMGgg remained low (mean for all head postures being 2.0% ± 0.2% and 0.6% ± 0.3% of maximum, respectively) and was unaffected by head posture (Figure 4). EMGgg activity was also unchanged by head posture in the 3 subjects in whom phasic EMGgg persisted at propofol effect site concentration of 6.0 μg/mL/kg.
Figure 4.
Effect of head posture on tonic and phasic genioglossus electromyogram (EMGgg) activity during severe flow limitation (i.e,. at a Pmask very close to Pcrit) (n = 10). The magnitude of the EMGgg was not significantly different between head postures. Error bars, Mean ± SD.
Site of Collapse
Regional pharyngeal pressure measurements were collected in 11 subjects. In the neutral head posture, pharyngeal collapse occurred in the retropalatal (nasopharyngeal) region in 8 subjects and in the retroglossal (oropharyngeal) region in 3 subjects. The site of collapse did not change between repeated measurements in the 10 subjects in whom measurements were obtained twice with the head in the neutral posture.
The site of collapse was unaffected by changing head posture in 6 subjects. In the remaining 5 subjects the site of collapse changed inconsistently: in 3 subjects who had nasopharyngeal collapse when neutral, the site of collapse changed to oropharyngeal with rotation (n = 1) and flexion (n = 1), and to hypopharyngeal with extension (n = 1); and in 2 subjects the site of collapse changed from oropharyngeal when neutral to nasopharyngeal with flexion (n = 1), and with both extension and rotation (n = 1)
DISCUSSION
This study shows that head extension and flexion have a marked influence on collapsibility of the passive upper airway. Compared to the neutral posture, head flexion increases and head extension decreases the propensity for the upper airway to collapse. The magnitude of the effect, expressed in terms of the difference in Pcrit between 20 ± 3 degrees of extension and 10 ± 2 degrees of flexion was 12.3 ± 3.5 cm H2O. The increase in Pcrit between head neutral and 10 ± 2 degrees of flexion was 4.9 ± 3.1 cm H2O. These differences are similar to the difference in Pcrit reported as being sufficient to discriminate between non-apneic snorers and individuals with OSA1 and between normal individuals and those with upper airway resistance syndrome during sleep.18 These findings indicate that, within an individual, head posture-related changes in upper airway anatomy can have substantial effects on patency of the hypotonic pharynx. Such a finding may have clinical implications for manipulating head position as a therapy for obstructive sleep apnea. Further, the findings highlight the need to standardize or account for head posture when measuring and interpreting measurements of upper airway collapsibility.
Neuromuscular Responses
Upper airway patency is determined by the precise balance between the neural control of the pharyngeal dilator muscles (neuromuscular factors) and the structural properties (anatomic factors) of the airway. In the present study we used propofol anesthesia, which activates γ-aminobutyric acid (GABA) neurons with direct and indirect inhibitory effects on the hypoglossal motor nucleus, to attenuate upper airway muscle activity but maintain spontaneous ventilation. This allows the mechanical properties of the airway to be studied with minimal neurogenic influence.16 In the majority of subjects we were able to obtain measurements of Pcrit in an upper airway that was essentially hypotonic: tonic and phasic EMGgg activity were, on average, 2.0% ± 0.2% and 0.6% ± 0.3% of maximum in the presence of severe inspiratory flow limitation. In the 3 subjects with persistent phasic activity (>5% of wakeful maximum), such activity appeared to have minimal effect on behavior of the upper airway, as the magnitude and pattern of change of Pcrit with changes in head posture in these individuals was not different from the remaining 12.
Measuring Upper Airway Collapsibility
Measurements of upper airway collapsibility were obtained by progressively decreasing mask pressure in order to elicit variable degrees of inspiratory flow limitation until V̇i approached zero. This technique was convenient as it allowed us to obtain multiple measurements of Pcrit over a relatively brief time, each measurement taking between 60 and 120 seconds. In the 10 subjects in whom measurements were obtained twice with the head in the neutral posture the difference in Pcrit was only 0.1 ± 1.4 cm H2O. This high reproducibility most likely reflects the highly standardized conditions under which the measurements were obtained. That is, the head, jaw, and body posture were tightly controlled and the depth of general anesthesia maintained constant.
Potentially confounding the measurement of Pcrit using a sequential pressure drop technique, as used in the present study, is progressive activation of upper airway muscles over the course of the pressure drop sequence as a consequence of progressively more severe flow limitation. Indeed a slight progressive increase in amplitude of the moving time averaged EMGgg was noted over the course of a pressure drop sequence (Figure 1). However we consider it unlikely that this activity significantly affected the measurement of Pcrit for the following reasons: First, even during the most severe flow limitation (i.e., at a Pmask very close to Pcrit) both tonic and phasic EMGgg remained very low and was unaffected by head posture (Figure 4). Second, the Pcrit for the group (−0.4 ± 4.4 cm H2O) was similar to what we have previously reported using an intermittent pressure drop technique (1.4 ± 3.5 cm H2O) in which mask pressure is reduced in 5-breath sequences before being returned to the maintenance pressure.16 These 2 techniques have recently been compared in healthy adults, revealing similar measurements of Pcrit.19 Third, if increased EMG activity at greater degrees of flow limitation had acted to “stiffen” the airway we would have expected greater flow for a given pressure at pressures near Pcrit and the appearance of an alinearity in the pressure-flow relationship at these pressures. This was not the case: the relationship between pressure and flow during this relatively lengthy period of progressive flow limitation remained linear at each head posture.
The Effect of Head Posture on Airway Collapsibility
Relative to the neutral posture, the passive upper airway was significantly more collapsible when the head was extended and significantly less collapsible when the head was flexed. These differences in collapsibility cannot be attributed to posture-induced changes in pharyngeal muscle activity, as it remained unchanged under the condition of propofol anesthesia. For this reason the mechanism is likely to be mechanical in origin.
The mechanism underlying the changes in pharyngeal collapsibility associated with changing head posture may relate to tracheal traction; an increase in tracheal length and traction accompanying head extension and decrease in length and traction with flexion may alter extraluminal tissue pressure and consequently influence transmural pressure and airway collapsibility.8,20–22 Posture-related displacement of the tongue and upper airway structures could also contribute to the differences in airway collapsibility by virtue of their effects on upper airway size.7,23–25 It is unlikely that changes in airway resistance contributed to the observed changes, as airway resistance upstream of the site of collapse was not different between head postures.
The magnitude of change in Pcrit with changes in head posture was substantial: Pcrit during head extension was 7.4 ± 3.7 cm H2O less than when the head was neutral and 12.3 ± 3.5 cm H2O less than when the head was flexed. These findings are consistent in direction but not magnitude with an endoscopic study in anesthetized and paralyzed OSA patients which found Pcrit to be approximately 3.5 cm H2O less during head extension than neutral and approximately 1 cm H2O greater during head flexion.11 Similarly, a study in 16 healthy male subjects sedated with midazolam (BIS 27 units) demonstrated a 4.3 cm H2O reduction in Pcrit during head extension relative to neutral.12 Our finding that rotation did not significantly alter Pcrit compared to neutral is similar to that reported in healthy midazolam-sedated subjects12 and infant cadavers,10 but differs from findings in anesthetized, paralyzed infants and small children in whom Pcrit increased during rotation relative to neutral.13 Differences between studies may be due to several factors including the degree of extension, flexion and rotation, study population ages, level of pharyngeal muscle activity during measurement, and measurement and anesthetic techniques. In the latter regard it is important to note that spontaneous respiration was preserved in our anesthesia model.
Translating these findings to the sleeping condition suggests that the upper airway may be particularly vulnerable to collapse during head flexion. Variations in head flexion/extension appear a plausible explanation for changes in severity of OSA observed in individuals that are not explained by change in whole body posture, sleep state, or mouth opening. Avoidance of head flexion during sleep may prove a useful adjunctive therapy for obstructive sleep apnea, although further investigation is warranted. The degree of flexion was substantial in our study (10 ± 2°) and it remains unknown how increase in collapsibility is related to magnitude of flexion, although a progressive change is suggested by findings from a study in infant cadavers.10
The magnitude of the reduction in airway collapsibility with head extension was also striking suggesting that head extension could reduce the severity of airway obstruction in sleep apneics. To date, the effect of head posture modification as a treatment modality for OSA has been reported in only a few studies, with mixed results. A cervical pillow purporting to promote head extension has been shown to improve AHI in patients with mild OSA26,27 but not severe OSA.26 More recently, Skinner et al showed that 40% of patients had complete or partial treatment success by using a cervicomandibular support collar designed to maintain 5° of head extension and prevent downward displacement of the mandible.28
Effect of Head Posture on the Site of Pharyngeal Collapse
When the head was in the neutral posture pharyngeal collapse occurred most frequently behind the soft palate (in 8 of 11 subjects). This tendency for the primary site of collapse to be in the velopharynx is consistent with many previous reports from studies conducted under a variety of conditions including anesthesia,29 sedation,30 and sleep.31 However, all previous studies have examined collapsibility under a single posture and/or with posture unspecified. Thus little is known about the effect of changes in head posture on the primary site of collapse.
In 6 of 11 subjects the site of collapse did not vary with changing head posture. However, in 5 subjects the primary site changed with changing head position. Nothing obvious separated these 5 subjects from those in whom the site of collapse was unaffected by head posture. The variability of site of collapse with head posture suggests that, in some individuals, measurements during sedation in a single position may not reflect behavior during sleep, with its potential for a variety of head positions. These findings raise the intriguing possibility of manipulating head posture during propofol sedation to uncover pharyngeal regions vulnerable to collapse for the purpose of guiding proposed surgical intervention or suitability for dental (mandibular repositioning) therapies.
DISCLOSURE STATEMENTS
This was not an industry supported study. Dr. Eastwood and Dr. Hillman have consulted for Inspiration Medical Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful for the assistance of the technical staff in the Department of Anesthesia and the West Australian Sleep Disorders Research Institute.
Financial Support: This study was supported by a NHMRC project grant (No. 303218) and the Sir Charles Gairdner Hospital Medical Research Foundation. PRE was supported by a NHMRC Senior Research Felowship (No. 513704).
Institution: This study was conducted in the Departments of Pulmonary Physiology and Anesthesia, Sir Charles Gairdner Hospital, Australia.
REFERENCES
- 1.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143:1300–3. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494–8. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz AR, Schubert N, Rothman W, et al. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1992;145:527–32. doi: 10.1164/ajrccm/145.3.527. [DOI] [PubMed] [Google Scholar]
- 4.Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996;110:1077–88. doi: 10.1378/chest.110.4.1077. [DOI] [PubMed] [Google Scholar]
- 5.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64:789–95. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 6.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet. 2002;359:1207–9. doi: 10.1016/S0140-6736(02)08224-7. [DOI] [PubMed] [Google Scholar]
- 7.Safar P, Escarraga LA, Chang F. Upper airway obstruction in the unconscious patient. J Appl Physiol. 1959;14:760–4. doi: 10.1152/jappl.1959.14.5.760. [DOI] [PubMed] [Google Scholar]
- 8.Thut DC, Schwartz AR, Roach D, Wise RA, Permutt S, Smith PL. Tracheal and neck position influence upper airway airflow dynamics by altering airway length. J Appl Physiol. 1993;75:2084–90. doi: 10.1152/jappl.1993.75.5.2084. [DOI] [PubMed] [Google Scholar]
- 9.Liistro G, Stanescu D, Dooms G, Rodenstein D, Veriter C. Head position modifies upper airway resistance in men. J Appl Physiol. 1988;64:1285–8. doi: 10.1152/jappl.1988.64.3.1285. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SL, Thach BT, Brouillette RT, Abu-Osba YK. Upper airway patency in the human infant: influence of airway pressure and posture. J Appl Physiol. 1980;48:500–4. doi: 10.1152/jappl.1980.48.3.500. [DOI] [PubMed] [Google Scholar]
- 11.Isono S, Tanaka A, Tagaito Y, Ishikawa T, Nishino T. Influences of head positions and bite opening on collapsibility of the passive pharynx. J Appl Physiol. 2004;97:339–46. doi: 10.1152/japplphysiol.00907.2003. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda H, Ayuse T, Oi K. The effects of head and body positioning on upper airway collapsibility in normal subjects who received midazolam sedation. J Clin Anesth. 2006;18:185–93. doi: 10.1016/j.jclinane.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa T, Isono S, Aiba J, Tanaka A, Nishino T. Prone position increases collapsibility of the passive pharynx in infants and small children. Am J Respir Crit Care Med. 2002;166:760–4. doi: 10.1164/rccm.200110-044OC. [DOI] [PubMed] [Google Scholar]
- 14.Schnider TW, Minto CF, Gambus PL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998;88:1170–82. doi: 10.1097/00000542-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol. 2003;94:1849–58. doi: 10.1152/japplphysiol.01017.2002. [DOI] [PubMed] [Google Scholar]
- 16.Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology. 2005;103:470–7. doi: 10.1097/00000542-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Mansour KF, Rowley JA, Meshenish AA, Shkoukani MA, Badr MS. A mathematical model to detect inspiratory flow limitation during sleep. J Appl Physiol. 2002;93:1084–92. doi: 10.1152/japplphysiol.01140.2001. [DOI] [PubMed] [Google Scholar]
- 18.Gold AR, Marcus CL, Dipalo F, Gold MS. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002;121:1531–40. doi: 10.1378/chest.121.5.1531. [DOI] [PubMed] [Google Scholar]
- 19.Marcus CL, Fernandes Do Prado LB, Lutz J, et al. Developmental changes in upper airway dynamics. J Appl Physiol. 2004;97:98–108. doi: 10.1152/japplphysiol.00462.2003. [DOI] [PubMed] [Google Scholar]
- 20.Jin-Hee K, Ro YJ, Seong-Won M, et al. Elongation of the trachea during neck extension in children: implications of the safety of endotracheal tubes. Anesth Analg. 2005;101:974–7. doi: 10.1213/01.ane.0000169330.92707.1e. table of contents. [DOI] [PubMed] [Google Scholar]
- 21.Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TC. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep. 2007;30:179–86. doi: 10.1093/sleep/30.2.179. [DOI] [PubMed] [Google Scholar]
- 22.Kairaitis K, Parikh R, Stavrinou R, et al. Upper airway extraluminal tissue pressure fluctuations during breathing in rabbits. J Appl Physiol. 2003;95:1560–6. doi: 10.1152/japplphysiol.00432.2003. [DOI] [PubMed] [Google Scholar]
- 23.Rubinstein I, McClean PA, Boucher R, Zamel N, Fredberg JJ, Hoffstein V. Effect of mouthpiece, noseclips, and head position on airway area measured by acoustic reflections. J Appl Physiol. 1987;63:1469–74. doi: 10.1152/jappl.1987.63.4.1469. [DOI] [PubMed] [Google Scholar]
- 24.Shorten GD, Armstrong DC, Roy WI, Brown L. Assessment of the effect of head and neck position on upper airway anatomy in sedated paediatric patients using magnetic resonance imaging. Paediatr Anaesth. 1995;5:243–8. doi: 10.1111/j.1460-9592.1995.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 25.Sivarajan M, Joy JV. Effects of general anesthesia and paralysis on upper airway changes due to head position in humans. Anesthesiology. 1996;85:787–93. doi: 10.1097/00000542-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Kushida CA, Rao S, Guilleminault C, et al. Cervical positional effects on snoring and apneas. Sleep Res. 1999;2:7–10. [PubMed] [Google Scholar]
- 27.Kushida CA, Sherrill CM, Hong SC, Palombini L, Hyde P, Dement WC. Cervical positioning for reduction of sleep-disordered breathing in mild-to-moderate OSAS. Sleep Breath. 2001;5:71–8. doi: 10.1007/s11325-001-0071-z. [DOI] [PubMed] [Google Scholar]
- 28.Skinner MA, Kingshott RN, Jones DR, Taylor DR. Lack of efficacy for a cervicomandibular support collar in the management of obstructive sleep apnea. Chest. 2004;125:118–26. doi: 10.1378/chest.125.1.118. [DOI] [PubMed] [Google Scholar]
- 29.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–26. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 30.Suto Y, Inoue Y. Sleep apnea syndrome. Examination of pharyngeal obstruction with high-speed MR and polysomnography. Acta Radiol. 1996;37:315–20. doi: 10.1177/02841851960371P166. [DOI] [PubMed] [Google Scholar]
- 31.Suratt PM, Dee P, Atkinson RL, Armstrong P, Wilhoit SC. Fluoroscopic and computed tomographic features of the pharyngeal airway in obstructive sleep apnea. Am Rev Respir Dis. 1983;127:487–92. doi: 10.1164/arrd.1983.127.4.487. [DOI] [PubMed] [Google Scholar]